Introduction
Flavonoids, extracted from traditional Chinese
herbs, vegetables and fruit, have attracted much attention due to
their antitumor activities. Several flavonoids have been shown to
inhibit the proliferation and migration of bladder cancer cells,
including curcumin, which has been found to induce apoptosis and
repress bladder tumor growth in vitro and in vivo
(1), and myricetin, which has been
shown to arrest T24 bladder cancer cells at G2/M through
the downregulation of cyclin B1 and the cyclin-dependent kinase
cell division control protein 2 homolog (2). Myricetin also suppresses the
migration of bladder cancer cells by decreasing matrix
metalloproteinase (MMP)-9 expression (2). Butein has been reported to inhibit
the migration and invasion of bladder cancer cells by reducing the
expression and activity of extracellular signal-regulated kinase
(ERK) 1/2 (3), and
epigallocatechin-3-gallate (EGCG) has been shown to induce
apoptosis in TSGH-8301 cells through the suppression of AKT
activity and the modulation of heat shock protein 27 expression
(4). Furthermore, EGCG has been
observed to reduce AKT activity, deactivate nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) and
downregulate MMP-9 expression, consequently reducing the migration
and invasion of T24 bladder cancer cells (5).
Naringenin, a bioactive flavonoid enriched in
grapefruits and citrus fruits, exhibits a wide range of
pharmacological activities. Frydoonfar et al (6) demonstrated that naringenin
significantly inhibited proliferation in HT29 colon cancer cells.
Naringenin treatment also decreased viability and lung metastasis
in B16-F10 melanoma cells, with metastasis reduced by 63% compared
with the non-treated groups (7).
Furthermore, naringenin has been found to inhibit
N-nitrosodiethylamine (NDEA)-induced hepatocarcinomas in rats
(8) and to exhibit anti-migration
effects. Naringenin inhibits the tumor necrosis factor-α-induced
migration of smooth vascular muscle cells by elevating heme
oxygenase-1 expression (9).
Recently, Lou et al (10)
demonstrated that naringenin repressed pancreatic cancer cell
migration and invasion through the downregulation of transforming
growth factor-β-induced epithelial to mesenchymal transition
markers, including vimentin, N-cadherin, MMP-2 and MMP-9.
Bladder cancer is one of the most common malignant
neoplasms worldwide and is associated with a high mortality rate
among urological neoplasms (11).
Emerging research has indicated that extracts from traditional
herbs exhibit beneficial effects for the treatment of bladder
cancer, including pro-apoptotic and anti-migratory effects.
However, the effects of naringenin on bladder cancer cells are yet
to be elucidated. The present study investigated the molecular
mechanisms underlying the effect of naringenin on the migration of
TSGH-8301 bladder cancer cells.
Materials and methods
Cell culture
Human bladder carcinoma TSGH-8301 cells
(Bioresources Collection and Research Center, Food Industry
Research and Development Institute, Hsinchu, Taiwan) were
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. Cells
were incubated at 37°C in a humidified atmosphere with 5%
CO2.
MTT assay
Cells were seeded on 24-well plates at a density of
2×104/ml and treated with the indicated concentrations
of naringenin for 24 h. Cells were then incubated with fresh medium
containing 5.0 g/l MTT at 37°C for an additional 3 h. Subsequent to
washing with phosphate-buffered saline (PBS), the purple-blue
sediments were dissolved in 1 ml isopropanol and the absorbance was
read at 563 nm. The relative proliferation rate was calculated
based on the optical density of each sediment at 563 nm compared
with the vehicle-treated groups.
Western blot analysis
Cell lysates were obtained from the
naringenin-treated TSGH-8301 cells. A total of 50 μg protein was
separated using 10% polyacrylamide gels and electrotransferred to
nitrocellulose membranes. Membranes were blocked using PBS
containing 0.5% non-fat milk for 1 h at room temperature and then
probed with anti-MMP2 (Millipore, Billerica, MA, USA), anti-NFκB
(BD Biosciences, Franklin Lakes, NJ, USA), anti-AKT,
anti-phospho-AKT, anti-ERK, anti-p38, and-phospho-p38 and
anti-β-actin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA)
primary antibodies at room temperature for 1 h. The membranes were
subsequently washed with PBS containing 0.1% Tween-20 and incubated
with horseradish peroxidase-conjugated bovine anti-goat
immunoglobulin G antibodies at a dilution of 1:5,000 (Santa Cruz
Biotechnology, Inc.). Membranes were washed with PBS, prior to
detection of the reactive signal using enhanced chemiluminescence
(Amersham Pharmacia Biotech, Amersham, UK). β-actin expression was
used as an internal control.
