Introduction
Autophagy is a conserved process (1–3) that
degrades the cytoplasmic contents with lysosomal enzymes (4–7). It
is a physiological function required for normal animal development
and survival in metabolic stress. The autophagy program is
activated when the organism suffers stress due to starvation.
Recent studies indicate that autophagy has important implications
during cancer initiation and progression. Autophagy functions as a
protective mechanism. This mechanism (8–10) is
utilized by normal cells and cancer cells, so autophagy is
considered to have varied roles in the development and treatment of
cancer (11–13). There are two ubiquitin-like
conjugation systems for autophagy, the Atg12 and Atg8 conjugation
systems. Atg7 is important in the process of autophagy as this E1
enzyme can activate Atg12 and Atg8. Currently, it is unclear how
Atg7 functions in breast cancer. It is well established that
3-methyladenine (3Ma) (14–16)
is an autophagy depressor. However, little is understood regarding
the effect of autophagy inhibition on cell viability and the cell
cycle in breast cancer, or if the mechanisms of 3Ma and Atg7 are
associated. In order to investigate the effect of autophagy
inhibition on MDA-MB-231 human breast cancer cells in the present
study, different cell models were designed and the changes in the
cell cycle and viability of the cells were assessed by flow
cytometry and MTT assay, respectively.
Materials and methods
Cell culture and model establishment
MDA-MB-231 human breast cancer cells were cultured
at 37°C in high-glucose Dulbecco’s modified Eagle’s medium (DMEM)
(Gibco-BRL, Carlsbad, CA, USA) with 10% fetal bovine serum, 100
U/ml penicillin and 100 μg/ml streptomycin (Gibco-BRL). All
cultures were maintained at 37°C in a humidified atmosphere with 5%
CO2. When cells reached ~80% confluence, cells were
seeded into 96-well plates at a density of 1.0×104
cells/well to a total of 200 μl/well and were incubated for 24 h in
routine culture. They were then divided into groups. The three
groups without Atg7 siRNA transfection were as follows: The control
(untreated) group; the starvation (S) group (replaced high-glucose
DMEM with no-glucose minimal essential medium and maintained for 4
h); and the S+3Ma group (starvation group treated with 0.05 mM/l
3Ma). The other three groups were obtained by transfecting Atg7
siRNA into half the cells of each of the three fundamental groups,
and were denoted as follows: The Atg7(-) group (controls with
Atg7-siRNA transfection); the S+Atg7(-) group (starvation group
treated with Atg7-siRNA); and the S+Atg7(-)+3Ma group (starvation
group treated with 0.05 mM/l 3Ma, following transfection with
Atg7-siRNA for 4 h). Each group was repeated in three different
wells under identical conditions.
Atg7 siRNA transfection
In the Atg7-deficiency models, Atg7 siRNA (RiboBio,
Guangzhou, China) was transfected into the corresponding groups
with Lipofectamine™ RNAiMAX Transfection reagent (Invitrogen, Life
Technologies, Carlsbad, CA, USA). Isolation of total RNA and cDNA
synthesis were performed with TRIzol® reagent and a cDNA
Synthesis kit purchased from Takara Biotechnology (Dalian, China).
All steps were implemented in accordance with the manufacturer’s
instructions. The Atg7 mRNA levels were detected by quantitative
polymerase chain reaction (qPCR) and transfection efficiency
reached >70%. The sequences of the Atg7 primers [Sangon Biotech
(Shanghai) Co., Ltd., Shanghai, China] were as follows: Human Atg7:
F, 5′-CTTTTTGCCAACATCCCTG-3′ and R, 5′-GGTCTCTGGTTGAATCTCCT-3′;
human β-actin primers: F, 5′-GAAGATCAAGATCATTGCTCCT-3′ and R,
5′-TACTCCTGCTTGCTGATCCA-3′. qPCR reaction conditions were as
follows: 2 min at 94°C, 40 cycles of 20 sec at 94°C, 16 sec at 54°C
and 30 sec at 72°C. The control group was transfected with
negative-control siRNA. Following transfection for 4 h, the medium
was removed from all groups and the cells were washed twice with
0.01 mol/l phosphate-buffered saline (PBS). The cells were then
recultured in high-glucose DMEM. The morphological changes in the
cells were observed with an inverted microscope at 24 h after
transfection (Eclipse TE2000-E; Nikon, Tokyo, Japan).
