Introduction
The renin-angiotensin-system (RAS) consists of
different axes and has been associated with a broad variety of
physiological and pathophysiological processes. Angiotensin II
(AngII) is the major effector peptide of the classical RAS axis
which is best known for its role in systemic volume homeostasis and
blood pressure control (1). AngII
is generated from AngI by angiotensin-converting enzyme (ACE) and
via binding to its preferred type 1 receptor, AT1R, affects
important processes, including proliferation, apoptosis, fibrosis
and inflammation (2). The
classical RAS has also been implicated in tumorigenesis (2), in part due to its strong angiogenic
activity mediated by the AngII/AT1R-dependent (3) induction of proangiogenic factors,
including vascular endothelial growth factor (VEGF) (4), angiopoietin 2 (5) and platelet-derived growth factor
(PDGF) (6). In ovarian carcinoma,
invasion and angiogenesis are correlated to AT1R expression levels
(7). Accordingly, ACE-inhibitors
have been demonstrated to suppress the growth and angiogenic
activity of head and neck carcinoma (8). In another study, ACE-inhibition and
blockade of AT1R reduced liver metastasis and angiogenesis in a
mouse model of colorectal carcinoma (9). The ACE insertion/deletion
polymorphism (ACE I/D), and specifically the DD-genotype, has also
been linked to the development of early gastric cancer (10) and has been associated with
increased lymph node metastasis (11). The AT1R expression status and ACE
I/D are independent risk factors for nodal spread in intestinal
type gastric cancer (12). For
osteosarcoma, a recent study demonstrated a reduction in tumor
growth and lung and liver metastasis by application of the AT1R
antagonist, CV11974, in a murine osteosarcoma model (13).
The non-classical, alternative ACE2/Ang(1–7)/Mas
axis in numerous aspects acts as a negative modulator of
AngII-mediated effects. In this axis, ACE2 facilitates the
formation of Ang(1–7) which is the high affinity ligand for
Mas (14). Ang(1–7) has
been demonstrated to inhibit migration and invasion of lung cancer
cells in vitro, possibly via the inhibition of p38 and c-Jun
N-terminal kinase (15) as well as
extracellular signal-regulated kinase 1/2 (16) MAP kinases. Accordingly,
overexpression of the Ang(1–7)-generating ACE2 has been demonstrated
to decrease proliferation, invasion and VEGF expression in lung
cancer (17). A previous study
demonstrated that ACE2 overexpression in A549 lung cancer cells
reduced metastasis in vivo and increased the expression of
E-cadherin in vivo and in vitro (18).
It is well established that in general, cancer cells
use glucose as their predominant energy substrate (19,20)
and it has been recently demonstrated that glucose transporter 1
(GLUT1) is the main glucose transporter in osteosarcoma cells
(21). Elevated glucose uptake as
measured by fluorodeoxyglucose (18F) uptake is associated with a
poor prognosis in patients with osteosarcoma (22,23).
Of note, Ang(1–7) has been implicated in regulating
glucose uptake into adipocytes and muscle and, therefore,
contributes to glucose homeostasis (24,25).
Thus, the present study aimed to elucidate if the ACE2/Ang(1–7)/Mas
axis of the RAS may also affect osteosarcoma cell
proliferation.
Materials and methods
Cell culture
The osteosarcoma cell lines U-2 OS and MNNG-HOS were
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). U-2 OS cells were maintained in McCoy’s 5A
medium (PAA Laboratories GmbH, Linz, Austria) and MNNG-HOS in MEM
with Earle’s Salt (Gibco, Darmstadt, Germany) and supplemented with
10% fetal calf serum (PAA Laboratories, Cölbe, Germany), 2 mM
l-glutamine (PAA Laboratories), 1 mM sodium pyruvate, 100 U/ml
penicillin and 100 μg/ml streptomycin (PAA Laboratories, Pasching,
Austria), at 37°C in a 5% CO2 humidified atmosphere. All
chemicals for the buffer preparation were obtained from
Sigma-Aldrich (Steinheim, Germany). Other providers were named
separately.
RNA preparation and quantitative
polymerase chain reaction (qPCR)
For experiments to investigate the influence of
interleukin (IL)-1β (PAN-Biotech GmbH, Aidenbach, Germany) and
angiotensin (1–7; Bachem Distribution Services GmbH, Weilam Rhein,
Germany), the cells were seeded at a density of 5×105
cells/well in a six-well plate (Sarstedt, Nümbrecht, Germany) at a
final volume of 3 ml and incubated for 24 h. RNA was prepared using
innuPrep RNA Mini kit (Analytik Jena AG, Jena, Germany) according
to the manufacturer’s instructions. cDNA-synthesis and qPCR were
performed exactly as described previously (31). The quantities of mRNA were
normalized to α-tubulin mRNA. Primers were designed with the aid of
Invitrogen’s OligoPerfect Designer and were obtained from
Invitrogen Life Technologies (Darmstadt, Germany).
