Introduction
Alzheimer’s disease (AD) is a regressive disease of
the central nervous system (CNS), characterized by progressive
cognitive dysfunction, including memory decline, with the
pathological features of senile plaques deposition, neurofibrillary
tangles (NFT) and neurodegeneration of the brain. These
pathological hallmarks occur predominantly in the temporal and
frontal lobes of the cerebral cortex, hippocampus and the basal
forebrain. Temporal lobe atrophy of AD patients is more severe than
that of healthy individuals. This is consistent with the clinical
features of AD patients, which include a decrease in intelligence
and the decline of short-term memory in the early stages.
Therefore, studies investigating neurodegeneration within the
temporal lobe will further elucidate the pathogenic mechanisms
behind AD (1,2).
Tanshinone IIA (Tan IIA), is an abietane-type
diterpene quinone, extracted from the traditional Chinese herbal
medicine Salvia miltiorrhiza (Danshen) which is a
well-established medicine in the treatment of cardiovascular
diseases (3). Currently, emerging
in vivo evidence is reporting that Tan IIA has
neuroprotective effects on the cholinergic system, and appears to
result in the improvements in the pathological changes caused by
AD, but the underlying mechanisms of these effects is largely
unclear (4). Previously, two
genes, inducible nitric oxide synthase (iNOS) and MMP-2, have been
reported as crucial in the development and pathogenesis of AD
(5,6). As a result, we hypothesized that Tan
IIA may reduce AD risk, through iNOS and MMP-2 signaling
pathways.
iNOS is an important enzyme that mediates
inflammatory processes. iNOS catalyzes the oxidative deamination of
L-arginine to produce NO at high concentrations. NO is
neuroprotective at low concentrations, but higher concentrations
are potently neurotoxic to brain cells (7). In AD patients, studies have
demonstrated that the number of iNOS-positive neurons is
significantly enhanced in the brain, and is accompanied by abundant
neuronal damage. This evidence suggested that iNOS may be
associated with the pathogenesis of AD (8). Matrix metalloproteinase 2 (MMP-2) is
a crucial zinc-dependent protease, involved in remodeling the
extracellular matrix and modifying cell-cell and cell-matrix
interactions (9). MMP-2
participates in the development of AD by regulating ionic
concentrations, which mediate the balance of amyloid beta protein
(Aβ) metabolism, therefore changes in MMP-2 levels are considered
as a potential diagnostic biomarker of AD.
iNOS and MMPs were activated by the NF-κB pathway in
the progression of cerebral aneurysm (10). However, few studies have
demonstrated the activation of iNOS and MMP-2 by NF-κB in AD
development. Nuclear transcription factor kappa B (NF-κB)
participates in multiple inflammatory immunoreactions. Furthermore,
it has a regulatory role in the transcriptional and modulatory
processes of numerous inflammatory mediator genes, including
adhesion molecules, cytokines, inflammatory mediators and
proteases. Accumulated evidence suggests that NF-κB is correlated
with AD pathogenesis, and involved in the mechanism of Aβ injury
(11), therefore, NF-κB may be the
upstream activator that regulates iNOS and MMP-2, and further
contributes to AD development induced by Aβ.
In the present study, we established AD model rats
with the method of direct Aβ injection, then administered a
interventional treatment with Tan IIA. Following this, the learning
and memory ability of rats was examined in each group, and the mRNA
and protein expression of iNOS, MMP-2 and NF-κBp65 in temporal
lobes was detected, respectively. The aim of the present study was
to investigate the protective effect of Tan IIA on the learning and
memory ability of AD rats, to confirm the potential correlation
between the three genes and AD pathogenesis at a transcriptional
and translational level, as well as to provide evidence for our
hypothesis that Tan IIA may reduce AD risk by inhibiting iNOS,
MMP-2 expression at a transcriptional and translational level,
through the NF-κB pathway.
Materials and methods
Reagents
All antibodies used in this study were purchased
from Cayman Chemical (Ann Arbor, MI, USA), including anti-iNOS,
anti-MMP-2, anti-NF-κBp65, goat polyclonal and anti-actin. One-step
Extract RNA kit (TRIzol reagent kit) was purchased from Promega,
Inc. (Madison, WI, USA). PrimeScript reverse-transcript reagent kit
and SYBR Premix Ex Taq™ enzyme were obtained from Takara Bio, Inc.
(Shiga, Japan); Aβ, Tan IIA (degree of purity, >99%) and other
biochemistry reagents were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Finally, the determination of protein content kit was
from Bio-Rad (Hercules, CA, USA).
Animals
The adult male Sprague-Dawley rats (n=60; weighing,
200–250 g; age range, 8–12 weeks) used in the present study were
obtained from the Laboratory Animal Center of the Second Military
Medical University (Shanghai, China). All rats were housed in a
climate-controlled room (temperature, 22–24°C; humidity, 65–80%)
with free access to food and water. The present study was conducted
following internationally recognized guidelines on animal welfare,
as well as the principles issued by the State Scientific and
Technological Commission (Beijing, China).
