Introduction
The mandibular condylar chondrocyte (MCC) is the key
functional cell of the temporomandibular joint (TMJ). A number of
TMJ diseases are caused by damage to these cells. Epidemiological
studies have identified a difference in TMJ morbidity between male
and female patients; TMJ morbidity in female patients is higher
than in male patients and data indicate that changes in estrogen
levels affect the metabolism of MCCs (1), suggesting that abnormal levels of
estrogen may be associated with TMJ disease (2). A previous study, which generated
different estrogen-level experimental environments and specifically
regulated the expression of estrogen receptors (ERs), found that ER
expression did not explain all phenomena observed in clinical and
animal studies (3). This suggests
that in addition to the ER classical signaling pathway, there may
be other signaling pathways, with and without ERα association,
involved in estrogen-related diseases.
Estrogen-related receptor α (ERRα) is a type of
orphan nuclear receptor that shares homology with ERs but without
the binding of estrogen (4). In
vitro studies have demonstrated that ERRα is expressed in the
torso cartilage tissues of humans and animals, and is involved in a
number of metabolic activities, including inflammation (5–10).
The regulatory response of ERRα to estrogen may depend on the
species, tissue and cell types (11,12),
and the expression and functions of ERRα in temporomandibular
tissues and cells remain unclear.
In the present study, the ERRα expression levels in
the MCCs of female Sprague-Dawley (SD) rats were investigated, to
analyze the impact of estrogen on the biological characteristics of
MCCs through the mediative effects of ERRα. This may enable further
understanding of MCC characteristics, as well as the mechanisms of
physiological and pathological changes in MCCs.
Materials and methods
Primary cultivation and identification of
MCCs
The present study was conducted in strict accordance
with the recommendations of the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The animal
use protocol was reviewed and approved by the Institutional Animal
Care and Use Committee of the Fourth Military Medical University
(Xi’an, China). Six female SD rats (age, eight weeks), were
sacrificed by cervical dislocation. The rats were then soaked in
75% ethanol and MCCs were separated under sterile conditions.
Serum-free RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) was
used to remove and rinse blood and other impurities adhered to the
articular cartilage. The articular cartilage was then repeatedly
cut with ophthalmic scissors to form sections of ~1 mm3.
A total of 2–3 ml of 0.25% trypsin solution (Sigma-Aldrich) was
added for digestion in the incubator at 37°C for 20 min; 3 ml of
0.2% type II collagenase solution (Sigma) was then added and the
mixture was further incubated for 2 h. The cell suspension was
pipetted, filtered using 200 mesh and centrifuged at 300 × g for 5
min, and the supernatant was removed. RPMI-1640 medium supplemented
with 10% fetal bovine serum was used to prepare the cell
suspension. The cells were counted, then seeded and incubated at
37°C, in 5% CO2 and saturated humidity. The cells were
then purified using the differential adhesion method (13). After 48 h, the medium was replaced
to remove any nonadherent cells. The remaining cells were
cultivated for 3–5 days, then the medium was replaced with the
common medium. When cell fusion had reached 80–90%, passage was
performed. The cell morphology was observed with an inverted
microscope (Olympus, Tokyo, Japan),.
Immunofluorescent staining
The third generation of logarithmically-growing MCCs
was prepared as a single cell suspension, then inoculated into the
Lab-Tek II chamber slide (NUNC, Roskilde, Denmark) with density of
~5×104 cells/chamber. The original culture medium was
removed after 24 h conventional cultivation. Following washes with
phosphate-buffered saline (PBS) solution for 3 times, 4%
paraformaldehyde was added for fixation and 0.25% Triton X
(Invitrogen Life Technologies, Carlsbad, CA, USA) was added for
15-min incubation at 37°C. After 15 min soaking in 3%
H2O2, goat serum (Boster, Wuhan, China) was
added to cover the cell-attached coverslip for blocking. The slide
was then incubated for 30 min at 37°C, followed by washing with PBS
three times. Type II collagen polyclonal antibody (titer 1:100;
Sigma-Aldrich) was added and the mixture was incubated for 2 h at
37°C. PBS was used to replace the primary antibody in the negative
control group. Following three washes with PBS, the secondary
FITC-conjugated goat anti-mouse IgG (Beyotime, Nantong, China) was
added in the dark and the slide was incubated for 1 h at 37°C then
washed with PBS three times. The residue fluid was drained and DAPI
was added for 15 min nuclear staining in the dark. Fluorescence
microscopy (Olympus) was then used to observe and capture images of
the staining situations.
Alcian blue staining
The MCCs were digested with 0.25% trypsin solution
at 37°C for 3 min, then seeded onto a 6-well plate and cultivated
using the normal cultivation method. When the wall-adherent cells
had grown to 80% confluence, the original culture medium was
removed, the cells were washed with PBS buffer three times and 4%
formaldehyde was used for fixation. The cells were again washed
three times with PBS buffer, Alcian blue (Sigma) was added for 30
min staining and the cells were washed with water. PBS was used to
replace the Alcian blue in the negative control group. A solution
of 95% alcohol was used to dehydrate until the background was
clear, then the cells were washed with water. The staining was then
observed and recorded.