Primer-downstream (DS) and upstream (US) sequences, the size of
amplified DNA fragments in base pairs (bp) and the optimized
annealing temperatures (AT) are listed in Table I.
 | Table IPrimers used for quantitative
polymerase chain reaction. |
Table I
Primers used for quantitative
polymerase chain reaction.
| Primer | Sequence (5′-3′) | Size (bp) | Annealing temperature
(°C) |
|---|
| h-ACE-DS |
GCAGAATCTTGCTGGTCTCTG | 262 | 63 |
| h-ACE-US |
CTCAAGTACTTCCAGCCAGTC | | |
| h-ACE2-DS |
TCCAGTACTGTAGATGGTGC | 372 | 55 |
| h-ACE2-US |
CTCCTTCTCAGCCTTGTTGC | | |
| h-APN-DS |
GCTGAGGGTGTAGTTGAGCTTC | 319 | 62 |
| h-APN-US |
CATCGGACTGTCAGTGGTGTAC | | |
| h-AT2R1-DS |
CTGCATTCTACAGTCACGTATGATG | 416 | 62 |
| h-AT2R1-US |
CTTCGACGCACAATGCTTGTAGCC | | |
| h-AT2R2-DS |
GTAAATCAGCCACAGCGAGG | 214 | 62 |
| h-AT2R2-US |
GGGCTTGTGAACATCTCTGG | | |
| h-IRAP-DS |
GACCTGAAGAGCCTGAACTG | 340 | 58 |
| h-IRAP-US |
CAGTGCAACTGGTTACAGGC | | |
| h-Mas1-DS |
CAATGCCGACTGGTACTTG | 407 | 62 |
| h-Mas1-US |
ACATCTCACTGGCAGGAAC | | |
| h-NEP-DS |
GAGGTTCTCCACCTCTGCTATC | 331 | 58 |
| h-NEP-US |
GCACTCTATGCAACCTACGATG | | |
| h-PEP-DS |
CCAAGACATGGTAGTAGAGC | 300 | 63 |
| h-PEP-US |
CCAACTACTGTCTGACGATG | | |
| h-α-Tubulin-DS |
CATTTCACCATCTGTTGGCTGGCTC | 528 | 58 |
| h-α-Tubulin-US |
CACCCGTCTTCAGGGTTCTTGGTTT | | |
Protein preparation and immunoblot
analyses
The cells were seeded at a density of
5×105 cells/well/3 ml in a six-well plate and incubated
for 24 h. Following incubation with IL-1β for a further 24 h, the
cells were harvested and suspended in lysis buffer, which contained
50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM EDTA, 0.5% Triton X-100,
10% glycerol, 10 mM K2HPO4, 0.5% NP-40, 1 mM
phenylmethanesulfonyl fluoride (Roche Diagnostics, Mannheim,
Germany), 1 mM sodium vanadate, 0.5% desoxycholate, 20 mM NaF, 20
mM glycerol-2-phosphate and a protease inhibitor cocktail (all from
Sigma, Heidelberg, Germany). Following an incubation period of 20
min on ice, the lysates were centrifuged at 15,000 × g for 30 min
and the resulting supernatants were stored at −80°C for further
analysis. A total of 22 μg protein in a final volume of 20 μl 1×
Laemmli-buffer (Bio-Rad, Hercules, CA, USA) were separated by
SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Millipore, Bedford, MA, USA). Following blocking with 1X
Roti® Block (Carl Roth GmbH & Co. KG, Karlsruhe,
Germany) the membranes were incubated with rabbit anti-Mas
(LS-B3564; LifeSpan Biosciences, Inc., Seattle, WA, USA), at a
dilution of 1:2,000 in phosphate-buffered saline (PBS)/5% skimmed
milk/0.03% NaN3 (Carl Roth GmbH & KG, Karlsruhe,
Germany; Merck, Darmstadt, Germany) or goat anti-actin (I-19,
SC-1616; Santa Cruz Biotechnology, Inc., Heidelberg, Germany), at a
dilution of 1:500 in Tris-buffered saline with Tween 20 (TBST)/5%
bovine serum albumin/0.03% NaN3 (fraction V; Carl Roth GmbH &
KG) overnight at 4°C. This was followed by incubation with
horseradish peroxidase-conjugated secondary antibodies: Anti-rabbit
immunoglobulin (Ig) G 1:5,000 or anti-goat IgG 1:10,000 (purchased
from Cell Signaling Technology, Inc., Frankfurt am Main, Germany)
in a 1x Roti® Block. For detection by enhanced
chemiluminescence, Super Signal West Dura Extended Duration
Substrate (Pierce Biotechnology, Inc., Rockford, IL, USA), was
used. The protein expression was quantified using Alpha Ease FC
software (Alpha Imager System; Alpha Innotech Corp., San Leandro,
CA, USA).
Proliferation assay
To investigate the effect of different agents on the
proliferation of U-2 OS or MNNG-HOS cells, the CyQUANT®
NF Cell Proliferation Assay kit (Invitrogen Life Technologies) was
used according to the manufacturer’s instructions. Osteosarcoma
cells were seeded into 96-well plates (Greiner Bio-One,
Frickenhausen, Germany) at a density of 1×104
cells/well/200 μl medium.
Migration assay
To investigate the potential effects on cell
migration, the Cultrex®-24 Well Cell Migration Assay
(AMS Biotechnology (Europe) Ltd., Abingdon, Oxfordshire, UK) was
used. The assay was performed according to the manufacturer’s
instructions. The principle of the assay is based on a simplified
Boyden chamber design with an 8-μm polyethylene terephthalate (PET)
membrane. Detection of cell invasion was quantified using Calcein
AM.
siRNA-mediated gene knockdown
To study the effect of Mas gene silencing, cells
were seeded into six-well plates at a density of 5×105
cells/well/3 ml and cultured for 24 h, respectively. For gene
silencing h-MAS1-small interfering (si)RNA, (SI03068898; Qiagen,
Hilden, Germany), control-siRNA (SI1027280; Qiagen) and SiLentFect
lipid reagent (Bio-Rad) were used, following the instructions by
Bio-Rad. A total of 24 h following transfection, the cells were
used for the experiments (the migration and proliferation assays).