Cell viability assay
Cell viability was measured by MTT (M-2128;
Sigma-Aldrich, St. Louis, MO, USA) method according to the
manufacturer’s instructions. All groups were detected at 6, 12, 24,
48 and 72-h time points.
Flow cytometry assay
MDA-MB-231 cells were seeded into 6-well plates at a
density of 5×104 cells/ml with a total of 2 ml/well then
treated individually based on the aforementioned groups. The cells
were harvested after 24-h incubation, and then they were fixed in
70% precooled ethanol for 1 h. Following two washes with PBS, the
cells were centrifuged and resuspended in 1 ml PBS (0.01 mol/l),
then stored at 4°C overnight. The cells were stained in PBS
containing 50 μg/ml RNase and 100 μg/ml propidium iodide in the
dark at room temperature for 30 min. Flow cytometry (FACSCanto II;
BD Biosciences, San Jose, CA, USA) was used to analyze the cell
cycle and levels of apoptosis with BD FACSDiva software (BD
Biosciences).
Statistical analysis
All data were analyzed using GraphPad Prism
software, version 5.01 (GraphPad, San Diego, CA, USA). The cell
viability detected by the MTT method was assessed by two-way
repeated measures analysis of variance with Bonferroni post-hoc
tests. P<0.05 was considered to indicate a statistically
significant difference. The data are expressed as the mean ±
standard deviation of experiments performed in triplicate.
Results
Transfection efficiency
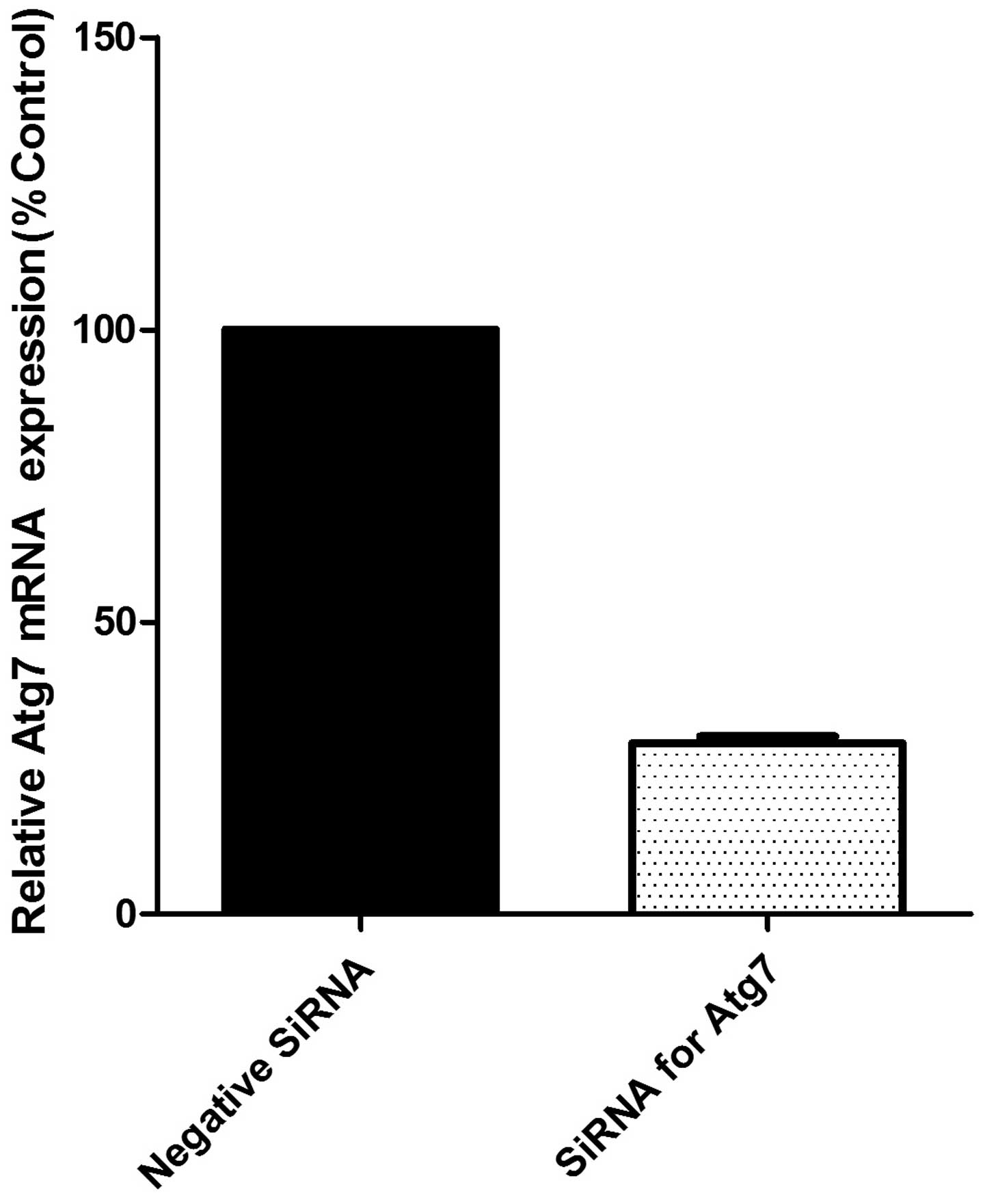
The measurement of Atg siRNA transfection efficiency
was repeated 12 times independently by qPCR with β-actin. Relative
Atg7 mRNA expression levels are presented in Fig. 1 compared with levels in the control
group, and the transfection efficiency reached >70%.