Immunocytochemical staining
The MCCs were seeded on coverslips at a density of
5×103 cells/ml for 48 h and then fixed with cold
acetone. Immunocytochemical analysis was performed using the
streptavidin-biotin complex method according to the manufacturer’s
instructions (Zhongshan Golden Bridge Biotechnology Co. Ltd.,
Beijing, China). 3,3′-Diaminobenzidine (Boster) served as the
chromogen. The primary antibody against ERRα was a monoclonal
rabbit anti-human ERRα (ab41868; Abcam, Cambridge, UK) at a 1:100
dilution. For the negative control, the primary antibody was
substituted with a commensurable volume of PBS. The samples were
counterstained with hematoxylin and examined under an Olympus
compound microscope (Olympus) equipped with a Nikon digital camera
(Nikon, Tokyo, Japan).
qPCR
Third-generation MCCs were prepared as a cell
suspension and inoculated into small culture flasks according to
the grouping. To investigate the effects of E2 on ERRα and
MCC-specific markers, MCCs were divided into an E2 group and
control group. The MCCs in the E2 group were treated with
10−8 M 17-β estradiol (E2; Sigma-Aldrich) for 48-h
stimulation, while the E2 was replaced by an equivalent solvent
(anhydrous ethanol) in the control group. To investigate the
effects of overexpression and inhibition of ERRα on MCCs, the cells
were transfected with the plasmid pcDNA3.1(+)-cont and pcDNA
pcDNA3.1(+)-ERRα, or treated with XCT790, respectively. After 48 h
10−8 M 17-β estradiol stimulation, the MCCs were
collected. The total RNA from MCCs was isolated using TRIzol
reagent (Invitrogen Life Technologies), according to the
manufacturer’s instructions. Complementary DNA was synthesized from
mRNA using Moloney murine leukemia virus reverse transcriptase
(Invitrogen Life Technologies). qPCR was conducted with a
Mastercycler EP Realplex4 (Eppendorf AG, Hamburg, Germany) and
SYBR-Green (Invitrogen Life Technologies). The primer sequences
used are shown in Table I. The
reactions were performed under the following cycling conditions:
95°C for 10 min, followed by 45 cycles of 95°C for 15 sec and 60°C
for 1 min. The expression of the target genes was calculated using
the formula 2−ΔΔCt. The expression data were normalized
to the expression levels of the β-actin gene.
 | Table IPrimer sequences used in the reaction
system. |
Table I
Primer sequences used in the reaction
system.
| Gene | Sequence |
|---|
| ERRα | F:
5′-GATGTGGCCTCTGGCTACCACTA-3′
R: 5′-CGGACAGCTGTACTCGATGCTC-3′ |
| Sox9 | F:
5′-TGAAGGGCTACGACTGGACG-3′
R: 5′-ACTTGTAATCGGGGTGGTCTTT-3′ |
| Col2a1 | F:
5′-TGGTGGAGCAGCAAGAGCA-3′
R: 5′-CCCTCAGTGGACAGTAGACGGA-3′ |
| GDF-5 | F:
5′-AGGGAGGTAACAGCAGCGT-3′
R: 5′-CTCCAAGGCACTGATGTCAAAC-3′ |
| Aromatase | F:
5′-CTTTTGAGACGATTCCAGGTGA-3′
R: 5′-GGATAAGTAATGCCCCAGAGTAGAT-3′ |
| β-actin | F:
5′-GGAGATTACTGCCCTGGCTCCTA-3′
R: 5′-GACTCATCGTACTCCTGCTTGCTG-3′ |
Western blotting
Briefly, cell extracts, containing 30 μg total
protein, were subjected to SDS-PAGE and then transferred to
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).
The membranes were blocked and then probed with primary antibodies
against ERRα (ab41868; Abcam), Sox9 (sc-20095; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), GDF-5 (sc-6901; Santa
Cruz Biotechnology, Inc.), aromatase (sc-14245; Santa Cruz
Biotechnology, Inc.), Col2a1 (ab34712; Abcam) and β-actin (ab8227;
Abcam). The secondary antibodies, which were selected according to
the species of origin of the primary antibodies, included mouse
anti-goat IgG-HRP (A0216; Beyotime), goat anti-rabbit IgG-HRP
(A0208; Beyotime), and donkey anti-goat IgG-HRP (A0181; Beyotime).
Following incubation, the luminescent signals were detected using
an enhanced chemiluminescence kit (Pierce, Rockford, IL, USA). The
Quantity One software (Bio-Rad, Hercules, CA, USA) protein bands
were used to measure the gray value of the target band
corresponding to β-actin and the gray value of the ratio of the
target protein in comparison with the strength of a sample
index.
MCC proliferation detection
The experimental groupings were as follows: E2
(10−8 M) treatment group, XCT790 (5 μM; Abcam) treatment
group, E2 (10−8 M)/XCT790 (5 μM) combined-treatment
group and blank control group. The third generation of
logarithmically-growing MCCs was prepared as a cell suspension,
then inoculated into 96-well plates, with seeding density at
1×104 cells/well. This was performed 5 times for each
treatment group. The cells were observed 24 h after inoculation; if
the cells were adherent to the wall, the original culture medium
was discarded. According to the instructions of the WST-1 kit
(Boster), WST-1 solution was added to the cells for 2 h-incubation
at 37°C and 5% CO2. The plate was then agitated for 1
min to fully mix the system for detection. The WST-1 treatment was
performed on the cells at 48 and 72 h, respectively, and then the
relevant absorbance was determined using a spectrophotometer
(BioPhotometer; Eppendorf). Absorbance was measured at 450 nm.
Construction of vector and
transfection
The target fragments were amplified using the
primers listed in Table II and
the pcDNA3.1(+) vector (Invitrogen Life Technologies) was then
digested with restriction enzymes: HindIII at the upstream
digestion site and EcoRI at the downstream digestion site.
Following identification, the endotoxin plasmid was extracted and
the vector was precipitated with ethanol. According to the
manufacturer’s instructions, Lipofectamine 2000 (Invitrogen Life
Technologies) was used to transfect the overexpressed ERRα; the
transfection was performed when the third generation of
logarithmically-growing MCCs had grown to 70–80% confluence.
 | Table IISequences of the target fragmental
primers. |
Table II
Sequences of the target fragmental
primers.
| Primer name | Primer sequence
(5′-3′) |
|---|
| ERRα-F |
TTAGGTACCGCCACCATGTCCAGCCAGGTGGTGG |
| ERRα-R |
CGGCGCTCGAGTCAGTCCATCATGGCCTCAAG |
After 48 h treatment, the cells were collected for
the detection of changes in MCC-related biological-characteristic
markers. All experiments were repeated three times.