AD model establishment
AD model rats were established with the method of
direct Aβ injection as previously described (12). Each rat received general
anaesthesia with intraperitoneal injection of 100 g/l chloral
hydrate (0.35 g/kg), which was then fixed by a stereotaxic
instrument on top of the head. The rat’s anterior fontanelle was
exposed following skin disinfection and incision on the top of the
head. With the use of a stereotaxically technique and rat brain
map, a 1 mm diameter hole was produced in the skull (3.0 mm behind
bregma, 2.0 mm to the right of the center line). A microsyringe was
vertically inserted to administer a 10 μl injection of Aβ into the
lateral ventricle (2.8 mm vertical from the brain surface). The
needle remained in that position for >5 min following injection
to ensure adequate diffusion of Aβ, after which it was slowly
withdrawn. Rats in AD and Tan IIA groups were injected with 10 μl
Aβ and the sham-operation group with 10 μl normal saline instead.
All procedures were performed with the strict aseptic
manipulation.
Rats in groups
The rats (n=60) were randomly assigned to four
groups: normal group (Control), sham-operation group (Sham), AD
group (AD) and Tan IIA group (Tan IIA), with 15 rats in each group.
After the AD model was established (24 h), rats in the Tan IIA
group were intragastrically administered with 50 mg/kg/day Tan IIA
(melted in corn oil) for 15 days. The rats in the other three
groups were fed with 50 mg/kg/day corn oil for 15 days. All the
rats were subjected to the Morris water maze test and their
behavioral indexes were recorded once daily from day 11–15
following the induction of AD. Following the test on the 15th day,
all the rats were sacrificed, and their temporal lobes were
extracted and placed onto ice and washed by icy normal saline. Half
of the temporal lobes were used in the western blot analysis and
the other in quantitative (q)PCR.
Morris water maze test
The Morris water maze was performed following the
commonly used procedure as described previously (13). All of the rats were subject to the
test once daily from day 11 to day 15 following the induction of
experimental AD. For the training, the rats were placed into water
at one of four quadrants around the pool’s perimeter, facing the
wall. Once the rat located the platform (escape latency; EL) within
60 sec, the training was terminated and EL was recorded as real
time. Otherwise, the rat was guided to the platform and allowed to
remain for 20 sec and EL was 1 min.
qPCR
The expression of genes was confirmed by qPCR.
Primers were designed and synthesized by Takara Bio, Inc. Total RNA
was isolated from iced temporal lobe tissues using RNAiso reagent.
First strand cDNA was synthesized from 0.5 μg of total cellular RNA
with random hexamers with the ExScript™ RT Reagents kit. qPCR
cycles were conducted for amplification of iNOS, MMP-2, NF-κBp65
and β-actin cDNA. The primer sequences are listed in Table I. Fluorescent dye used in qPCR
reactions was SYBR Premix Ex Taq™. All reactions were performed in
triplicate with the TaqMan EZ (reverse transcriptase) RT-PCR core
kit in an ABI-7000 machine (Ambion, Applied Biosystems Foster City,
CA, USA) according to the manufacturer’s instructions. PCR was
performed in a 25 μl solution containing 400 nM of the primer, 200
nM probe, 300 μM each of deoxynucleotide triphosphate (dNTP), 3.0
mM manganese acetate, 2.5 U DNA polymerase and the PCR buffer. The
threshold cycle changes (ΔCt) denote the difference in Ct of iNOS,
MMP-2 or NF-κBp65 from the Ct level of β-actin within the sample.
Following incubation for 15 min at 42°C, the PCR cycling program
was set for one cycle of pre-denaturation at 95°C for 60 sec and
then 40 cycles at 95°C for 30 sec, 95°C for 5 sec, 60°C plate
reading for 30 sec, melting curve from 55–95°C read every 0.2°C,
holding for 1 sec between reads. The relative copies of iNOS, MMP-2
and NF-κBp65 mRNA on the internal control β-actin are in arbitrary
units.
 | Table IPrimers of iNOS, MMP-2, NF-κBp65 and
β-actin. |
Table I
Primers of iNOS, MMP-2, NF-κBp65 and
β-actin.