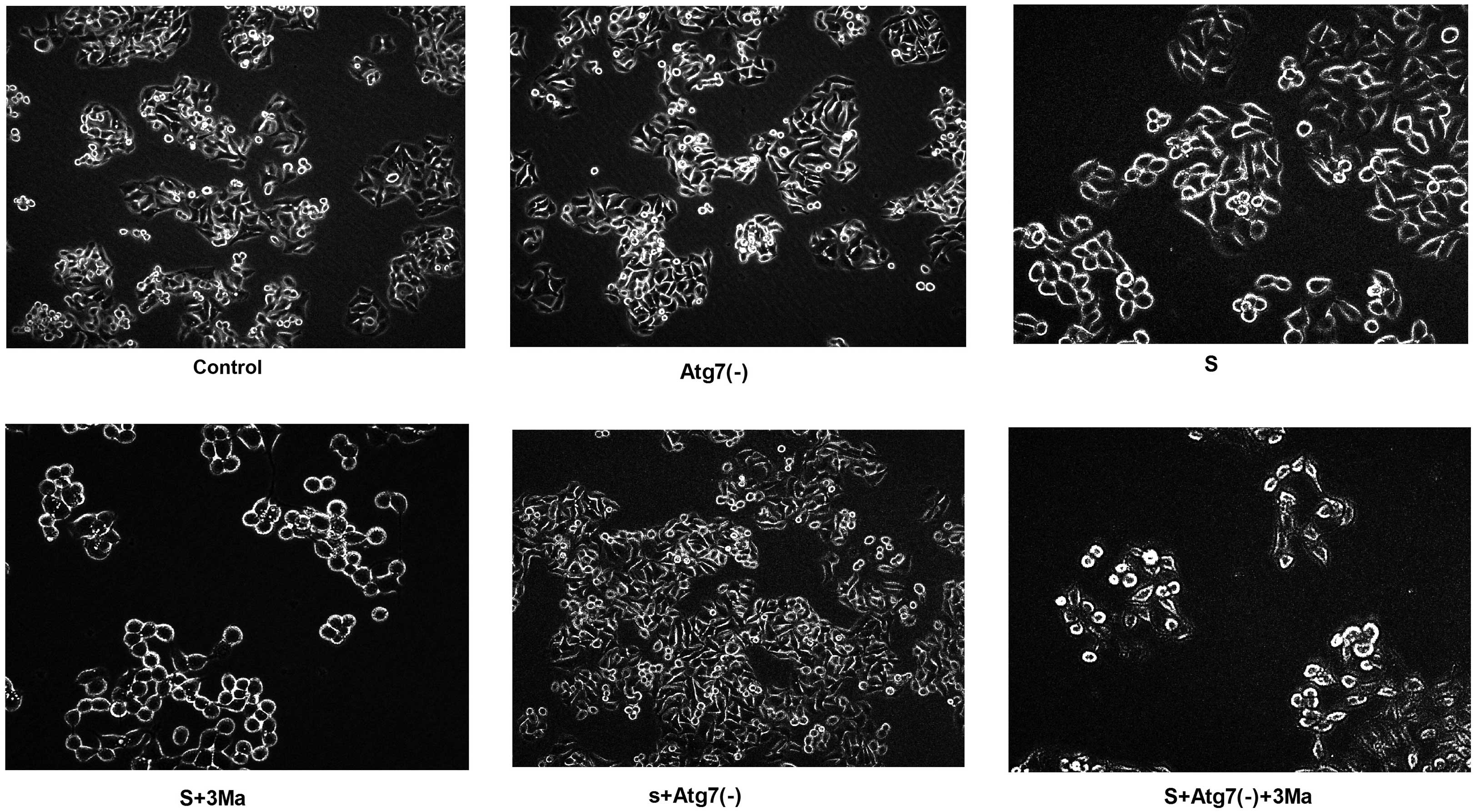
Cytomorphological changes vary between
groups inhibited by 3Ma and groups with Atg7 deficiency
Following 24-h transfection with Atg7 siRNA, the
cells exhibited more protuberances. In addition, the cells
congregated and adhered to the wall of the culture plate more
easily [control vs. Atg7(-); S vs. S+Atg7(-); S+3Ma vs.