Statistical analysis
The collected experimental data were processed with
SPSS 18.0 statistical software (SPSS, Inc., Chicago, IL, USA) and
Student’s t-test was used for intergroup comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of MCCs
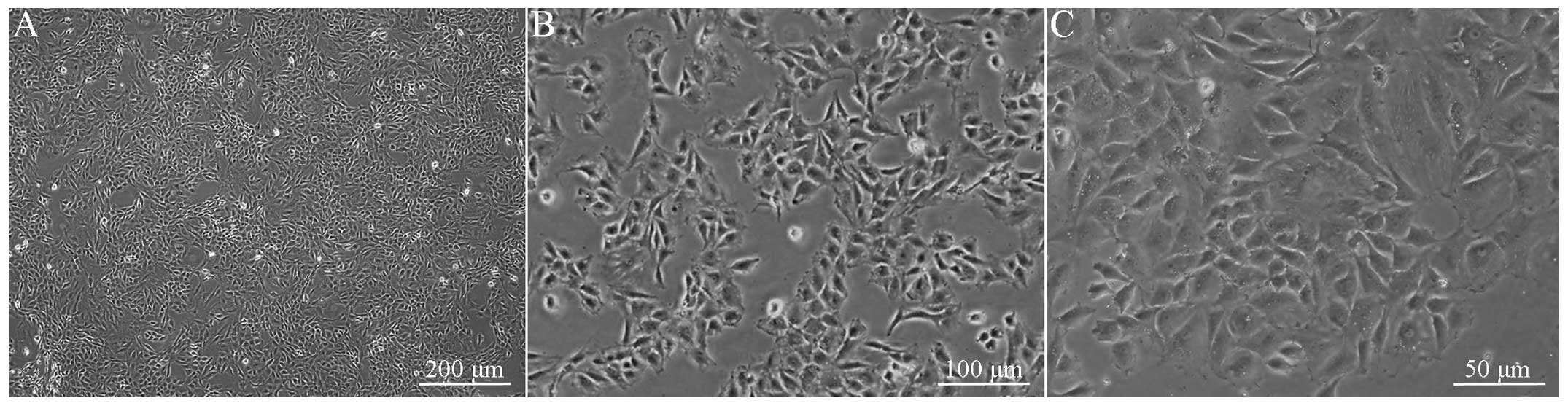
Observed under an inverted microscope, the primary
cultured rat MCCs appeared spherical with a refractive
extracellular matrix within 12 h and the cells were adherent to the
wall within 12 to 48 h. Following adherence, the cells stretched
and exhibited polygon- or spindle-shaped morphology, with blunt
cell bodies (Fig. 1). The cells
aggregated in ~1 week, confluenced in ~10 days and formed a
monolayer in ~14 days, and secreted large quantities of refractive
extracellular matrix. After three weeks, overlapping growth was
observed in certain cells, with multilayer-clump-like growth zones
in certain areas. The cells that exhibited positive
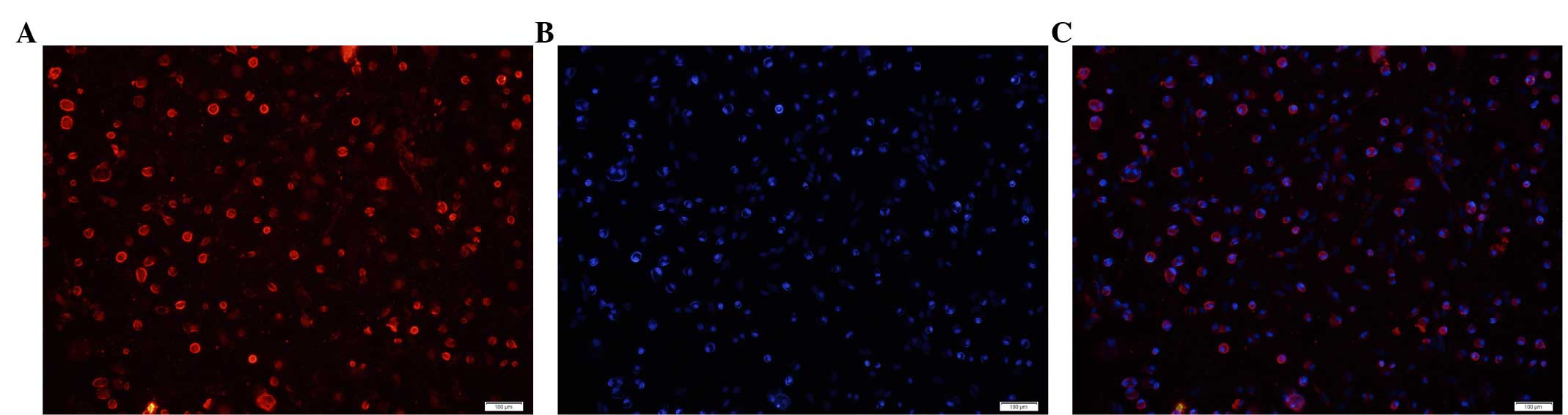
immunofluorescent staining for cytoplasmic type II collagen
accounted for >90% cells in the random view field, while DAPI
blue staining was observed, only in the nucleus (Fig. 2).
Alcian blue staining
Alcian blue staining revealed that the cultured
cells were positively blue stained (Fig. 3A), exhibiting characteristics of
cartilage-specific extracellular matrix, while the PBS control
group exhibited no positive staining (Fig. 3B).