| Gene | Primer |
|---|
| iNOS | F: CTC ACT GTG GCT
GTG GTC ACC TA
R: GGG TCT TCG GGC TTC AGG TTA |
| MMP-2 | F: GAT CCG TGG TGA
GAT CTT CTT C
R: AGA ACA CAG CCT TCT CTT CCT G |
| NF-κBp65 | F: AGG ACC CAA GCA
CCT TCT TT
R: GGG ATT TTG TCG TTG CTT GT |
| β-actin | F: TGA CAG GTG CAG
AAG GAG A
R: TAG AGC CAC CAA TCC ACA CA |
Western blot analysis
Temporal lobe samples were homogenized in an
ice-cold lysis buffer (pH 7.5) containing 20 mM Tris, 150 mM NaCl,
10 mM MgCl2, 1.0 mM EDTA, 1.0 mM EGTA, 1% Triton X-100
and a mixture of protease inhibitors. The homogenate was
centrifuged at 15,000 × g at 4°C for 30 min and the supernatant was
stored at −80°C for the western blot analysis. Briefly, samples
were incubated with a sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) sample buffer for 5 min at 95°C. Each
sample (40 μg/lane) was then subjected to SDS-PAGE and the
separated proteins were transferred for 60 min to a PVDF membrane
(GE Healthcare, Buckinghamshire, UK). The membrane was first
incubated with a blocking solution containing 0.3–2% skimmed milk,
25 mM Tris, 150 mM NaCl and 0.1% Tween-20 (pH 7.5) for 60 min, then
with the corresponding primary antibodies overnight (4°C). The
primary antibodies were a rabbit polyclonal antibody against iNOS
(1:500), a goat polyclonal antibody against MMP-2 (1:400), a goat
polyclonal antibody against NF-κBp65 (1:400) and mouse monoclonal
antibodies against β-actin (1:1,000). Following this, the membranes
were placed into the secondary antibody solution and incubated for
60 min. The corresponding secondary antibody was a goat anti-rabbit
IgG-HRP conjugate (1:2,000) for iNOS, a donkey anti-goat IgG-HRP
conjugate (1:2,000) for MMP-2 and NF-κBp65 and a sheep anti-mouse
IgG-HRP conjugate (1:2,000) for β-actin. Final detection was
performed with the enhanced chemiluminescence methodology (Amersham
ECL western blotting detection reagents and analysis system; GE
Healthcare) using a lumino imaging analyzer (LAS-3000; FUJIFILM,
Tokyo, Japan). To normalize for protein loading, chemiluminescence
of the bands in each lane was standardized to the intensity of the
β-actin band in the same lane.
Statistical analysis