S+Atg7(-)+3Ma]. On the contrary, in the S+3Ma and S+Atg7(-)+3Ma
groups, the cell mass died and cell quantity markedly reduced. The
cells were not easily observed with the microscope. Furthermore,
the cells appeared more round and did not easily adhere to the wall
of culture plate following 3Ma treatment (Fig. 2).
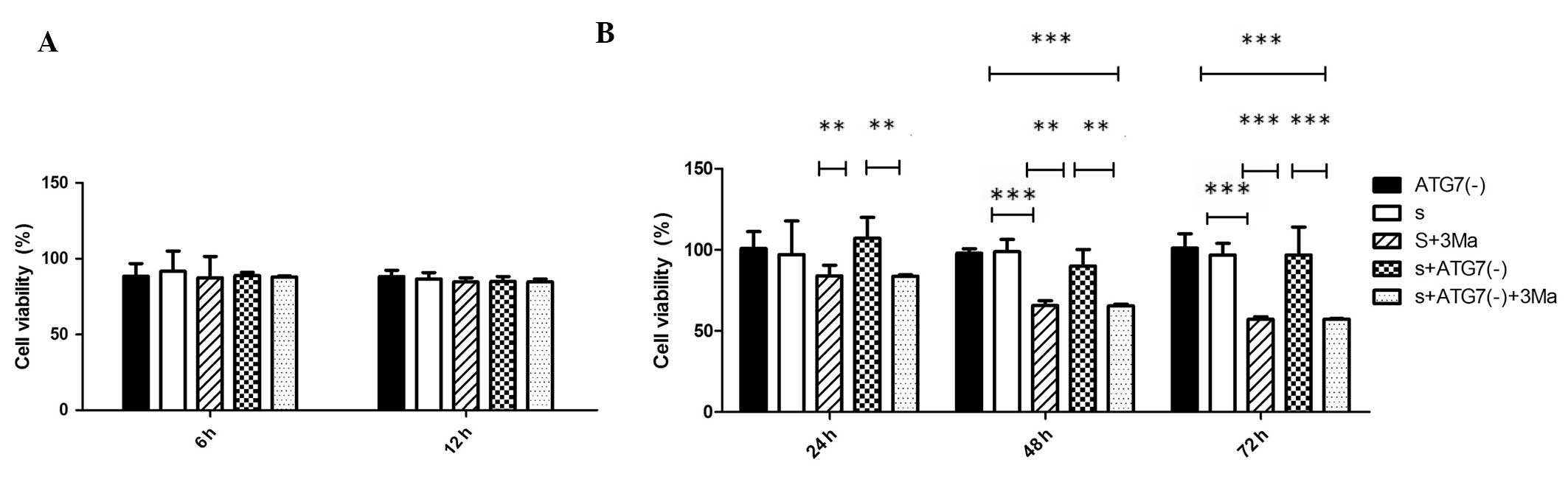
3Ma treatment reduces viability of
MDA-MB-231 cells while Atg7 deficiency does not
Cell viabilities of all groups at different time
points (6, 12, 24, 48 and 72 h) were analyzed (Fig. 3). The percentage cell viability was
not significantly different between Atg7 siRNA-transfected groups
and untransfected groups at any time point (P>0.05). 3Ma
treatment did not significantly affect cell viability at the 6 and
12 h time points in any groups (P>0.05). In cells without Atg7
deficiency, the 3Ma treatment led to significantly reduced
viability at 48 and 72 h (P<0.001, S vs. S+3Ma); whilst in
Atg7-deficient cells, 3Ma treatment led to a significant reduction
in viability at 24, 48 (P<0.01) and 72 h (P<0.001) [S+Atg(-)
vs. S+Atg7(-)+3Ma]. When comparing the difference between the
effects of Atg7 deficiency and 3Ma treatment, the S+3Ma group
exhibited lower cell viability compared with the S+Atg7(-) group at
24 and 48 h time points (P<0.01) and at the 72 h time point
(P<0.001) (Fig. 3B).