Expression of ERRα and MCC-related
biological characteristic markers
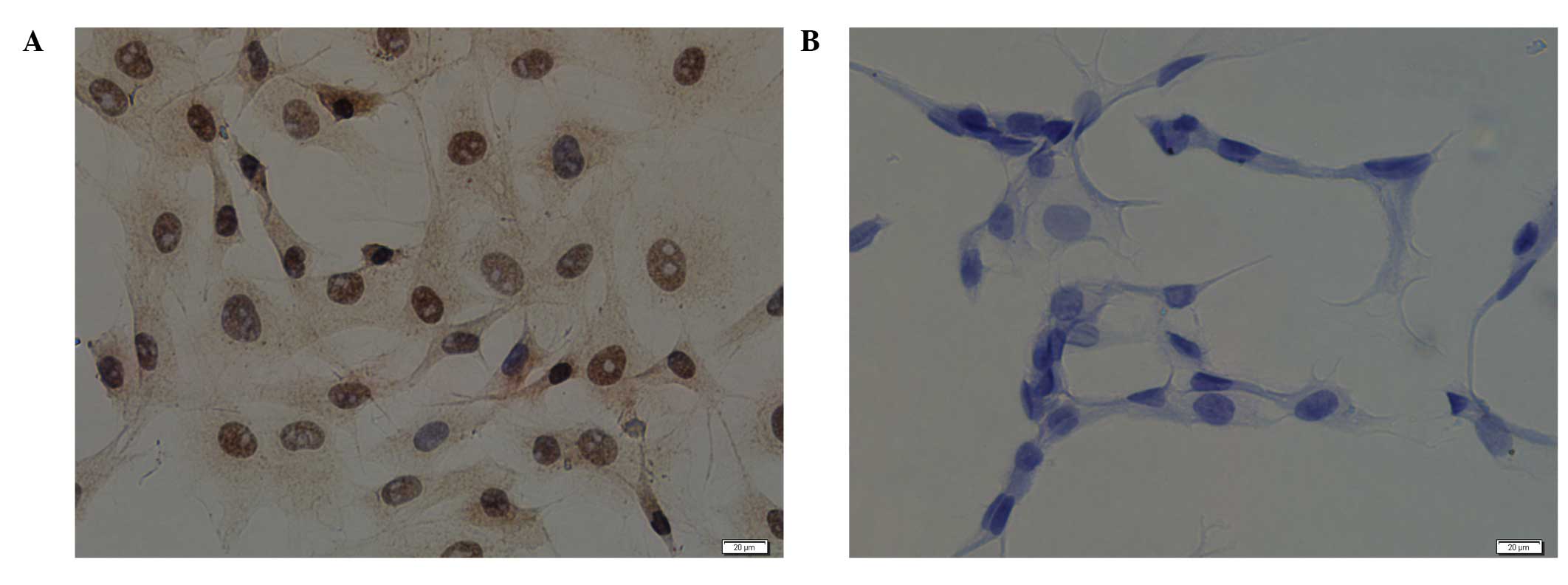
As shown in Fig.
4A, ERRα was expressed in the MCCs; the nuclei and cytoplasm
were brown-stained, although the expression in the nucleus was
markedly greater. Fig. 4B shows
the PBS negative control staining.
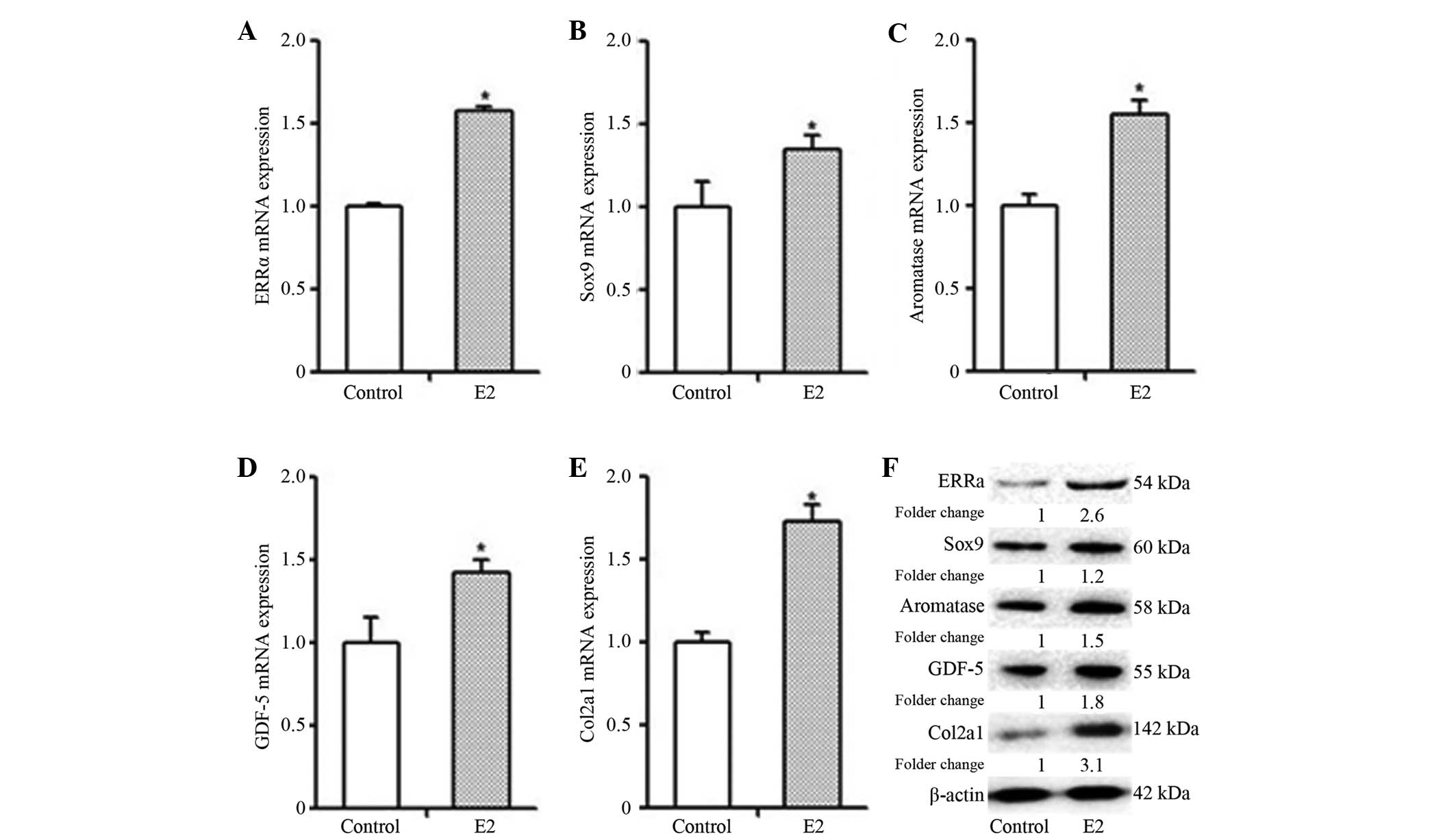
In order to investigate the impact of E2 on the
biological characteristics of MCCs, different markers of MCC
proliferation and differentiation were analyzed. Following E2
(10−8 M) stimulation for 48 h, the mRNA and protein
expression levels of ERRα, Sox9, GDF-5, aromatase and Col2a1 in the
MCC cells were significantly higher than those in the cells of the
control group (Fig. 5; P<0.05).