Data are presented as the mean ± SD and one-way
analysis of variance (ANOVA) was used to determine the effect of AD
or Tan IIA. Association analysis among iNOS, MMP-2, NF-κBp65
expression at mRNA and protein level was performed by Spearman’s
correlation analysis. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
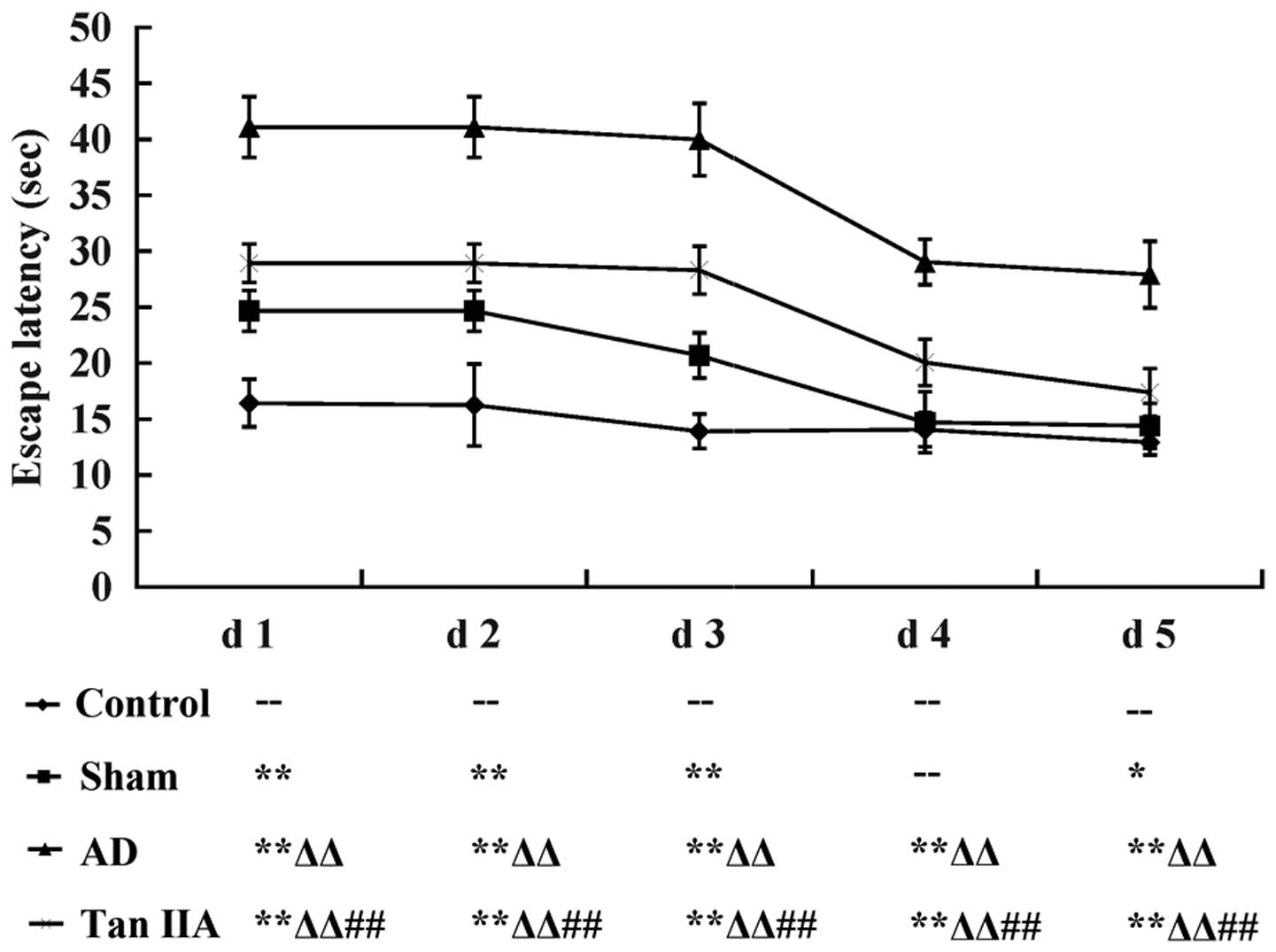
Tan IIA improves the learning and memory
ability of AD rats
The Morris water maze test was conducted to
investigate the learning and memory ability of each rat in the four
groups. Mean EL was considered as the quantitative indicator of the
behavioral observations representative of cognitive function. Data
was recorded as the mean ± SD and then transformed to the line
chart illustrated in Fig. 1. Each
group demonstrated a progressive decline in EL from day 1–5
(P<0.05). Compared with the Control group, EL was significantly
longer in the Sham group from day 1–5 except for day 4 (P>0.05).
However, the AD group had a steeper increase in EL than the Sham
group from day 1–5 (P<0.01, respectively). The results indicated
that the AD model was successfully established in rats by the
direct injection of Aβ and therefore were used in the experiments.
When the comparison was performed between AD and Tan IIA groups, it
was identified that EL reduced markedly in the Tan IIA group from
day 1–5 (P<0.01 respectively), which suggested that Tan IIA
improved the cognitive dysfunction in learning and memory exhibited
by the AD rats.
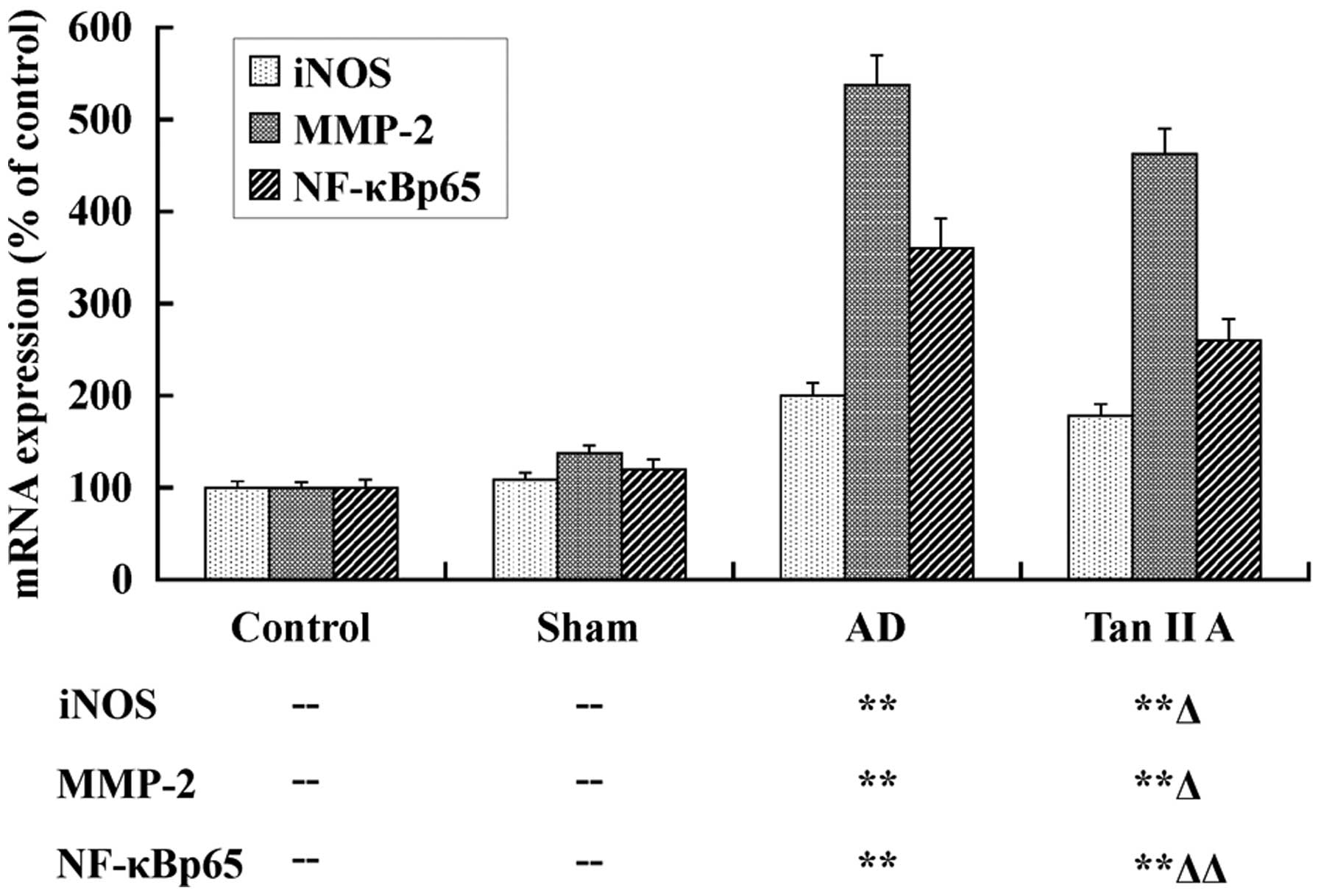
Tan IIA inhibits the mRNA expression of
iNOS, MMP-2 and NF-κBp65
The mRNA expression of iNOS, MMP-2 and NF-κBp65 was
detected, respectively, in temporal lobe tissues of the four groups
utilizing qPCR, to elucidate the protective effect of Tan IIA at a
transcriptional level. The results are presented as a column
diagram in Fig. 2. There was no
significant difference in expression of all three genes between the
Sham and Control groups (P>0.05). However, in the AD group, the
mRNA expression of iNOS, MMP-2 and NF-κBp65 were evidently
upregulated (P<0.01, respectively). These data demonstrated that
the higher mRNA expression levels of iNOS, MMP-2 and NF-κBp65 in