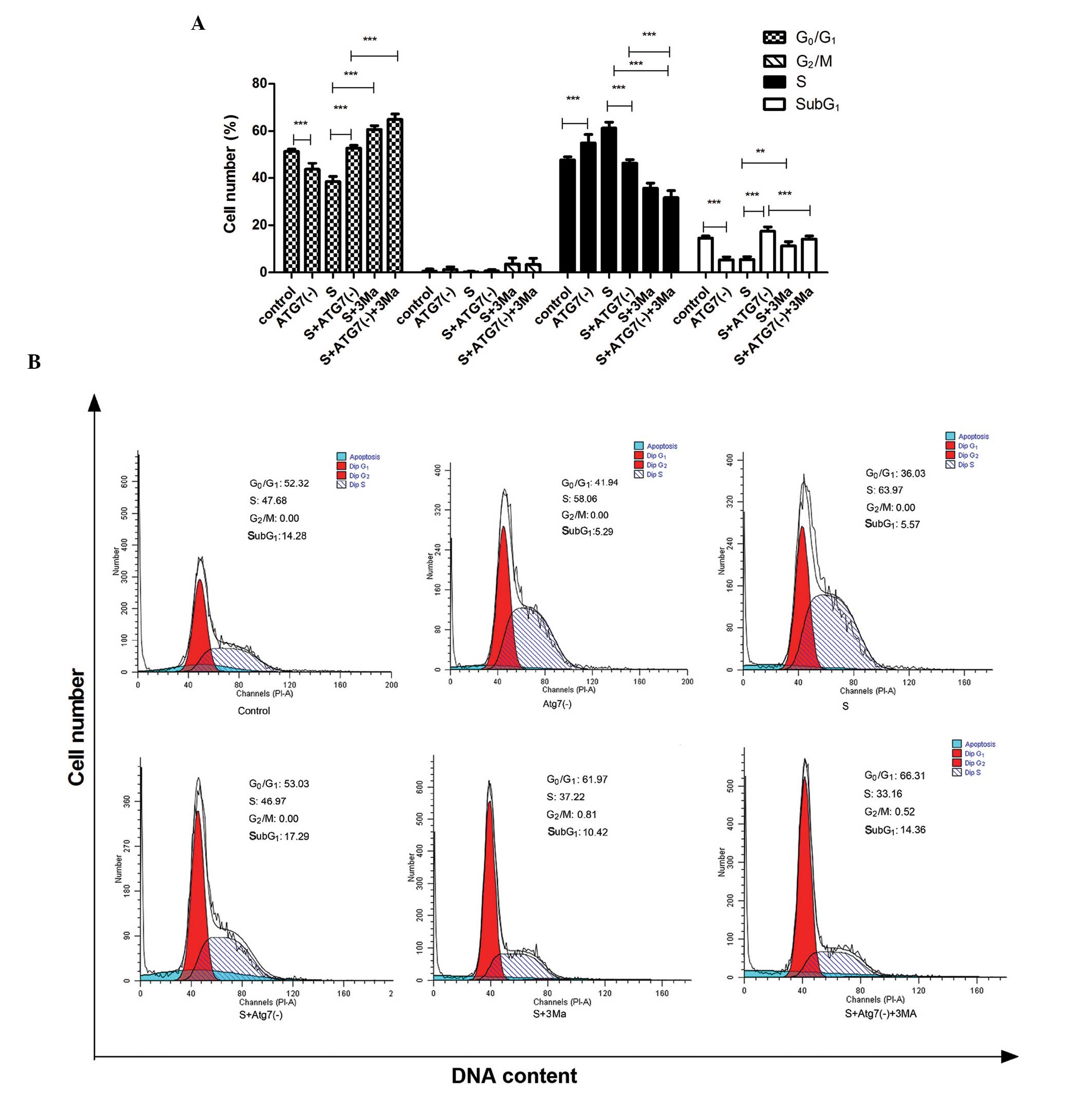
Effects of Atg7 siRNA interference and
3Ma treatment on apoptosis and cell cycle in MDA-MB-231 cells
The cells in the SubG1-phase represent
cells undergoing apoptosis (Fig.