Therefore, E2 promoted the gene and protein expression of ERRα,
Sox9, GDF-5, aromatase and Col2a1 in the MCCs.
Effects of XCT790 on MCC
proliferation
In order to reveal the impact of E2 on MCC
proliferation and the possible regulatory effects of ERRα, E2
(10−8 M) and an inverse agonist of ERRα, XCT790 (5 μM),
were used to treat the MCCs. WST-1 was performed at different time
points to detect cell proliferation. Statistical analysis (Fig. 6) demonstrated that MCC
proliferative capacity in the E2 (10−8 M) treatment
group was higher than that in the control group and the E2
(10−8 M)/XCT790 (5 μM) treatment group. In addition, the
MCC proliferative capability of the XCT790 (5 μM) treatment group
was lower than that of the control group and the E2
(10−8 M) treatment group; and the MCC proliferative
capability in the E2 (10−8 M)/XCT790 (5 μM) treatment
group was lower than that in the E2 (10−8 M) but higher
than that in the XCT790 (5 μM) treatment groups.
Construction of the ERRα eukaryotic
expression vector
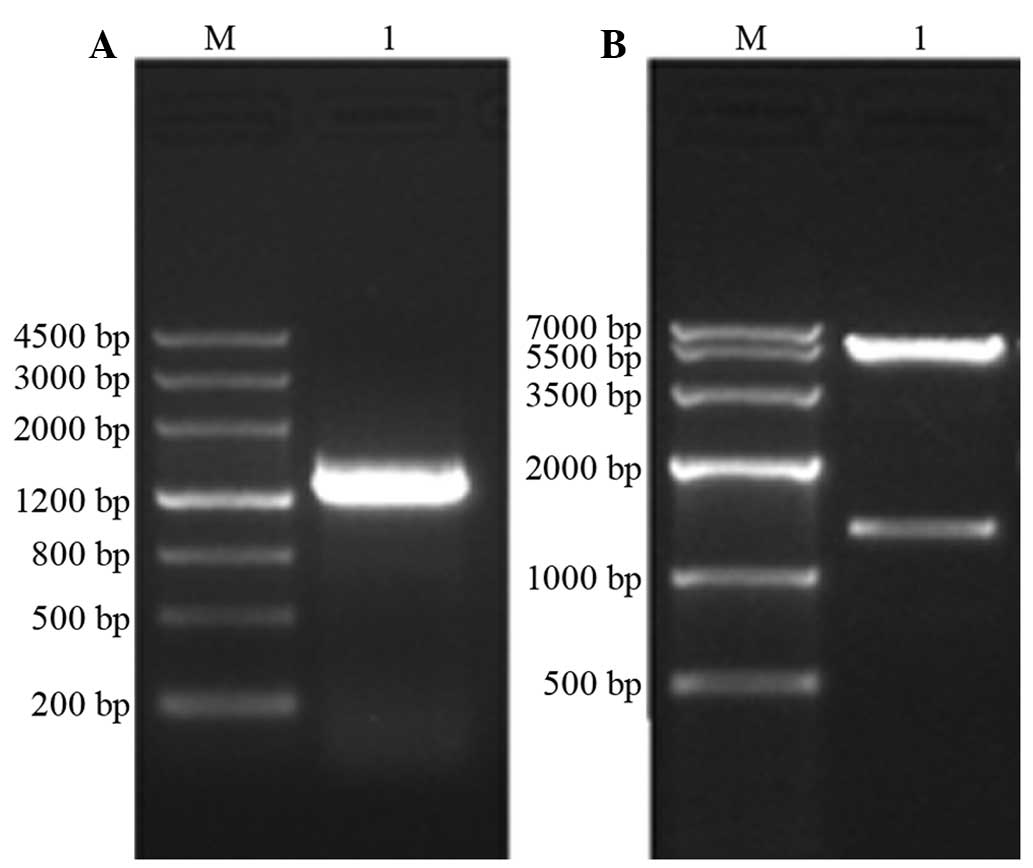
For electrophoretic identification of the PCR
products, agarose gel electrophoresis was used to separate the
samples and the 1,300 bp band of ERRα was observed.
For the electrophoretic identification of
recombinant plasmids, the recombinant plasmid pcDNA3.1 (+)-ERRαby
was obtained following enzyme digestion and ligation reactions. The
minipreparation-extracted plasmids were double-enzyme digested and
agarose gel electrophoresis was performed, which revealed two DNA
bands (Fig. 7), one at ~5.4 kb,
matching the size of the pcDNA3.1 (+) vector, and the other at
~1,300 bp, matching the base pair number of ERRα.
DNA sequence analysis of the recombinant
plasmids
The sequencing results of pcDNA3.1-ERRα were
performed using BLAST analysis following the correction. The
nucleic acid sequences were consistent with the coding sequences of
ESRRA in the GenBank database.