temporal lobe tissues were associated with AD. Most importantly, in
our further investigations, the mRNA expression of iNOS, MMP-2 and
NF-κBp65 was distinctly reduced in the Tan IIA group compared with
the AD group (P<0.05, P<0.05 and P<0.01,
respectively).
Tan IIA reduces the protein expression of
iNOS, MMP-2 and NF-κBp65
Western blot analysis was utilized to determine the
expression at a translational level. The protein expression of
iNOS, MMP-2 and NF-κBp65 was compared among the four groups
(Fig. 3). There was a significant
upregulation in the protein expression of iNOS, MMP-2 and NF-κBp65
in the Sham group compared with the Control group (P<0.05,
respectively). The AD group had a highly significant increase in
all three protein expression levels compared with the Sham group
(P<0.01, respectively). The findings suggested that the elevated
protein expression of iNOS, MMP-2 and NF-κBp65 in the temporal lobe
tissues was also associated with AD. Furthermore, the expression of
the three proteins in the Tan IIA group reduced markedly compared
with the AD group (P<0.01, respectively), which indicated that
Tan IIA reduced the protein expression of iNOS, MMP-2 and NF-κBp65
in the temporal lobe tissues of AD rats.
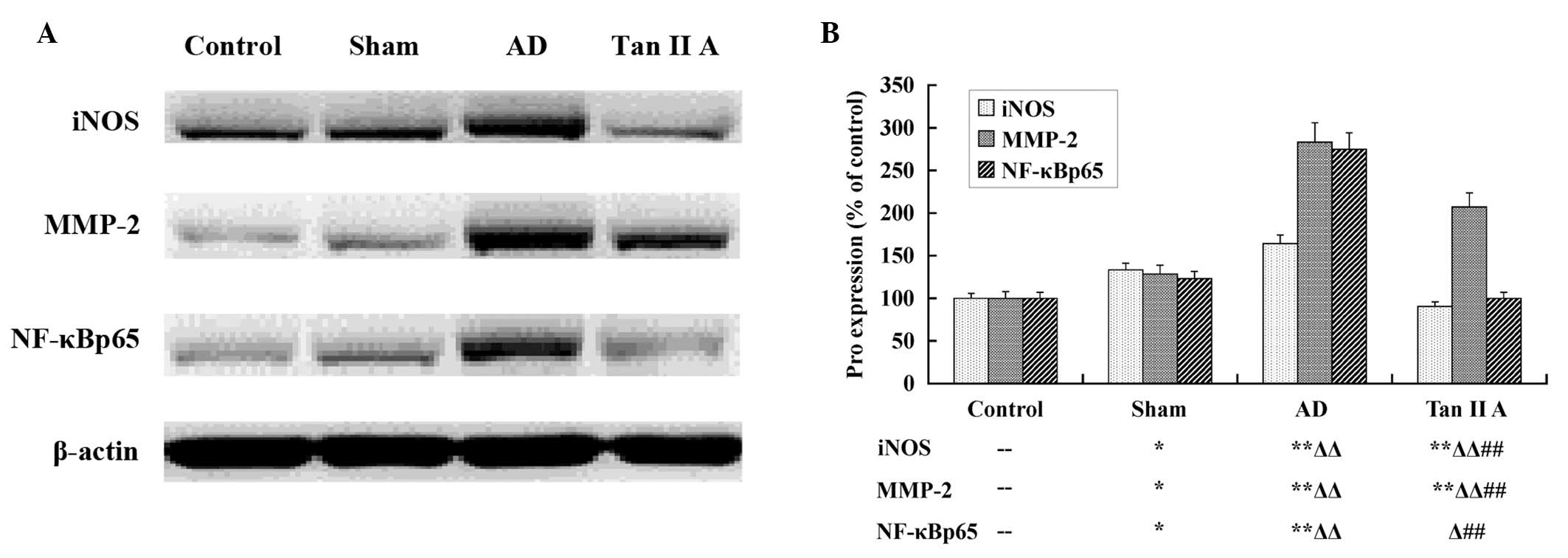 | Figure 3Tan IIA inhibits the translation of
iNOS, MMP-2 and NF-κBp65. The results of the western blot analysis
are presented; (A) the image produced by western blot assay and (B)
the column diagram calculated and transformed from the gamma value
of each stripe. Aβ injection induced the upregulation of iNOS,
MMP-2 and NF-κBp65 proteins in the AD group, and Tan IIA
significantly inhibited the enhanced translation of the three
proteins (P<0.01, respectively) in the Tan IIA group.
--P>0.05 vs. Control group; *P<0.05 vs.
Control group; **P<0.01 vs. Control group;
ΔP<0.05 vs. Sham group; ΔΔP<0.05 vs.
Sham group; #P<0.05 vs. AD group;
##P<0.01 vs. AD group. Aβ, amyloid β; AD, Alzheimer’s
disease; Tan IIA, Tanshinone IIA; iNOS, inducible nitric oxide
synthase; MMP-2, matrix metalloproteinase-2; NF-κB, nuclear
transcription factor-κB. |
Association between iNOS, MMP-2 and
NF-κBp65 at transcriptional and translational levels