Wound healing assay
For the wound healing experiments, cells were seeded
at a density of 1×106/ml. A wound was created by
scraping the cell monolayer with a sterilized tip. Following the
removal of the detached cells, the cell monolayer was treated with
the indicated concentrations of naringenin for 24 h. Migration was
assessed by counting the cell numbers in the cell-free region. The
value was presented as the mean ± standard deviation (SD) of three
independent experiments.
Gelatin zymography assay
Cells were incubated in serum-free medium and
cultured in the presence of the indicated naringenin concentrations
for 24 h. Supernatants were then collected and separated using 8%
SDS-polyacrylamide gels containing 0.1% gelatin. The gels were
washed with washing buffer (2.5% Triton X-100) twice with gentle
agitation at room temperature for 30 min, prior to incubation with
the reaction buffer [40 mM Tris-HCl (pH 8.0) 10 mM CaCl2
and 0.01% NaN3] for 12 h. The gels were then stained
with Coomassie Brilliant Blue R250 and destained. The degraded
zones, representing MMP-2 activity, were quantified using a
densitometer.
Migration assay
Cells were treated with different concentrations of
naringenin for 24 h and seeded at a density of 5×105/ml
in the upper chamber of the 48-well Boyden chamber. The lower
chamber contained 20% FBS. The chamber was incubated at 37°C for 24
h. Cells that migrated to the lower surface of the membrane were
fixed in methanol for 10 min and stained with 10% Giemsa for 1 h.
The number of cells was quantified by counting three random
microscopic fields (magnification, ×400).
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments and were evaluated using the Student’s
t-test with SPSS statistical software (SPSS, Inc., Chicago, IL,
USA). A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
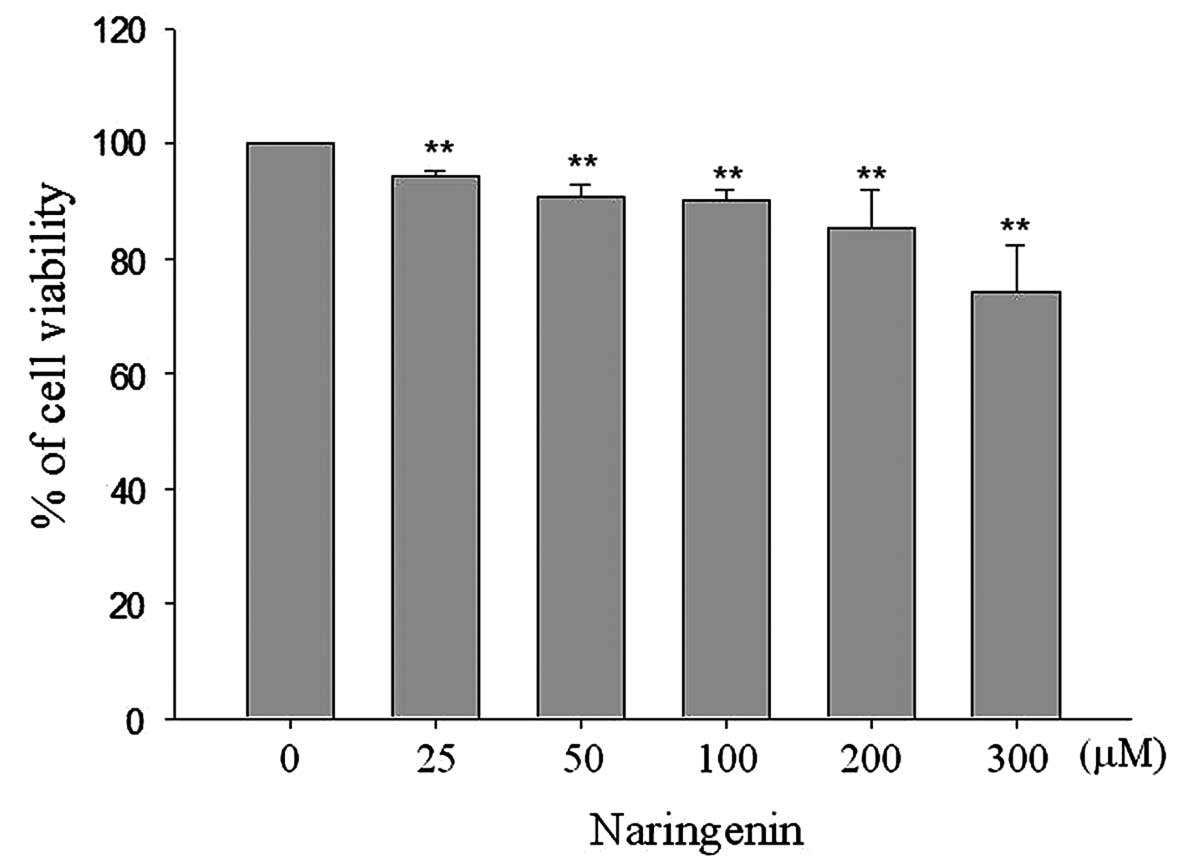
Naringenin reduces TSGH-8301 cell
viability
In order to determine the effect of naringenin on
the proliferation of bladder cancer cells, an MTT assay was
conducted. As shown in Fig. 1,
exposure to 25, 50, 100, 200 and 300 μM naringenin for 24 h was