4). Atg7 siRNA transfection led to increased percentages of
cells in apoptosis and in the G0/G1-phase;
while the percentage of cells in the S-phase reduced [P<0.001, S
vs. S+Atg7(-)]. In the untransfected cells, 3Ma treatment led to an
increase in percentage of cells in apoptosis (P<0.01) and in the
G0/G1-phase (P<0.001), while the
percentage in the S-phase decreased (P<0.001) (S vs. S+3Ma).
However, in the Atg7 deficient cells, 3Ma led to reduced levels of
apoptosis and S-phase cells, while there was an increased
percentage of cells in the G0/G1-phase
[P<0.001, S+Atg7(-) vs. S+Atg7(-)+3Ma].
Discussion
Following analysis of the cellular morphology of the
groups, autophagy inhibition by Atg7 deficiency functions to
maintain the adhesion of MDA-MB-231 cells, however, another
autophagy inhibitor, 3Ma, functions to deprive the adhesion of
MDA-MB-231 cells.
The results of the cell viability assay indicated
that transfection of Atg7-siRNA had no significant effect on the
survival of MDA-MB-231 human breast cancer cells. This result
supports those of previous clinical studies. Chang et al
(17) reported that the loss of
Atg7 or Atg3 function did not influence cellular processes during
intestinal cell death and they hypothesized that there is an Atg7-
and Atg3-independent autophagy pathway which is used in certain
cells. Qin et al (18)
systematically screened 14 functional polymorphisms in six
autophagy-related genes including Atg7 and identified no
association of Atg7 gene variants with the risk of breast cancer in
a Chinese population.
In the current study, the results of the flow
cytometry revealed that autophagy inhibition by transfection of
Atg7 siRNA increased the percentages of cells in apoptosis and
G0/G1-phase, while it reduced the percentage
of cells in S-phase in the starvation groups. The cell cycle was
arrested in the G0/G1-phase. These results
support the theory that Atg7 deficiency inhibits proliferation and
promotes apoptosis in MDA-MB-231 human breast cancer cells;
however, no effect of Atg7 deficiency on cell viability was
observed.
The results of the present study imply that the
effect of autophagy inhibition by Atg7 deficiency is not sufficient
to reduce cell viability, or that there is another pathway that
counteracts its effect on cell viability. Thus, the effect of Atg7
in the breast cancer requires further study. Compared with the
control cells, the two factors of starvation and Atg7 deficiency
each reduced the percentage of cells in apoptosis and
G0/G1-phase and elevated the percentage of
cells in S-phase. This implies the effect of Atg7 deficiency on the
cell cycle may associate with glucose starvation. The data supports
a study by Balmer et al (19) which indicated that low
glucose-induced apoptosis with inhibition of autophagy may lead to
cell death in 661W photoreceptor cells. Ramírez-Peinado et
al (20) hypothesized that
autophagic flux induced by other stimuli is inhibited in the
absence of glucose. Data from the present study suggest the
inhibition of autophagy by Atg7 deficiency may promote apoptosis
and cell cycle arrest in the G0/G1-phase
under conditions of glucose starvation. Following Atg7 siRNA
transfection without the glucose starvation, the percentage of
cells in the S-phase increased while those in apoptosis reduced.
Further study is required to validate these results, and confirm
the lack of effect by Atg7 deficiency on cell viability.
According to the results of the current study,
inhibition of autophagy by Atg7 deficiency promotes apoptosis and
inhibits cell proliferation in cells maintained in low-glucose
medium but does not influence the viability of these cells.
As another method of autophagy inhibition, 3Ma has
an opposing functional effect to Atg7 deficiency on the cellular
morphology. Treated by 3Ma, the cells became rounder and aggregated
less easily. The autophagy inhibitor 3Ma was found to reduce the
viability of breast cancer cells. With Atg7 deficiency, the P-value
related to the effect of 3Ma was larger than that without Atg7
deficiency and was significant at an earlier time point
(significant difference at 24-h time point). Therefore, the present
study provides reasonable grounds to infer that Atg7 deficiency may
potentiate the effect of 3Ma on cell viability (but it may also be
counteracted by 3Ma). The data from the present study demonstrated
that an interaction existed between the method of autophagy
inhibition occurring following 3Ma treatment and that occurring in
Atg7 deficiency.
A number of studies indicate that 3Ma (21–23)
potentiates the effects of anticarcinogens by inhibiting autophagy
and increasing apoptosis. It has been suggested that 3Ma may be a
good therapeutic strategy for use in drug-resistant cancer cells.