Spearman’s correlation analysis on the mRNA
expression of iNOS, MMP-2 and NF-κBp65 suggested that any two of
the three genes had an evident positive correlation, as summarized
in Table II. The strongest
correlation was identified between iNOS and MMP-2 with
rs=0.998 and P<0.01. At a translation level, a
significantly positive correlation was identified between iNOS and
NF-κBp65 with rs=0.974 and P <0.05, as shown in
Table III.
 | Table IISpearman’s correlation analysis on the
mRNA expression of iNOS, MMP-2 and NF-κBp65. |
Table II
Spearman’s correlation analysis on the
mRNA expression of iNOS, MMP-2 and NF-κBp65.
| Index | iNOS | MMP-2 | NF-κBp65 |
|---|
| iNOS | - |
rs=0.998** |
rs=0.992** |
| MMP-2 |
rs=0.998** | - |
rs=0.985* |
| NF-κBp65 |
rs=0.992** |
rs=0.985* | - |
 | Table IIISpearman’s correlation analysis on
protein expressions of iNOS, MMP-2 and NF-κBp65. |
Table III
Spearman’s correlation analysis on
protein expressions of iNOS, MMP-2 and NF-κBp65.
| Index | iNOS | MMP-2 | NF-κBp65 |
|---|
| iNOS | - |
rs=0.822 |
rs=0.974* |
| MMP-2 |
rs=0.822 | - |
rs=0.911 |
| NF-κBp65 |
rs=0.974* |
rs=0.911 | - |
Discussion
Patients with AD consistently suffer from symptoms
of cognitive impairment, with memory dysfunctions occurring at an
early stage in the disease. Compared with the healthy brain, AD
patients have significant atrophy of the temporal lobe, lower
bleeding through part of temporal lobes. Studies that focus on
neurodegeneration in the temporal lobe will certainly contribute to
the elucidation of AD pathogenesis. A number of animal experiments
have demonstrated that Tan IIA may protect the cholinergic system
in the brains of AD rats and improve the pathological alterations
induced by AD, but the mechanism underlying this effect was largely
unclear. In the present study, the protective effect of Tan IIA on
the learning and memory ability of AD rats was investigated, by
means of the Morris water maze behavioral test. We further analyzed
the effect of Tan IIA on the expression of the three genes closely
associated with AD in the temporal lobe tissues of AD rats, and
attempted to clarify the protective mechanism at a transcriptional
and translational level.