observed to reduce cell viability to 94.38±0.93, 90.59±2.29,
89.92±1.98, 85.34±6.63 and 74.09±8.27%, respectively, compared with
the non-treated groups. To further assess the effect of naringenin
on bladder cancer cell cycle distribution, flow cytometric analysis
was performed. Naringenin treatment was not found to affect cell
cycle distribution in TSGH-8301 cells (data not shown).
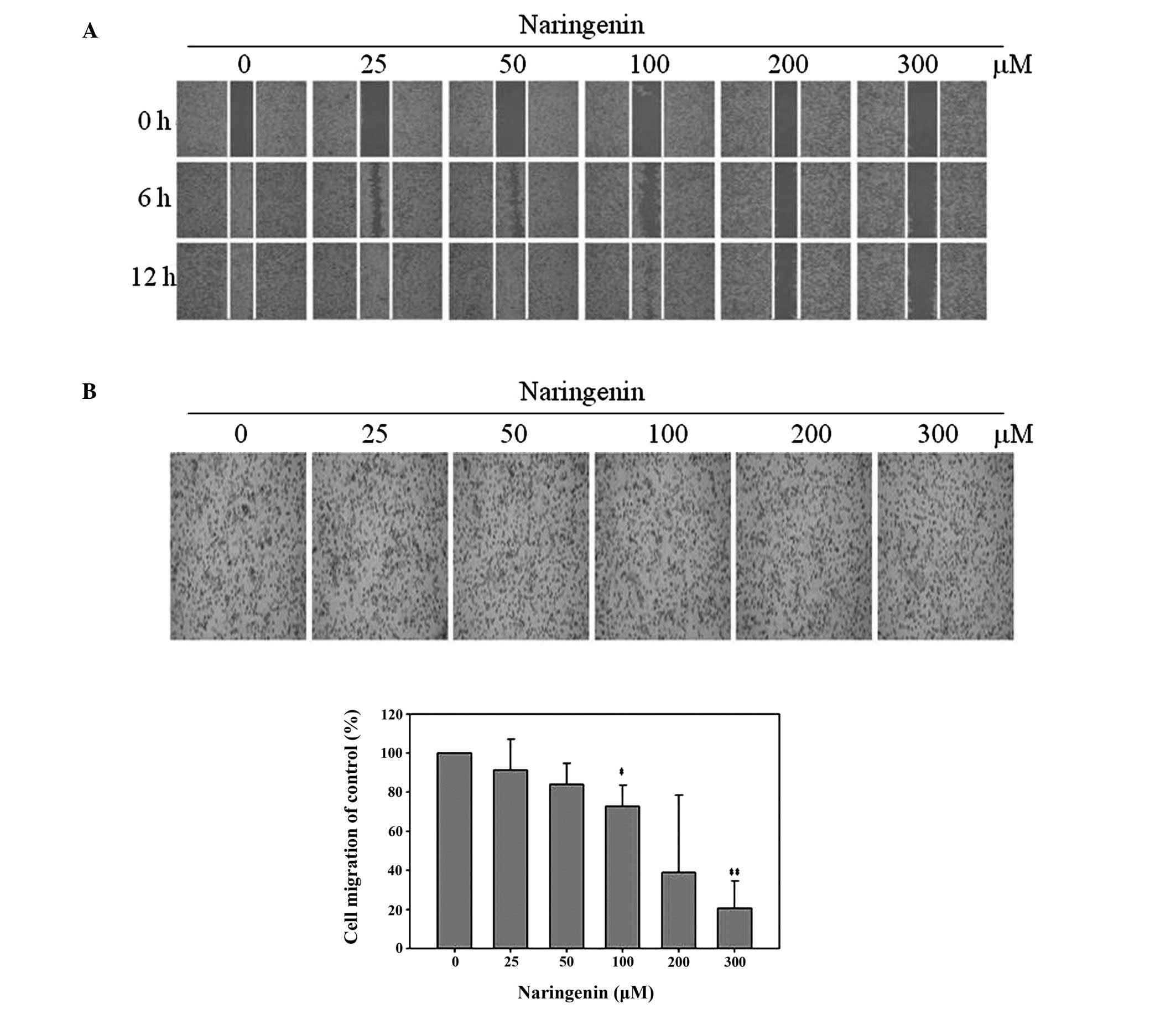
Naringenin reduces TSGH-8901 cell
migration
In order to investigate the effect of naringenin on
the migration of bladder cancer cells, wound healing and Boyden
chamber assays were performed. Exposure to 100, 200 and 300 μM
naringenin for 12 h was observed to significantly decrease the
motility of TSGH-8301 cells, consequently reducing the number of
cells in the wound zone, compared with vehicle-treated control
groups (Fig. 2A). Boyden chamber
analysis indicated that migration was decreased to 91.40±15.78,
84.10±10.75, 72.75±10.76, 39.00±39.44 and 20.74±13.93% in cells
treated with 25, 50, 100, 200 and 300 μM naringenin, respectively