The current study produced a similar result; 3Ma increased
apoptosis in MDA-MB-231 human breast cancer cells. As an autophagy
inhibitor, 3Ma significantly increased the percentage of the cells
in apoptosis and the G0/G1-phase while it
reduced the percentage of cells in the S-phase. This effect of 3Ma
on the cell cycle resembled that of Atg7 deficiency, indicating
that the two methods of autophagy inhibition by 3Ma treatment and
Atg7 deficiency can each promote apoptosis and cell arrest in the
G0/G1-phase. However, in combination with
Atg7 deficiency, 3Ma reduced the effect of Atg7 deficiency alone on
apoptosis and cell arrest in G0/G1-phase.
Therefore, 3Ma may abrogate the effect of Atg7 deficiency on the
cell cycle to a certain extent; whereas, with regards to cell
viability, Atg7 deficiency had no influence and 3Ma treatment led
to a significant reduction.
The current study confirmed that 3Ma treatment or
Atg7 deficiency can exert effects on the cell cycle to promote
apoptosis and cell arrest in G0/G1-phase in
MDA-MB-231 human breast cancer cells.
In conclusion, the current study indicated an
interaction between the mechanism of autophagy inhibition following
3Ma treatment and that occurring in response to Atg7 deficiency.
3Ma strongly reduced the viability of MDA-MB-231 human breast
cancer cells and the effect of 3Ma was potentiated by Atg7
deficiency. In the present study, the absence of glucose was
demonstrated to be important on the effect of Atg7 deficiency on
the cell cycle. However, Atg7 deficiency did not cause changes in
cell viability. Atg7 deficiency may not be sufficient to reduce the
cell viability or it is possible that there is another pathway that
counteracts its effect on cell viability. Whether affecting cell
viability or the cell cycle, there exists negative interaction
between mechanisms occurring in Atg 7 deficiency and those
following 3Ma treatment.
Overall, Atg7 appears to be involved in a different
pathway to 3Ma in the process of autophagy. Inhibition of autophagy
may influence cell viability and the cell cycle through different
pathways in MDA-MB-231 human breast cancer cells.
Acknowledgements
The authors thank Liuqi Yang (National Key
Laboratory of the West China School of Sichuan University) for
providing MDA-MB-231 cells, and Qijie Li for technical assistance
in flow cytometry. The current study was supported by the National
Natural Science Foundation of China, study code H0815, and was
funded by the fostering project of Sichuan province Science and
Technology Innovation Seedling Engineering, project number 20132048
and the Health Department of Sichuan province, project number
110328.
References
|
1
|
Ahn JH and Lee M: Autophagy-dependent
survival of mutant B-raf melanoma cells selected for resistance to
apoptosis induced by inhibitors against oncogenic B-raf. Biomol
Ther (Seoul). 21:114–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo JY, Karsli-Uzunbas G, Mathew R, Aisner
SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, et
al: Autophagy suppresses progression of K-ras-induced lung tumors
to oncocytomas and maintains lipid homeostasis. Genes Dev.
27:1447–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu L, Du H, Shi M, Chen Z and Hang J:
ATG7 deficiency promote apoptotic death induced by Cisplatin in
human esophageal squamous cell carcinoma cells. Bull Cancer.
100:15–21. 2013.PubMed/NCBI
|
|
4
|
Shen J, Zheng H, Ruan J, Fang W, Li A,
Tian G, Niu X, Luo S and Zhao P: Autophagy inhibition induces
enhanced proapoptotic effects of ZD6474 in glioblastoma. Br J
Cancer. 109:164–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tong Y, You L, Liu H, Li L, Meng H, Qian Q
and Qian W: Potent antitumor activity of oncolytic adenovirus
expressing Beclin-1 via induction of autophagic cell death in
leukemia. Oncotarget. 4:860–874. 2013.PubMed/NCBI
|
|
6
|
Basit F, Cristofanon S and Fulda S:
Obatoclax (GX15-070) triggers necroptosis by promoting the assembly
of the necrosome on autophagosomal membranes. Cell Death Differ.
20:1161–1173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yue W, Hamaï A, Tonelli G, Bauvy C,
Nicolas V, Tharinger H, Codogno P and Mehrpour M: Inhibition of the
autophagic flux by salinomycin in breast cancer
stem-like/progenitor cells interferes with their maintenance.
Autophagy. 9:714–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar S, Kumar A, Pathania AS, Guru SK,
Jada S, Sharma PR, Bhushan S, Saxena AK, Kumar HM and Malik F:
Tiron and trolox potentiate the autophagic cell death induced by
magnolol analog Ery5 by activation of Bax in HL-60 cells.