Knockdown of Mas expression was confirmed by qPCR in separate
aliquots.
Statistical analysis
Mann-Whitney U tests were applied in the case of
n≥4. Non-parametric data were illustrated as boxplots with medians,
quartiles and an interquartile range (IQR) ± 1.5 × IQR. Data are
expressed as the median ± quartile 1 and 3 (Q1 and Q3). P<0.05
was considered to indicate a statistically significant
difference.
Results
mRNA expression of essential components
of the classical axes in osteocarcinoma cell lines
The classical RAS axis has been implicated in the
development and metastasis of various tumor types and cohort
studies revealed that ACE inhibitors reduce the risk of tumors
(26,27). Less is known about the expression
and function of the alternative ACE2/Ang(1–7)/Mas
axis in osteosarcoma. Thus, the present study aimed to determine
the basic and cytokine-dependent expression of RAS components in
U-2 OS and MNNG-HOS cells. As revealed in Fig. 1, the cell lines demonstrated mRNA
expression for the proteolytic enzymes ACE, ACE2, membrane
alanyl-aminopeptidase (APN), neutral endopeptidase 24.11 (NEP) and
prolyl-endopeptidase (PEP), as well as for the different
angiotensin receptor subtypes, AT1R, AT2R, Mas and AT4R; the latter
is also known as insulin-regulated aminopeptidase (IRAP). MNNG-HOS
cells exhibited higher expression levels of all RAS components
investigated.
 | Figure 1Detection of the mRNA expression of
essential components of the classical (ACE, AT1R) or alternative
(ACE2, AT2R, PEP, NEP, Mas, APN, IRAP) RAS axes in the osteosarcoma
cell lines U-2 OS and MNNG-HOS. ACE, angiotensin-converting enzyme;
ATR1, angiotensin II type 1 receptor; RAS,
renin-angiotensin-system; PEP, prolyl-endopeptidase; NEP, neutral
endopeptidase 24.11; APN, alanyl-aminopeptidase; IRAP,
insulin-regulated aminopeptidase. |
Effect of IL-1β on the mRNA expression
levels of RAS components in osteocarcinoma cell lines
In response to IL-1β, a significant increase in the
mRNA expression levels of the Ang(1–7)
receptor, Mas and the Ang(1–7)-generating enzymes NEP and ACE2 was
observed in both U-2 OS and MNNG-HOS cells. However, PEP-mRNA
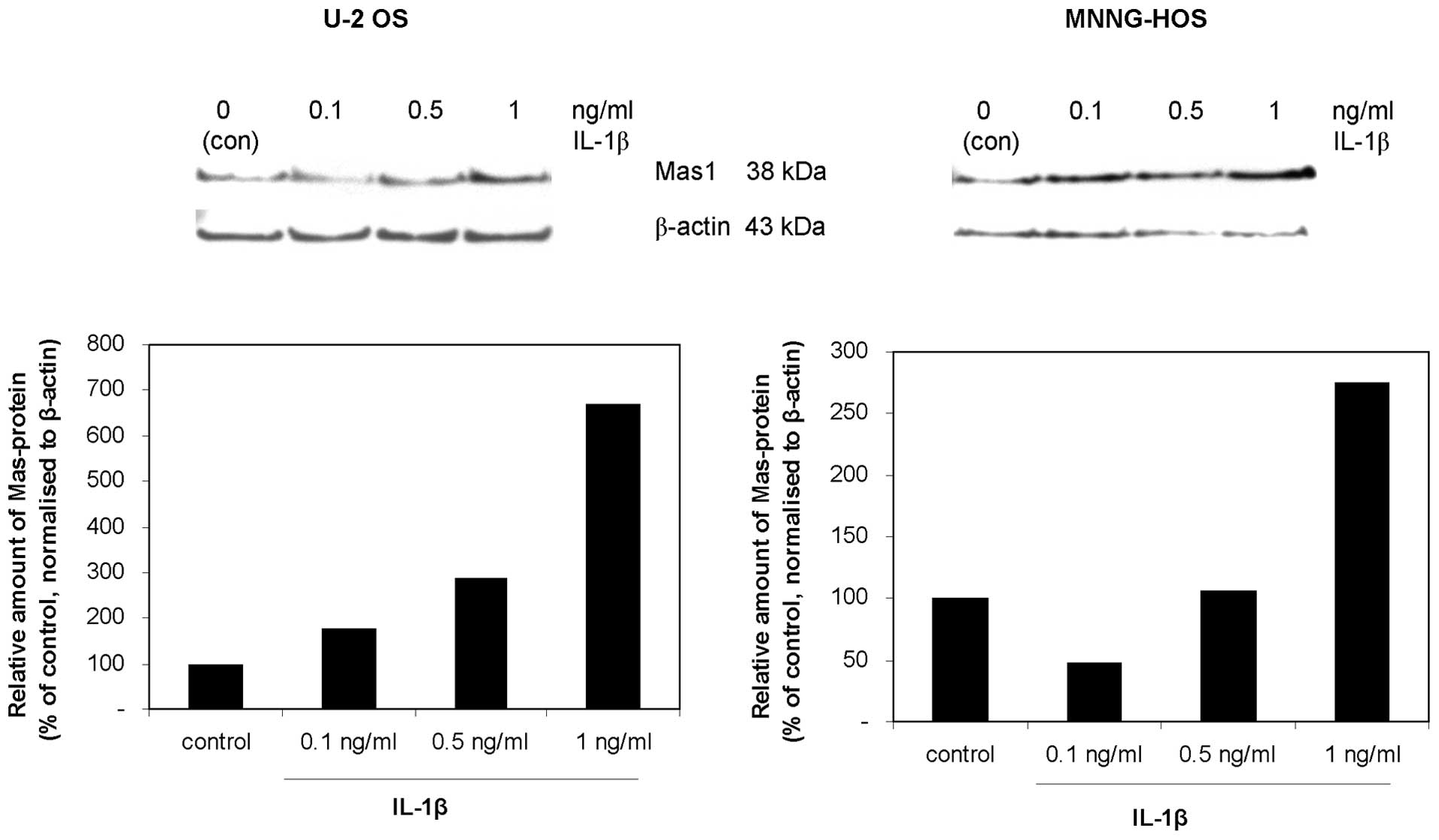
expression levels increased in U-2 OS cells only (Fig. 2). The dose-dependent increase of
Mas expression in response to IL-1β was confirmed at the protein
level in U-2 OS and MNNG-HOS cells (Fig. 3). Furthermore, a similar increase
of Mas expression was observed in response to TNFα (data not
shown), suggesting the involvement of NF-κB signalling in Mas
induction.