(Fig. 2B).
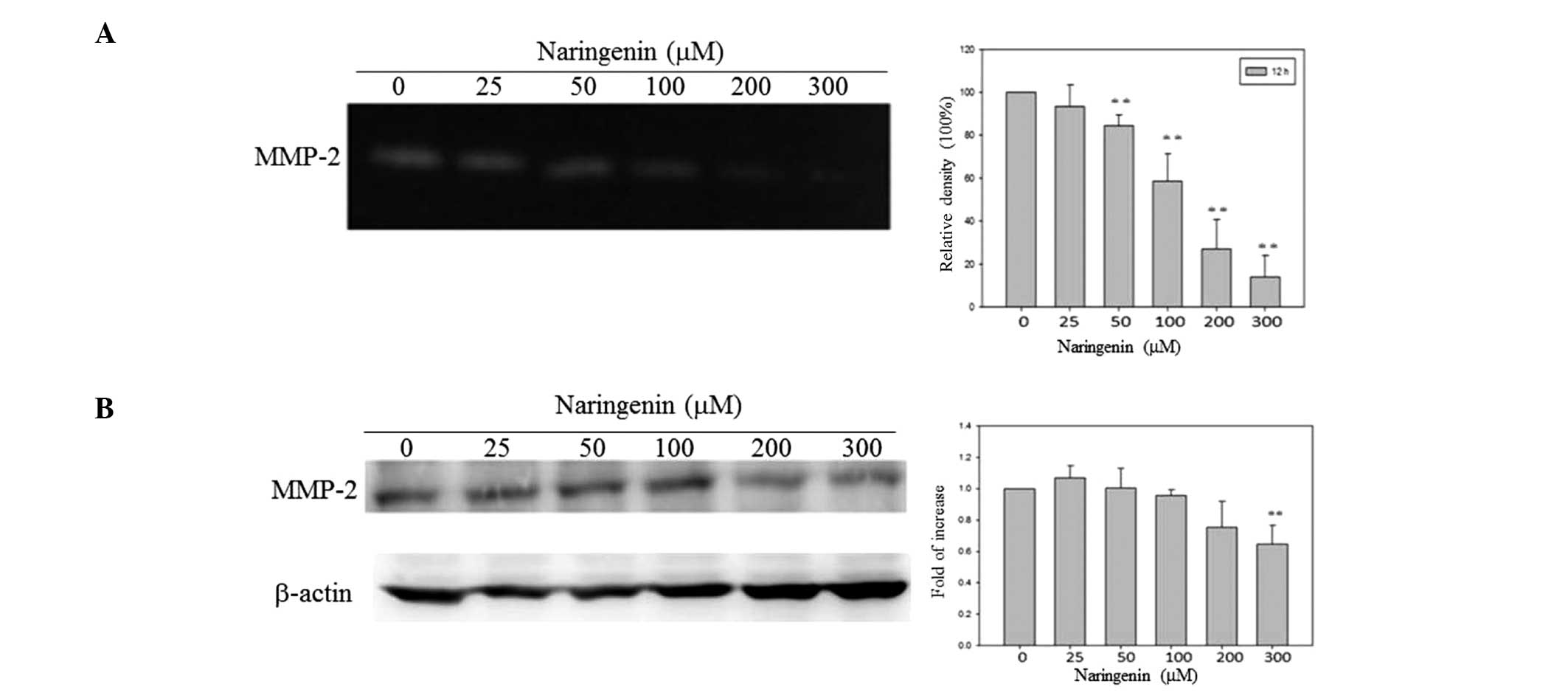
Naringenin inhibits the expression and
activity of migration-related proteins
It is well established that MMP-2 and urokinase-type
plasminogen activator play an important role in cancer migration.
To investigate whether naringenin modulates the expression and
activity of MMP-2, western blot analysis and zymography assays were
conducted. Naringenin was found to inhibit MMP-2 activity in a
dose-dependent manner, as revealed by zymography assay (Fig. 3A). However, a statistically
significant decrease in MMP-2 expression was only observed in the
TSGH-8301 cells treated with a high concentration of naringenin
(300 μM) (Fig. 3B).
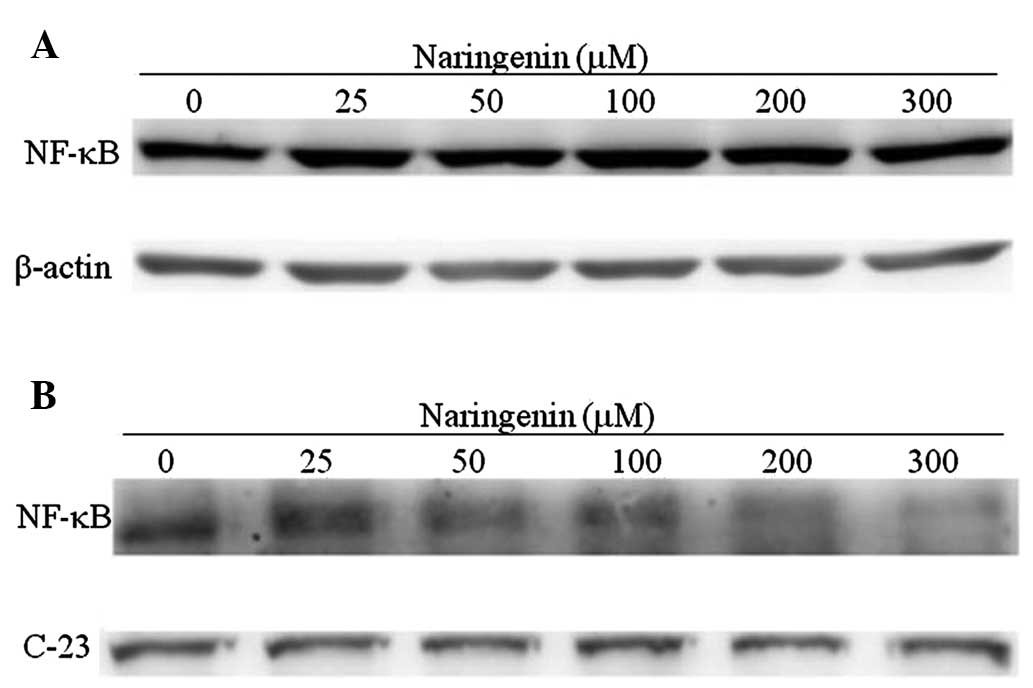
The NF-κB transcription factor regulates numerous
genes involved in proliferation, apoptosis and migration upon
translocation to the nucleus (12). In the present study, western blot
analysis was performed using whole cell or nuclear extracts to
assess the effect of naringenin on NF-κB expression and nuclear
translocation. As shown in Fig.