An in vivo AD model was established in rats
by direct Aβ protein injection. The AD rats demonstrated an evident
dysfunction in learning and memory in the Morris water maze test,
while, Tan IIA distinctly reduced this effect. Consequently, the
results confirmed that Tan IIA had a protective effect on learning
and memory ability of AD rats.
At a transcriptional and translational level, iNOS
and MMP-2 in the temporal lobe tissues of AD rats was significantly
upregulated, which was consistent with the results of our previous
study in hippocampus of AD rats (14). From these findings, we make several
conjectures. First, MMPs are one of the most important
metalloenzymes, which are closely associated with Aβ metabolism. Aβ
metabolism disorder in AD rats induces changes in the
concentrations of metal irons, which further induces enhanced
expression of MMP-2 in temporal lobes. Secondly, iNOS is highly
correlated to neurotoxicity induced by Aβ according to previous
studies. Aβ injection activates the NO/NOS pathway, in which higher
concentrations of NO produced by iNOS mediate delayed neuronal
death following damage. Then the loss of neurons in brain causes
cognitive decline, particularly dysfunctions of learning and memory
(12,15). In the present study, these data
demonstrated an enhanced expression of iNOS in temporal lobe
tissues of AD rats, accompanied with the dysfunction of learning
and memory, providing evidence for the above conjectures.
Furthermore, the results that Tan IIA inhibited the expression of
iNOS and MMP-2 at a transcriptional and translational level in
temporal lobe tissues, confirmed the involvement of iNOS and MMP-2
pathways in AD pathogenesis and development.
In order to further elucidate the mechanisms
involved, we detected the alterations of the potential upstream
molecular pathway, NF-κBp65. As the most general subtype of NF-κB
protein family, NF-κBp65 is expressed in almost all cells and not
only participates in normal immune and inflammatory reactions, but
is also important in the regulation of various genes which are
involved in AD pathology (16).
Furthermore, the p65 subtype may improve the transcriptional
activation of target genes with the transcription-activating domain
located in the C-terminal, or exert its effects through being
coupled with other IκB members (17). Enhanced NF-κBp65 activity has been
identified in the brain tissue of AD patients, including in
degenerating neurons and in neurons adjacent to senile plaques and
glial cells. NF-κBp65 is highly associated with the pathogenesis of
AD. Furthermore, as NF-κB in neurons may be activated by Aβ
deposition, the NF-κB pathway could be involved in Aβ damage
mechanism in AD (18). In the
present study, NF-κBp65 were upregulated in temporal lobe tissues
of AD rats at a transcriptional and translational level, which
indicated that NF-κBp65 is involved in AD development induced by Aβ
injection. In a similar manner, the inhibitory effects of Tan IIA
on the mRNA and protein expression of NF-κBp65 was accompanied by
restrained AD risk and this further demonstrated that NF-κB pathway
participated in AD development in the AD rats. Furthermore, the
results suggest that Tan IIA may reduce AD risk through the NF-κB
pathway.
In the present study, it was identified that iNOS,
MMP-2 and NF-κBp65 are involved in AD development, as well as the
neuroprotective effects of Tan IIA on AD. To determine whether the
activation of iNOS and MMP-2 in AD rats was induced by the NF-κB
pathway, an association analysis among iNOS, MMP-2 and NF-κBp65 was
performed. As a result, a highly positive correlation between any
two of the three genes was identified, as was a positive
association between iNOS and the NF-κBp65 protein. According to
several other studies, there is a κB element in the promoter of
iNOS and MMP-2, respectively, which provides the binding site for
the signal molecules of the NF-κB pathway (17). Consequently, we can infer that, the
upregulation of mRNA and protein expression of NF-κBp65 activates
iNOS transcription and translation to produce high concentrations
of NO, which may lead to the learning and memory dysfunction of AD
rats. By contrast, the activated NF-κBp65 targets the κB sequence
of MMP-2 promoter to upregulate MMP-2 gene expression, which
aggravates the neurological disorder in AD rats. Furthermore, Tan
IIA inhibits the activation of NF-κB pathway, thus inhibiting the
iNOS and MMP-2 signaling pathways to reduce the risk of AD
stimulated by Aβ injection.
Based on the findings of the present study, we
conclude that iNOS, MMP-2 and NF-κBp65 are involved in the
development of AD induced by Aβ injection, and Tan IIA may reduce
the risk of AD by inhibiting iNOS, MMP-2 and NF-κBp65 expression at
the transcriptional and translational levels in temporal lobe
tissues of AD rats. Additionally, these results provide further
evidence for the hypothesis that Tan IIA may reduce AD risk by
inhibiting iNOS and MMP-2 expression through the NF-κB pathway.