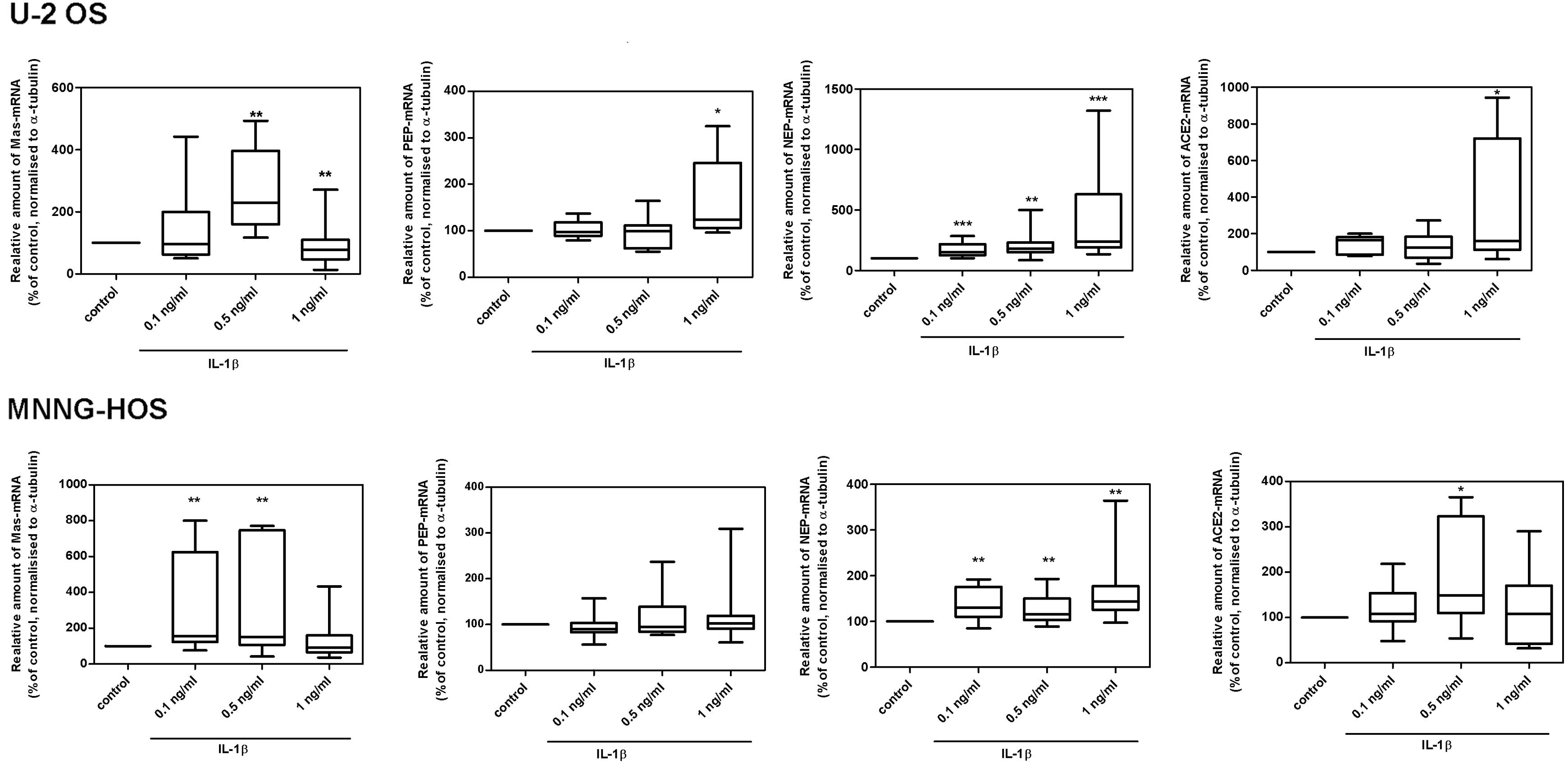 | Figure 2Effect of IL-1β on the mRNA expression
levels of RAS components in U2-OS and MNNG-HOS cells. IL-1β
provoked a dose-dependent increase in the mRNA expression of Mas,
ACE2, PEP, and NEP following 24 h. Data are expressed as the median
± Q1 and Q3; n=6; *P<0.05, **P<0.01 and
***P<0.001 compared with the control. IL-1β,
interleukin-1β; RAS, renin-angiotensin-system; ACE,
angiotensin-converting enzyme; PEP, prolyl-endopeptidase; NEP,
neutral endopeptidase 24.11; Q, quartile. |
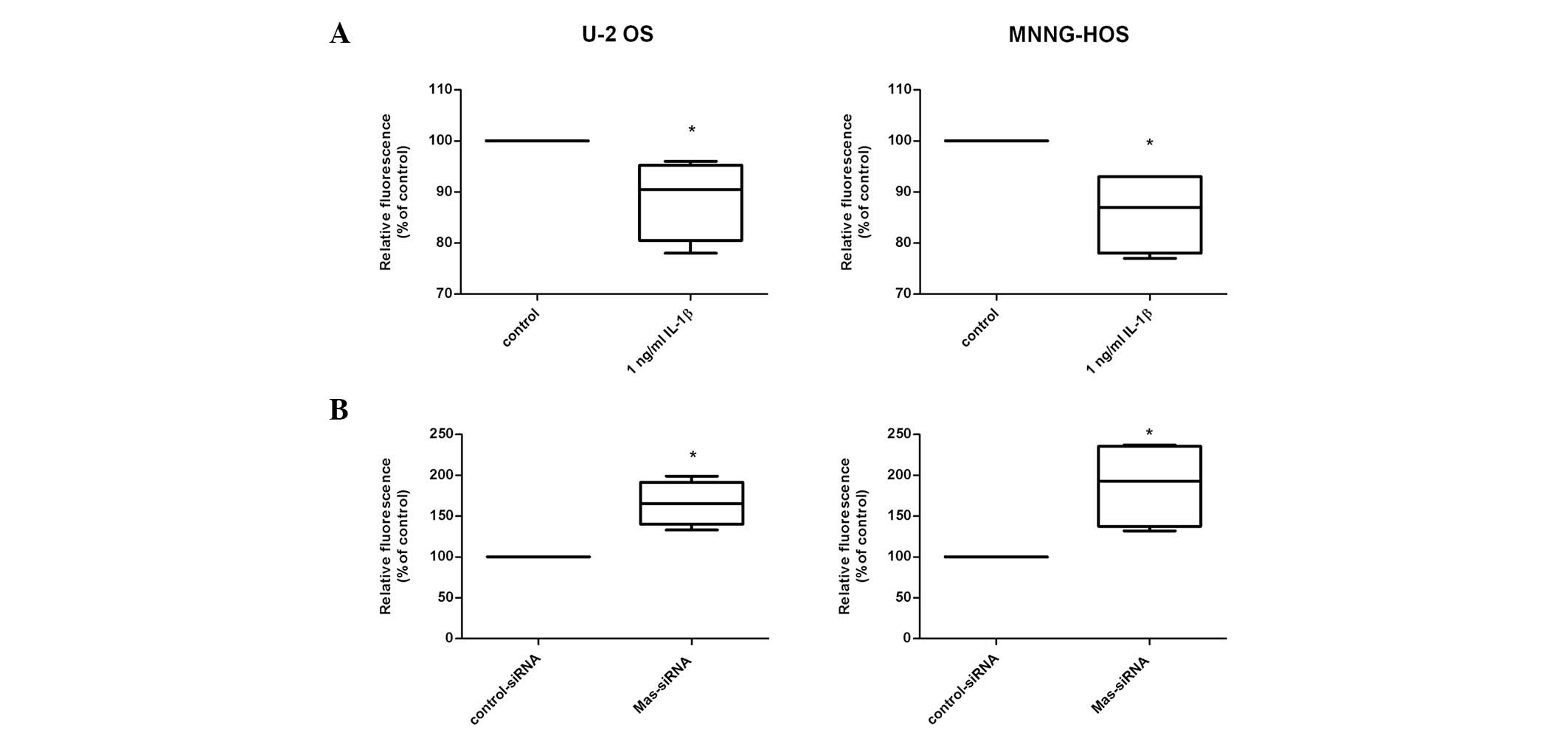
Effects of IL-1β-dependent increase of
Mas expression on cell proliferation and migration
Next, the question whether the IL-1β-dependent
increase of Mas expression had functional implications was
addressed by assessing cell proliferation and migration. As
demonstrated in Fig. 4A, IL-1β
provoked a moderate but significant reduction in proliferation of
the two cell lines (U-2 OS: 90% of control, P=0.027; MNNG-HOS: 88%
of control, P=0.026) in parallel with an observed induction of Mas
expression. To further substantiate a possible linkage between Mas
expression and osteosarcoma cell proliferation, siRNA-mediated
knockdown of Mas was performed. Fig.
4B demonstrates that the decrease in Mas expression lead to
increased proliferation of U-2 OS (1.7-fold; P<0.03) and
MNNG-HOS cells (1.9-fold; P=0.03).