4A, naringenin treatment was not found to significantly alter
NF-κB expression. However, naringenin treatment was observed to
significantly decrease the nuclear translocation of NF-κB in a
dose-dependent manner (Fig.
4B).
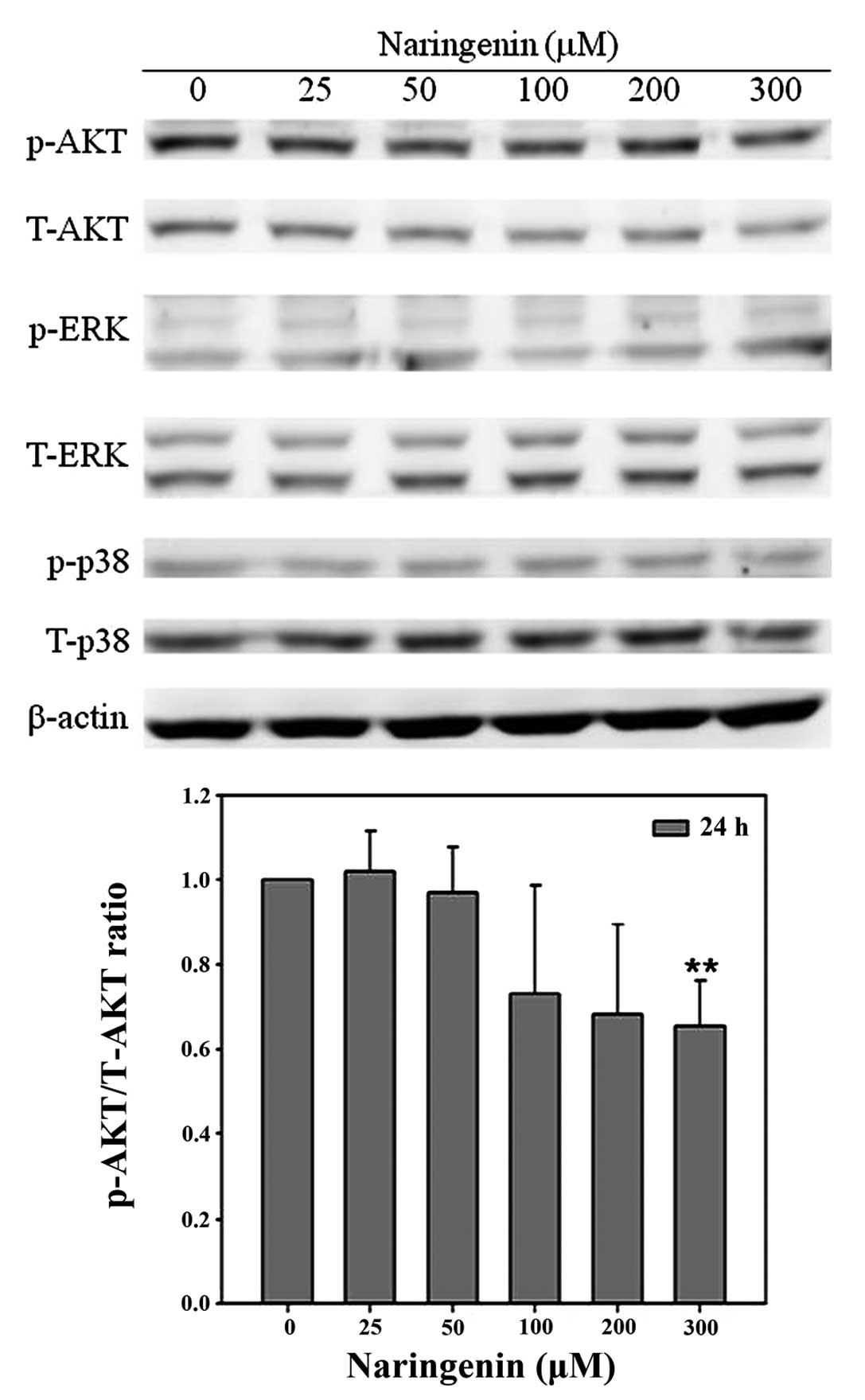
AKT and mitogen-activated protein kinase (MAPK)
signaling, occurring in response to extracellular stimuli, have an
important role in cell proliferation and migration. In order to
investigate the effect of naringenin on AKT and MAPK activity,
western blot analysis was performed. In the presence of 25, 50,
100, 200, 300 mM naringenin, the p-AKT/AKT ratio was 0.94, 0.90,
1.16, 0.69 and 0.66 compared with the vehicle group. Naringenin was
observed to significantly decrease AKT activity, as shown by the
decreased p-AKT/T-AKT ratio in Fig.
5. However, naringenin was not identified to significantly
alter the activity of p38 or ERK (Fig.
5).
Discussion
Bladder cancer is one of the primary urothelial
malignancies, spreading into proximal or distal tissues through
migration and invasion into the blood or lymphatic systems and
causing high mortality rates. The suppression of migration may
enhance the survival rate of patients with bladder cancer. In the
present study, naringenin treatment was found to significantly
repress the migration of bladder cancer cells through the
downregulation of MMP-2 and the inhibition of AKT and NF-κB
activities.
It is well established that degradation of the
extracellular matrix (ECM) causes metastasis by enabling the spread
of cancer cells through blood and lymphatic vessels (13). Levels of MMPs, a family of
zinc-dependent ECM endopeptidases, have been shown to be