Expression levels of MCC-associated
biological characteristic markers
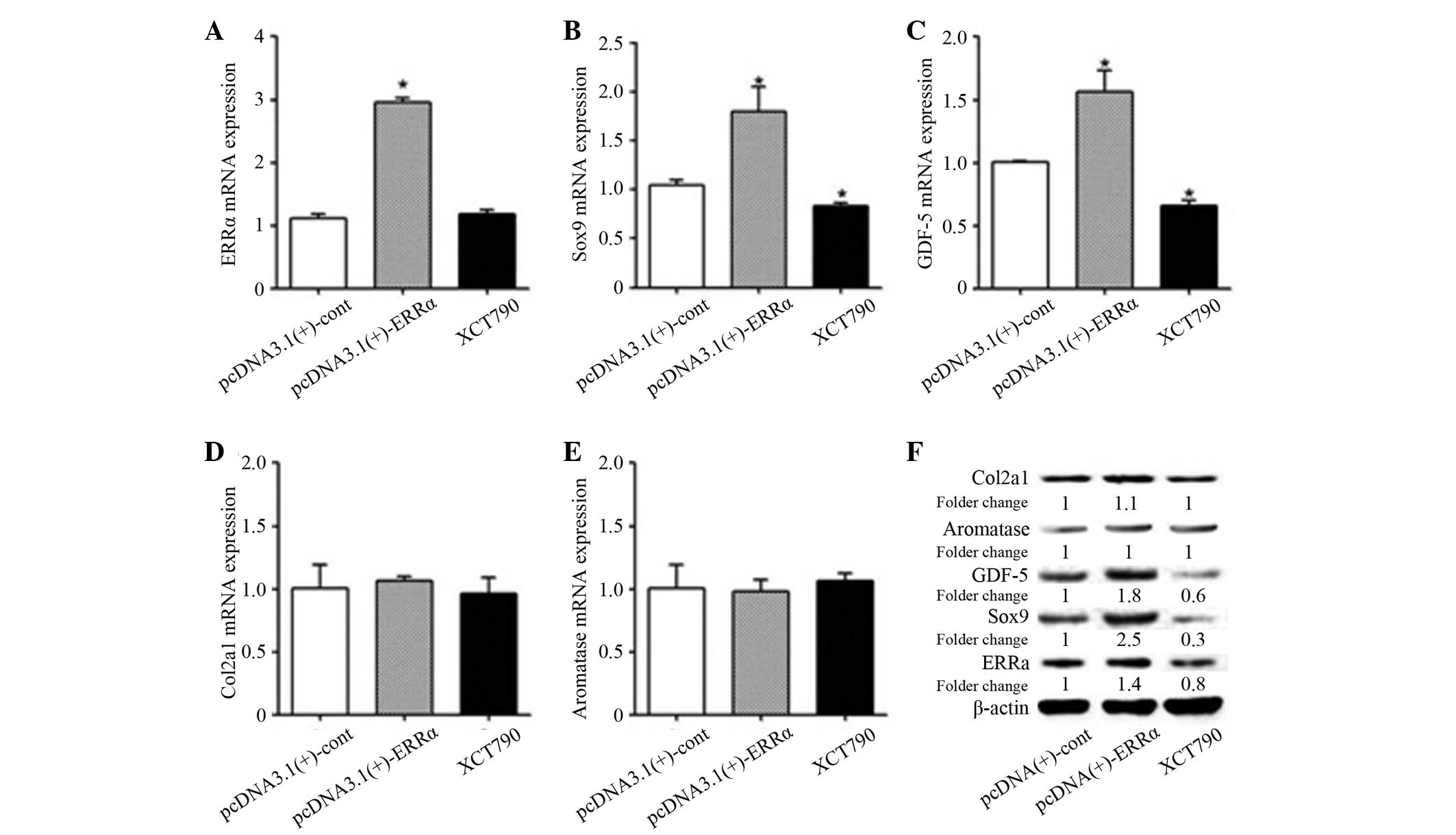
Once ERRα overexpression in the transfected MCCs was
confirmed, statistical analysis of the ERRα group compared with the
control group revealed that the expression levels of the ERRα mRNA
and protein were significantly increased (P<0.05) and the
expression levels of Sox9 and GDF-5 mRNA and protein were also
significantly increased (P<0.05). However, no significant
difference was identified in the change in mRNA and protein
expression levels of Col2a1 and aromatase (Fig. 8). The results showed that the
transfection was successful, resulting in overexpression of ERRα
mRNA and protein, and that ERRα overexpression exibited a positive
regulatory role on the early proliferation and differentiation
markers of MCCs, namely Sox9 and GDF-5, although showed no
significant correlation with Col2a1 and aromatase, which were
expressed by the mature MCCs.
In order to further clarify the role of ERRα, XCT790
(5 μM) was used to inhibit ERRα activity in MCCs. The changes in
gene and protein expression levels of ERRα, Sox9, GDF-5, Col2a1 and
aromatase after 48 h were detected by qPCR and western blotting.
The results revealed that compared with the control group, no
significant difference was detected in the expression levels of
ERRα mRNA in the XCT790 (5 μM) treatment group, although the
protein expression level was reduced. In addition, the mRNA and
protein expression levels of Sox9 and GDF-5 were reduced, but no
significant changes were identified in the expression levels of
Col2a1 and aromatase mRNA and protein. The results indicated that
the inhibition of ERRα in MCCs with XCT790 (5 μM) occurred mainly
at the protein level, not at the gene level. In conclusion, these
results suggest that XCT790 (5 μM) inhibited the mRNA and protein
expression of Sox9 and GDF-5 through downregulation of ERRα protein
expression.
Discussion
In the present study, the bodies of the primary MCCs
were obtuse, appearing polygonal or spindle-like. Type II collagen
immunofluorescent staining revealed that the positive staining
cells covered >90% of the area in the view field, indicating
that the cultivated cells maintained the ability to synthesize type
II collagen. The positive Alcian blue staining revealed that the
cultured cells possessed the ability to secrete proteoglycans,
thus, exhibiting the phenotype of MCCs (14,15).
A study demonstrated that the expression of ERRα in
tissues and cells is important with regard to the metabolic
activities and biological functions of the body (16). ERRα is expressed in the bone and
cartilage of the limbs and trunk, and may mediate the regulation of
bone metabolism balance (17,18).