Apoptosis. 18:605–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JH, Lee JE, Lee SJ, Park SJ, Park KH,
Jeong M and Koh HC: Potential autophagy enhancers protect against
fipronil-induced apoptosis in SH-SY5Y cells. Toxicol Lett.
223:25–34. 2013. View Article : Google Scholar
|
|
10
|
Qu W, Xiao J, Zhang H, Chen Q, Wang Z, Shi
H, Gong L, Chen J, Liu Y, Cao R and Lv J: B19, a novel monocarbonyl
analogue of curcumin, induces human ovarian cancer cell apoptosis
via activation of endoplasmic reticulum stress and the autophagy
signaling pathway. Int J Biol Sci. 9:766–777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Polewska J, Skwarska A, Augustin E and
Konopa J: DNA-damaging imidazoacridinone C-1311 induces autophagy
followed by irreversible growth arrest and senescence in human lung
cancer cells. J Pharmacol Exp Ther. 346:393–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei YM, Li X, Xu M, Abais JM, Chen Y,
Riebling CR, Boini KM, Li PL and Zhang Y: Enhancement of autophagy
by simvastatin through inhibition of Rac1-mTOR signaling pathway in
coronary arterial myocytes. Cell Physiol Biochem. 31:925–937. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye L, Zhao X, Lu J, Qian G, Zheng JC and
Ge S: Knockdown of TIGAR by RNA interference induces apoptosis and
autophagy in HepG2 hepatocellular carcinoma cells. Biochem Biophys
Res Commun. 437:300–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheng Y, Sun B, Guo WT, Zhang YH, Liu X,
Xing Y and Dong DL: 3-Methyladenine induces cell death and its
interaction with chemotherapeutic drugs is independent of
autophagy. Biochem Biophys Res Commun. 432:5–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei FR, Li XQ, Liu H, Zhu RD, Meng QY and
Rong JJ: Rapamycin and 3-methyladenine regulate apoptosis and
autophagy in bone-derived endothelial progenitor cells. Chin Med J
(Engl). 125:4076–4082. 2012.PubMed/NCBI
|
|
16
|
Lin YC, Kuo HC, Wang JS and Lin WW:
Regulation of inflammatory response by 3-methyladenine involves the
coordinative actions on Akt and glycogen synthase kinase 3β rather
than autophagy. J Immunol. 189:4154–4164. 2012.PubMed/NCBI
|
|
17
|
Chang TK, Shravage BV, Hayes SD, Powers
CM, Simin RT, Wade Harper J and Baehrecke EH: Uba1 functions in
Atg7- and Atg3-independent autophagy. Nat Cell Biol. 15:1067–1078.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin Z, Xue J, He Y, Ma H, Jin G, Chen J,
Hu Z, Liu X and Shen H: Potentially functional polymorphisms in
ATG10 are associated with risk of breast cancer in a Chinese
population. Gene. 527:491–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balmer D, Emery M, Andreux P, Auwerx J,
Ginet V, Puyal J, Schorderet DF and Roduit R: Autophagy defect is
associated with low glucose-induced apoptosis in 661W photoreceptor
cells. PLoS One. 8:e741622013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramírez-Peinado S, León-Annicchiarico CL,
Galindo-Moreno J, Iurlaro R, Caro-Maldonado A, Prehn JH, Ryan KM
and Muñoz-Pinedo C: Glucose-starved cells do not engage in
prosurvival autophagy. J Biol Chem. 288:30387–30398.
2013.PubMed/NCBI
|
|
21
|
Pliyev BK and Menshikov M: Differential
effects of the autophagy inhibitors 3-methyladenine and chloroquine
on spontaneous and TNF-α-induced neutrophil apoptosis. Apoptosis.
17:1050–1065. 2012.PubMed/NCBI
|
|
22
|
Tseng HC, Liu WS, Tyan YS, Chiang HC, Kuo
WH and Chou FP: Sensitizing effect of 3-methyladenine on
radiation-induced cytotoxicity in radio-resistant HepG2 cells in
vitro and in tumor xenografts. Chem Biol Interact. 192:201–208.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q,
Wenk MR, Ong CN, Codogno P and Shen HM: Dual role of
3-methyladenine in modulation of autophagy via different temporal
patterns of inhibition on class I and III phosphoinositide
3-kinase. J Biol Chem. 285:10850–10861. 2010. View Article : Google Scholar : PubMed/NCBI
|