significantly elevated in metastatic human cancers (14). Citrus flavonoids suppress cancer
cell migration through the downregulation of MMP expression.
Nobiletin, a major bioactive citrus flavonoid, is capable of
reducing the MMP-7 proenzyme (proMMP-7) expression by inhibiting
the binding of activator protein-1 to the proMMP-7 promoter in
colorectal cancer cells (15). In
addition, nobiletin exhibits anti-metastatic effects by modulating
chemokine receptor-4 and MMP-9 expression in breast cancer cells
(16). Consistent with these
observations, the present study revealed that naringenin reduced
MMP-2 expression and activity, which attenuated the migration of
bladder cancer cells. Furthermore, treatment with naringenin
significantly reduced the nuclear translocation of NF-κB. The NF-κB
transcription factor regulates numerous cellular functions,
including adhesion, inflammation and cancer metastasis, by
controlling the expression of several genes, such as
cyclooxygenase-2 (COX-2), MMP-2 and MMP-9 (17). In the normal state, NF-κB is bound
to the cytoplasmic region, but is translocated into the nucleus in
response to certain stimuli (17).
Inhibiting the function of natural bioactive compounds may be a
promising chemotherapeutic strategy (17). A recent study demonstrated that
naringenin administration downregulated NDEA-induced NF-κB, COX-2,
MMP-2 and MMP-9 expression and inhibited NDEA-induced
hepatocellular carcinomas in rats (8). In the present study, naringenin was
found to reduce NF-κB nuclear translocation and inhibit MMP-2
expression, subsequently decreasing bladder cancer cell
migration.