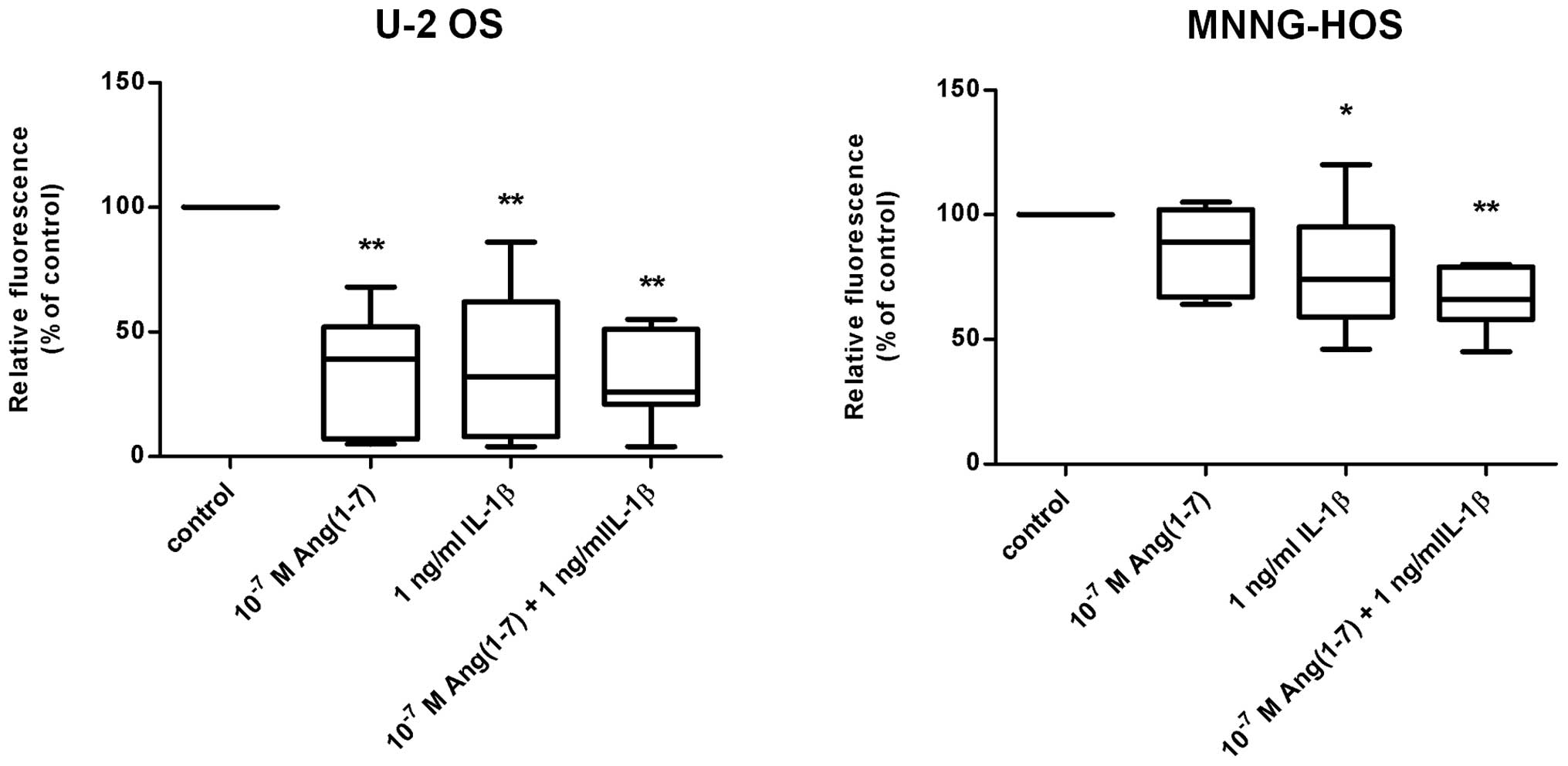
Interleukin-1β inhibited the migration of U-2 OS and
MNNG-HOS cells (Fig. 5). This
effect was more prominent in U-2 OS (40% of control; P<0.01) as
compared with the MNNG-HOS cell line (80% of control; P=0.024). In
support of the hypothesis that these effects on migration are due
to altered Mas expression, a similar inhibitory effect on cell
migration was observed in response to the administration of the
natural agonistic ligand for the Mas receptor, Ang(1–7). As
demonstrated in Fig. 5,
Ang(1–7) reduced cell migration to 48% in U-2 OS
cells (P<0.01), whereas MNNG-HOS cells revealed a tendency
towards reduced migration only. However, Ang(1–7)
applied together with IL-1β induced a highly significant reduction
of migration also in the MNNG-HOS cell line (62% of control;
P<0.01). As expected, the siRNA-mediated knockdown of Mas led to
an increase in cell migration which appeared to be more pronounced
in U-2 OS cells (1.4-fold) compared with MNNG-HOS cells
(1.1-fold).
Discussion
The aim of the present study was to elucidate the
expression and possible functional relevance of the alternative
ACE2/Ang(1–7)/Mas RAS axis in osteosarcoma cells. The
results of this study demonstrated that all essential components of
this axis, including Ang(1–7)-generating
proteases and the putative Ang(1–7)
receptor, Mas, are expressed in U-2 OS and MNNG-HOS osteosarcoma
cell lines.
The Mas gene was first cloned in 1986 from carcinoma
cells (28), and was identified to
provoke the development and growth of tumors upon transfer into
nude mice. Accordingly, Mas was regarded initially as a
proto-oncogene. The anti-proliferative activity of Mas transgene
expression or the preferred Mas ligand, Ang (1–7) was
revealed in later studies (29).
The results of the present study demonstrated that in the
osteosarcoma cell lines utilized, there was an IL-1β-dependent
increase in Mas expression, which was associated with decreased
cell proliferation. By contrast, the opposite effect was observed
in response to a siRNA-mediated knockdown of Mas. Although these
findings are consistent with the proposed anti-proliferative
effects of Mas, it should be mentioned that there is no direct
evidence supporting the causality between IL-1β-dependent Mas
induction and diminished proliferation of osteosarcoma.