However, further studies should be conducted to elucidate the
mechanism underlying this effect in detail. Nevertheless, iNOS,
MMP-2 and NF-κBp65 are potential targets in AD prophylaxis and
treatment because they are not only accurate biomarkers that
indicate the progression of AD, but are also key targets against Aβ
deposition (19,20). Furthermore, we conclude Tan IIA is
an effective neuroprotective agent for AD therapy, and iNOS, MMP-2
and NF-κBp65 may be the potential targets molecular targets for
manipulating this effect therapeutically.
Acknowledgements
The present study was supported by grants from
Shanghai Health Bureau Scientific Research Project (no. 20114297)
and Shanghai Jiaotong University School of Medicine Nature Science
Fund Project (no. 11XJ21068).
References
|
1
|
Taly A, Corringer PJ, Guedin D, Lestage P
and Changeux JP: Nicotinic receptors: allosteric transitions and
therapeutic targets in the nervous system. Nat Rev Drug Discov.
8:733–750. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beagley KW, Huston WM, Hansbro PM and
Timms P: Chlamydial infection of immune cells: altered function and
implications for disease. Crit Rev Immunol. 29:275–305. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mei Z, Zhang F, Tao L, et al:
Cryptotanshinone, a compound from Salvia miltiorrhiza modulates
amyloid precursor protein metabolism and attenuates beta-amyloid
deposition through upregulating alpha-secretase in vivo and in
vitro. Neurosci Lett. 452:90–95. 2009. View Article : Google Scholar
|
|
4
|
Wang Q, Yu X, Patal K, Hu R, Chuang S,
Zhang G and Zheng J: Tanshinones inhibit amyloid aggregation by
amyloid-β peptide, disaggregate amyloid fibrils, and protect
cultured cells. ACS Chem Neurosci. 4:1004–1015. 2013.PubMed/NCBI
|
|
5
|
Malinski T: Nitric oxide and nitroxidative
stress in Alzheimer’s disease. J Alzheimers Dis. 11:207–218.
2007.
|
|
6
|
Durrant JD, de Oliveira CA and McCammon
JA: Including receptor flexibility and induced fit effects into the
design of MMP-2 inhibitors. J Mol Recognit. 23:173–182.
2010.PubMed/NCBI
|
|
7
|
Steinert JR, Chernova T and Forsythe ID:
Nitric oxide signaling in brain function, dysfunction, and
dementia. Neuroscientist. 16:435–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolk DA and Klunk W: Update on amyloid
imaging: from healthy aging to Alzheimer’s disease. Curr Neurol
Neurosci Rep. 9:345–352. 2009.
|
|
9
|
Romi F, Helgeland G and Gilhus NE: Serum
levels of matrix metalloproteinases: implications in clinical
neurology. Eur Neurol. 67:121–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aoki T, Kataoka H, Shimamura M, et al:
NF-kappaB is a key mediator of cerebral aneurysm formation.
Circulation. 116:2830–2840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Zhen YF, Pu-Bu-Ci-Ren, et al:
Salidroside attenuates beta amyloid-induced cognitive deficits via
modulating oxidative stress and inflammatory mediators in rat
hippocampus. Behav Brain Res. 244:70–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Limpeanchob N, Jaipan S, Rattanakaruna S,
Phrompittayarat W and Ingkaninan K: Neuroprotective effect of
Bacopa monnieri on beta-amyloid-induced cell death in primary
cortical culture. J Ethnopharmacol. 120:112–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakashima Y, Ohsawa I, Konishi F, et al:
Preventive effects of Chlorella on cognitive decline in
age-dependent dementia model mice. Neurosci Lett. 464:193–198.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang P, Chen M, Lv J, Chen C and Jiao BH:
Effect of tanshinone II A on MMP-2 and iNOS expression and free
radical release in hippocampus of rat Alzheimer’s disease model.
Academic Journal of Second Military Medical University. 31:380–384.
2010.(In Chinese).
|
|
15
|
Silveira LR, Pereira-Da-Silva L, Juel C
and Hellsten Y: Formation of hydrogen peroxide and nitric oxide in
rat skeletal muscle cells during contractions. Free Radic Biol Med.
35:455–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ascolani A, Balestrieri E, Minutolo A, et
al: Dysregulated NF-κB pathway in peripheral mononuclear cells of
Alzheimer’s disease patients. Curr Alzheimer Res. 9:128–137.
2012.
|
|
17
|
Nomura Y: NF-kappaB activation and IkappaB
alpha dynamism involved in iNOS and chemokine induction in
astroglial cells. Life Sci. 68:1695–1701. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akhand AA, Du J, Liu W, et al:
Redox-linked cell surface-oriented signaling for T-cell death.
Antioxid Redox Signal. 4:445–454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malinski T: Nitric oxide and nitroxidative
stress in Alzheimer’s disease. J Alzheimers Dis. 11:207–218.
2007.
|
|
20
|
Rosenberg GA: Matrix metalloproteinases
and their multiple roles in neurodegenerative diseases. Lancet
Neurol. 8:205–216. 2009. View Article : Google Scholar : PubMed/NCBI
|