The TMJ is involved in speech, chewing and other functions
(19). Due to its special
structures, the characteristics of the TMJ are different from that
of other joints in the trunk and limbs. MCCs are an important
component of TMJ, which undergoes constant change to respond to the
external stress stimuli and internal hormone levels. To the best of
our knowledge, the present study was the first study to reveal
positive ERRα expression in the MCCs. ERRα expression in the nuclei
and cytoplasm of MCCs further suggests that ERRα may act as a
transcription factor, shuttling between the nucleus and cytoplasm,
and actively regulating signal transduction in cells.
Estrogen exhibits extensive effects on body
metabolism, and is closely associated with cell proliferation,
differentiation and maturation (20). An increasing number of studies have
demonstrated that estrogen is important in the metabolism of
bone/cartilage tissue, and that its biological effects are
associated with the estrogen dose and response pathways (21–25).
The imbalance in estrogen regulation depends largely on the changes
in its response pathways. In addition to the traditional ER
signaling pathway, there are a number of associated by-pathways.
Thus, to investigate the impact of E2 on the biological
characteristics of MCCs and ERRα expression, the physiological
concentration of E2 (10−8 M) (26) was used in the present study to
process in vitro cultured MCCs. The mRNA and protein
expression levels of ERRα, Sox9, GDF-5, aromatase and Col2a1, were
elevated following stimulation of MCCs by E2 (10−8 M)
for 48 h, indicating that the physiological concentration of E2
exhibited the capacity to promote early proliferation and
differentiation, and late maturation of in vitro-cultured
MCCs.
Considering that the ERRα expression levels
increased in the MCCs following E2 (10−8 M) treatment
and that ERRα has been reported to promote cell proliferation
(27), the physiological
concentration of E2 and the specific ERRα inverse agonist, XCT790,
were used in the present study to treat the MCCs, and WST-1 was
used to observe the changes in cell proliferation. The WST-1
results revealed that E2 promoted the proliferation of MCCs and
that when XCT790 (5 μM) was used to inhibit ERRα expression, the
proliferative capacity of the MCCs was reduced. Therefore, ERRα
promoted MCC proliferation and mediated the E2-induced promotion of
cell proliferation. The results suggest that the ERRα expression in
MCCs was directly or indirectly influenced by E2, and that ERRα
mediated the signal transduction through which E2 affected MCC
proliferation, differentiation and maturation, although the
specific signaling pathway for these effects remains unclear.
ERRα has been confirmed to regulate different
metabolic activities of humans and multiple animals under different
conditions, and is crucial in controlling metabolic balance
(28). In the present study,
following plasmid transfection to overexpress ERRα, the gene and
protein expression levels of Sox9 and GDF-5 were found to
significantly increase. The gene and protein expression of Col2a1
and aromatase increased marginally, although not significantly. In
the XCT790 treatment group, ERRα protein expression, but not mRNA
expression, was significantly inhibited, consistent with the
results of a study of the mechanism of XCT790 inhibition of ERRα
(29). Following XCT790 treatment,
the gene and protein expression levels of Sox9 and GDF-5 declined
along with the reduction in ERRα protein expression levels,
although no significant changes were identified in the gene and
protein expression levels of Col2a1 and aromatase.
In MCCs, the observation that changes in ERRα
protein expression levels may increase or reduce mRNA and protein
expression levels of Sox9 and GDF-5 suggested that Sox9 and GDF-5
were regulated by ERRα, and that these genes were downstream
responsive genes. Activation of the Sox9 gene may promote
proliferation and aggregation of cartilage precursor cells, and
further differentiation towards chondrocytes (30). The changes in Sox9 expression
levels were consistent with those of ERRα, indicating that ERRα may
be a direct regulator of Sox9 in MCCs. In addition, ERRα affected
the signal response of cell proliferation and accumulation in the
early stages of MCC metabolic change. GDF-5 is the predominant
regulator of cartilage growth and differentiation, and it was also
positively regulated by ERRα. In the early stages of bone/cartilage
metabolism, changes in the expression levels of Sox9 may induce
changes in the subsequent bone/cartilage metabolism (17). Whether the effects of ERRα on GDF-5
were mediated through Sox9 remains to be confirmed by further
studies.
Col2a1 and aromatase are expressed by mature
cartilage, and are important in secreting type II collagen and
topically synthesizing estrogen to maintain the stable chondrocytes
function. Col2a1 and aromatase expression levels were not sensitive
to ERRα regulation, further suggesting that the effect of ERRα on
MCCs is mainly reflected in upper-end regulation, namely
proliferation, differentiation and maturation. This does not
exclude the possibility that ERRα may exert indirect effects on
Col2a1 and aromatase. As the course of cell proliferation,
differentiation, maturation and apoptosis is a continuous metabolic
process, all stages influence each other; the associations are
close rather than isolated. In order to maintain normal biological
functions, the balance of cell proliferation, differentiation and
maturation are precisely regulated by hormone levels, transcription
factors and numerous other factors in the body (31). The interactions among ERRα and
other transcription factors remain to be elucidated.
In conclusion, ERRα was identified as an important
regulator in the early proliferation and differentiation of MCCs,
positively regulating Sox9. Overexpressed ERRα promoted
proliferation of MCCs, although the impact on cell maturation is
unclear. ERRα expression may be affected by multiple factors,
including estrogen levels. Further studies regarding the
ERRα-mediated biological characteristic changes of MCCs may aid
further understanding of the impact of changes in the body estrogen
levels on the physiological and pathological changes in the
TMJ.
References
|
1
|
Chander CL and Desa FM: The effects of
estrogens on cartilage degradation using in vivo and in vitro
models. Agents Actions. 34:282–284. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meisler JG: Chronic pain conditions in
women. J Womens Health. 8:313–320. 1999. View Article : Google Scholar
|
|
3
|
Sims NA, Dupont S, Krust A,
Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M and
Baron R: Deletion of estrogen receptors reveals a regulatory role
for estrogen receptors-beta in bone remodeling in females but not
in males. Bone. 30:18–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giguère V, Yang N, Segui P and Evans RM:
Identification of a new class of steroid hormone receptors. Nature.