Previous studies demonstrated that blocking of IL-1β
activity, by means of IL-1 receptor antagonist (IL-1Ra) together
with administration of the green tea-derived epigallocatechin
gallate, results in effective downregulation of pro-inflammatory
and pro-angiogenic cytokines, including IL-6, IL-8 and VEGF in U-2
OS cells (30). This evidence
implicated a role of IL-1β in tumor progression and immune escape
mechanisms, and simultaneously suggested that IL-1β-dependent Mas
induction may act as compensatory and growth-limiting mechanisms.
This indicated that the cytokine/Mas function in osteosarcoma is
highly complex. In parallel to increasing Mas expression levels,
IL-1β provoked a substantial increase in ACE2 expression.
Therefore, it is possible to hypothesize, that increased ACE2
expression results in higher levels of Ang(1–7),
which would then be able to bind to the more abundant Mas receptor
with ease. This would represent a shift from the detrimental
classical ACE/AngII/AT1R axis to the counter-acting and, possibly
beneficial, ACE2/Ang(1–7)/Mas axis of the RAS. Such a shift from
classical to alternative RAS axes would also be facilitated by the
observed increase in the expression of NEP.
By contrast to the tumor-promoting activity of
AngII/AT1R described above, Ang(1–7) has
been demonstrated to inhibit migration and invasion of lung
carcinoma cells and lung cancer (15,16).
Similar effects were observed in response to ACE2 overexpression
(17,18).
In summary, the present study demonstrated the
expression of essential components of classical and alternative RAS
axes in osteosarcoma cell lines. Activation of the
ACE2/Ang(1–7)/Mas axis compromised osteosarcoma
growth and migration, and may therefore represent a promising
therapeutic option. Furthermore, it was demonstrated that
(pro-inflammatory) cytokines are capable of modulating the
expression/activity of RAS axes, and in particular, that of the
ACE2/Ang(1–7)/Mas axis, which implicates the
existence of a number of more complex humoral networks that are
involved in net tumor growth, progression and metastasis.
Additional studies are required to further elucidate this
complexity to translate these results into clinical
applications.
Acknowledgements
The authors are grateful to Manja Möller and Ines
Schultz for excellent technical assistance.
References
|
1
|
Peach MJ: Renin-angiotensin system:
biochemistry and mechanisms of action. Physiol Rev. 57:313–370.
1977.PubMed/NCBI
|
|
2
|
Deshayes F and Nahmias C: Angiotensin
receptors: a new role in cancer? Trends Endocrinol Metab.
16:293–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamarat R, Silvestre JS, Durie M and Levy
BI: Angiotensin II angiogenic effect in vivo involves vascular
endothelial growth factor- and inflammation-related pathways. Lab
Invest. 82:747–756. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pupilli C, Lasagni L, Romagnani P, Bellini
F, Mannelli M, Misciglia N, Mavilia C, Vellei U, Villari D and
Serio M: Angiotensin II stimulates the synthesis and secretion of
vascular permeability factor/vascular endothelial growth factor in
human mesangial cells. J Am Soc Nephrol. 10:245–255.
1999.PubMed/NCBI
|
|
5
|
Otani A, Takagi H, Oh H, Koyama S and
Honda Y: Angiotensin II induces expression of the Tie2 receptor
ligand, angiopoietin-2, in bovine retinal endothelial cells.
Diabetes. 50:867–875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujita M, Hayashi I, Yamashina S, Itoman M
and Majima M: Blockade of angiotensin AT1a receptor signaling
reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys
Res Commun. 294:441–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suganuma T, Ino K, Shibata K, Kajiyama H,
Nagasaka T, Mizutani S and Kikkawa F: Functional expression of the
angiotensin II type 1 receptor in human ovarian carcinoma cells and
its blockade therapy resulting in suppression of tumor invasion,
angiogenesis, and peritoneal dissemination. Clin Cancer Res.
11:2686–2694. 2005. View Article : Google Scholar
|
|
8
|
Yasumatsu R, Nakashima T, Masuda M, Ito A,
Kuratomi Y, Nakagawa T and Komune S: Effects of the angiotensin-I
converting enzyme inhibitor perindopril on tumor growth and
angiogenesis in head and neck squamous cell carcinoma cells. J
Cancer Res Clin Oncol. 130:567–573. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neo JH, Malcontenti-Wilson C, Muralidharan
V and Christophi C: Effect of ACE inhibitors and angiotensin II
receptor antagonists in a mouse model of colorectal cancer liver
metastases. J Gastroenterol Hepatol. 22:577–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ebert MP, Lendeckel U, Westphal S, Dierkes
J, Glas J, Folwaczny C, Roessner A, Stolte M, Malfertheiner P and
Röcken C: The angiotensin I-converting enzyme gene
insertion/deletion polymorphism is linked to early gastric cancer.
Cancer Epidemiol Biomarkers Prev. 14:2987–2389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Röcken C, Lendeckel U, Dierkes J, Westphal
S, Carl-McGrath S, Peters B, Krüger S, Malfertheiner P, Roessner A
and Ebert MP: The number of lymph node metastases in gastric cancer
correlates with the angiotensin I-converting enzyme gene
insertion/deletion polymorphism. Clin Cancer Res. 11:2526–2530.
2005.PubMed/NCBI
|
|
12
|
Röcken C, Röhl FW, Diebler E, Lendeckel U,
Pross M, Carl-McGrath S and Ebert MP: The angiotensin
II/angiotensin II receptor system correlates with nodal spread in
intestinal type gastric cancer. Cancer Epidemiol Biomarkers Prev.