331:91–94. 1988.
|
|
5
|
Bonnelye E and Aubin JE: Estrogen
receptor-related receptor alpha: a mediator of estrogen response in
bone. J Clin Endocrinol Metab. 90:3115–3121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonnelye E, Zirngibl RA, Jurdic P and
Aubin JE: The orphan nuclear estrogen receptor-related
receptor-alpha regulates cartilage formation in vitro: implication
of Sox9. Endocrinology. 148:1195–1205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujimoto J and Sato E: Clinical
implication of estrogen-related receptor (ERR) expression in
uterine endometrial cancers. J Steroid Biochem Mol Biol. 116:71–75.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sonoda J, Laganière J, Mehl IR, et al:
Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors
of IFN-gamma-induced host defense. Genes Dev. 21:1909–1920. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonnelye E, Saltel F, Chabadel A, Zirngibl
RA, Aubin JE and Jurdic P: Involvement of the orphan nuclear
estrogen receptor-related receptor alpha in osteoclast adhesion and
transmigration. J Mol Endocrinol. 45:365–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajalin AM, Pollock H and Aarnisalo P:
ERRalpha regulates osteoblastic and adipogenic differentiation of
mouse bone marrow mesenchymal stem cells. Biochem Biophys Res
Commun. 396:477–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu D, Zhang Z, Gladwell W and Teng CT:
Estrogen stimulates estrogen-related receptor alpha gene expression
through conserved hormone response elements. Endocrinology.
144:4894–4904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shigeta H, Zuo W, Yang N, DiAugustine R
and Teng CT: The mouse estrogen receptor-related orphan receptor
alpha 1: molecular cloning and estrogen responsiveness. J Mol
Endocrinol. 19:299–309. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan IM, Bishop JC, Gilbert S and Archer
CW: Clonal chondroprogenitors maintain telomerase activity and Sox9
expression during extended monolayer culture and retain
chondrogenic potential. Osteoarthritis Cartilage. 17:518–528. 2009.
View Article : Google Scholar
|
|
14
|
Lin Z, Willers C, Xu J and Zheng MH: The
chondrocyte: biology and clinical application. Tissue Eng.
12:1971–1984. 2006. View Article : Google Scholar
|
|
15
|
Schulze M, Kuettner KE and Cole AA: Adult
human chondrocytes in alginate culture. Preservation of the
phenotype for further use in transplantation models. Orthopade.
29:100–106. 2000.(In German).
|
|
16
|
Lu D, Kiriyama Y, Lee KY and Giguère V:
Transcriptional regulation of the estrogen-inducible pS2 breast
cancer marker gene by the ERR family of orphan nuclear receptors.
Cancer Res. 61:6755–6761. 2001.PubMed/NCBI
|
|
17
|
Bonnelye E, Reboul P, Duval N, Cardelli M
and Aubin JE: Estrogen receptor-related receptor alpha regulation
by interleukin-1beta in prostaglandin E(2)- and cAMP-dependent
pathways in osteoarthritic chondrocytes. Arthritis Rheum.
63:2374–2384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gallet M and Vanacker JM: ERR receptors as
potential targets in osteoporosis. Trends Endocrinol Metab.
21:637–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pirttiniemi P, Kantomaa T and Sorsa T:
Effect of decreased loading on the metabolic activity of the
mandibular condylar cartilage in the rat. Eur J Orthod. 26:1–5.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gruber CJ, Tschugguel W, Schneeberger C
and Huber JC: Production and actions of estrogens. N Engl J Med.
346:340–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jakob F, Ebert R, Ignatius A, Matsushita
T, Watanabe Y, Groll J and Walles H: Bone tissue engineering in
osteoporosis. Maturitas. 75:118–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bay-Jensen AC, Slagboom E, Chen-An P,
Alexandersen P, Qvist P, Christiansen C, Meulenbelt I and Karsdal
MA: Role of hormones in cartilage and joint metabolism:
understanding an unhealthy metabolic phenotype in osteoarthritis.
Menopause. 20:578–586. 2013.PubMed/NCBI
|
|
23
|
Martín-Millán M and Castañeda S:
Estrogens, osteoarthritis and inflammation. Joint Bone Spine.
80:368–373. 2013.
|
|
24
|
Lee HR, Kim TH and Choi KC: Functions and
physiological roles of two types of estrogen receptors, ERα and
ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res.
28:71–76. 2012.
|
|
25
|
Orajärvi M, Puijola E, Yu SB, Liu X,
Tiilikainen P, Wang M, Raustia A and Pirttiniemi P: Effect of
estrogen and dietary loading on condylar cartilage. J Orofac Pain.
26:328–336. 2012.PubMed/NCBI
|
|
26
|
Yasuoka T, Nakashima M, Okuda T and
Tatematsu N: Effect of estrogen replacement on temporomandibular
joint remodeling in ovariectomized rats. J Oral Maxillofac Surg.
58:189–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bianco S, Lanvin O, Tribollet V, Macari C,
North S and Vanacker JM: Modulating estrogen receptor-related
receptor-alpha activity inhibits cell proliferation. J Biol Chem.
284:23286–23292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ranhotra HS: The estrogen-related receptor
alpha: the oldest, yet an energetic orphan with robust biological
functions. J Recept Signal Transduct Res. 30:193–205. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lanvin O, Bianco S, Kersual N, Chalbos D
and Vanacker JM: Potentiation of ICI182,780 (Fulvestrant)-induced
estrogen receptor-alpha degradation by the estrogen
receptor-related receptor-alpha inverse agonist XCT790. J Biol
Chem. 282:28328–28334. 2007. View Article : Google Scholar
|
|
30
|
Zhou G, Zheng Q, Engin F, et al: Dominance
of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad
Sci USA. 103:19004–19009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Desvergne B, Michalik L and Wahli W:
Transcriptional regulation of metabolism. Physiol Rev. 86:465–514.
2006. View Article : Google Scholar : PubMed/NCBI
|