16:1206–1212. 2007.PubMed/NCBI
|
|
13
|
Wasa J, Sugiura H, Kozawa E, Kohyama K,
Yamada K and Taguchi O: The tumor suppressive effect of angiotensin
II type 1 receptor antagonist in a murine osteosarcoma model.
Anticancer Res. 31:123–127. 2011.PubMed/NCBI
|
|
14
|
Santos RA, Simoes e Silva AC, Maric C,
Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV,
Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ,
Schultheiss HP, Speth R and Walther T: Angiotensin-(1–7) is an
endogenous ligand for the G protein-coupled receptor Mas. Proc Natl
Acad Sci USA. 100:8258–8263. 2003.
|
|
15
|
Ni L, Feng Y, Wan H, Ma Q, Fan L, Qian Y,
Li Q, Xiang Y and Gao B: Angiotensin-(1–7) inhibits the migration
and invasion of A549 human lung adenocarcinoma cells through
inactivation of the PI3K/Akt and MAPK signaling pathways. Oncol
Rep. 27:783–790. 2012.
|
|
16
|
Gallagher PE and Tallant EA: Inhibition of
human lung cancer cell growth by angiotensin-(1–7). Carcinogenesis.
25:2045–2052. 2004.
|
|
17
|
Feng Y, Ni L, Wan H, Fan L, Fei X, Ma Q,
Gao B, Xiang Y, Che J and Li Q: Overexpression of ACE2 produces
antitumor effects via inhibition of angiogenesis and tumor cell
invasion in vivo and in vitro. Oncol Rep. 26:1157–1164.
2011.PubMed/NCBI
|
|
18
|
Qian YR, Guo Y, Wan HY, Fan L, Feng Y, Ni
L, Xiang Y and Li QY: Angiotensin-converting enzyme 2 attenuates
the metastasis of non-small cell lung cancer through inhibition of
epithelial-mesenchymal transition. Oncol Rep. 29:2408–2414.
2013.PubMed/NCBI
|
|
19
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu W and Zhao S: Metabolic changes in
cancer: beyond the Warburg effect. Acta Biochim Biophys Sin
(Shanghai). 45:18–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cifuentes M, García MA, Arrabal PM,
Martínez F, Yañez MJ, Jara N, Weil B, Domínguez D, Medina RA and
Nualart F: Insulin regulates GLUT1-mediated glucose transport in
MG-63 human osteosarcoma cells. J Cell Physiol. 226:1425–1432.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Costelloe CM, Macapinlac HA, Madewell JE,
Fitzgerald NE, Mawlawi OR, Rohren EM, Raymond AK, Lewis VO,
Anderson PM, Bassett RL Jr, Harrell RK and Marom EM: 18F-FDG PET/CT
as an indicator of progression-free and overall survival in
osteosarcoma. J Nucl Med. 50:340–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Franzius C, Bielack S, Flege S, Sciuk J,
Jürgens H and Schober O: Prognostic significance of (18)F-FDG and
(99m)Tc-methylene diphosphonate uptake in primary osteosarcoma. J
Nucl Med. 43:1012–1017. 2002.PubMed/NCBI
|
|
24
|
Liu C, Lv XH, Li HX, Cao X, Zhang F, Wang
L, Yu M and Yang JK: Angiotensin-(1–7) suppresses oxidative stress
and improves glucose uptake via Mas receptor in adipocytes. Acta
Diabetol. 49:291–299. 2012.
|
|
25
|
Giani JF, Mayer MA, Muñoz MC, Silberman
EA, Höcht C, Taira CA, Gironacci MM, Turyn D and Dominici FP:
Chronic infusion of angiotensin-(1–7) improves insulin resistance
and hypertension induced by a high-fructose diet in rats. Am J
Physiol Endocrinol Metab. 296:E262–E271. 2009.
|
|
26
|
Lever AF, Hole DJ, Gillis CR, McCallum IR,
McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL and
Robertson JW: Do inhibitors of angiotensin-I-converting enzyme
protect against risk of cancer? Lancet. 352:179–184. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friis S, Sørensen HT, Mellemkjaer L,
McLaughlin JK, Nielsen GL, Blot WJ and Olsen JH:
Angiotensin-converting enzyme inhibitors and the risk of cancer: a
population-based cohort study in Denmark. Cancer. 92:2462–2470.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Young D, Waitches G, Birchmeier C, Fasano
O and Wigler M: Isolation and characterization of a new cellular
oncogene encoding a protein with multiple potential transmembrane
domains. Cell. 45:711–719. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alenina N, Xu P, Rentzsch B, Patkin EL and
Bader M: Genetically altered animal models for Mas and
angiotensin-(1–7). Exp Physiol. 93:528–537. 2008.PubMed/NCBI
|
|
30
|
Hönicke A-S, Ender SA and Radons J:
Combined administration of EGCG and IL-1 receptor antagonist
efficiently downregulates IL-1-induced tumorigenic factors in U-2
OS human osteosarcoma cells. Int J Oncol. 41:753–758. 2012.
|
|
31
|
Chilukotia RK, Mostertz J, Bukowska A,
Aderkast C, Felix SB, Busch M, Völker U, Goette A, Wolke C, Homuth
G and Lendeckel U: Effects of irbesartan on gene expression
revealed by transcriptome analysis of left atrial tissue in a
porcine model of acute rapid pacing in vivo. Int J Cardiol.
168:2100–2108. 2013. View Article : Google Scholar : PubMed/NCBI
|