Phosphorylation-induced AKT activation leads to the
phosphorylation of downstream targets, including glycogen synthase
kinase-3β, forkhead transcription factors and NF-κB, which in turn
mediate the effects of AKT on cell proliferation, apoptosis and
migration (18). AKT
phosphorylation has been found in several human cancers and
correlates with poor prognosis (19). Nobiletin has been shown to
attenuate the migration and invasion of gastric adenocarcinomas by
downregulating AKT signals (20).
Similarly, fisetin exhibits antimetastatic effects by deactivating
AKT and reducing MMP-23 expression in PC-3 prostate cancer cells
(21). The present study revealed
that naringenin inhibited AKT phosphorylation, which suggests that
the targeting of AKT phosphorylation may have a role in the
anticancer effects of naringenin.
In conclusion, the present study has provided the
first evidence, to the best of our knowledge, that naringenin is a
novel MMP-2 inhibitor that inhibits bladder cancer cell migration
and, thus, may have the potential to suppress bladder cancer
metastasis.
Acknowledgements
This study was supported by grants from the National
Science Council of Taiwan (nos. NSC-101-2311-B-040-001 and
CSMU-CMMC-102-01).
References
|
1
|
Tian B, Wang Z, Zhao Y, et al: Effects of
curcumin on bladder cancer cells and development of urothelial
tumors in a rat bladder carcinogenesis model. Cancer Lett.
264:299–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun F, Zheng XY, Ye J, Wu TT, Wang JI and
Chen W: Potential anticancer activity of myricetin in human T24
bladder cancer cells both in vitro and in vivo. Nutr Cancer.
64:599–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Chen W and Li X: A novel
anticancer effect of butein: inhibition of invasion through the
ERK1/2 and NF-kappaB signaling pathways in bladder cancer cells.
FEBS Lett. 582:1821–1828. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen NG, Lu CC, Lin YH, et al: Proteomic
approaches to study epigallocatechin gallate-provoked apoptosis of
TSGH-8301 human urinary bladder carcinoma cells: roles of AKT and
heat shock protein 27-modulated intrinsic apoptotic pathways. Oncol
Rep. 26:939–947. 2011.
|
|
5
|
Qin J, Wang Y, Bai Y, et al:
Epigallocatechin-3-gallate inhibits bladder cancer cell invasion
via suppression of NF-κB mediated matrix metalloproteinase-9
expression. Mol Med Rep. 6:1040–1044. 2012.PubMed/NCBI
|
|
6
|
Frydoonfar HR, McGrath DR and Spigelman
AD: The variable effect on proliferation of a colon cancer cell
line by the citrus fruit flavonoid Naringenin. Colorectal Dis.
5:149–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lentini A, Forni C, Provenzano B and
Beninati S: Enhancement of transglutaminase activity and polyamine
depletion in B16-F10 melanoma cells by flavonoids naringenin and
hesperitin correlate to reduction of the in vivo metastatic
potential. Amino Acids. 32:95–100. 2007. View Article : Google Scholar
|
|
8
|
Subramanian P and Arul D: Attenuation of
NDEA-induced hepatocarcinogenesis by naringenin in rats. Cell
Biochem Funct. 31:511–517. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen S, Ding Y, Tao W, Zhang W, Liang T
and Liu C: Naringenin inhibits TNF-α induced VSMC proliferation and
migration via induction of HO-1. Food Chem Toxicol. 50:3025–3031.
2012.
|
|
10
|
Lou C, Zhang F, Yang M, et al: Naringenin
decreases invasiveness and metastasis by inhibiting TGF-β-induced
epithelial to mesenchymal transition in pancreatic cancer cells.
PLoS One. 7:e509562012.PubMed/NCBI
|
|
11
|
Kim JJ: Recent advances in treatment of
advanced urothelial carcinoma. Curr Urol Rep. 13:147–152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klein T and Bischoff R: Physiology and
pathophysiology of matrix metalloproteases. Amino Acids.
41:271–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawabata K, Murakami A and Ohigashi H:
Nobiletin, a citrus flavonoid, down-regulates matrix
metalloproteinase-7 (matrilysin) expression in HT-29 human
colorectal cancer cells. Biosci Biotechnol Biochem. 69:307–314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baek SH, Kim SM, Nam D, et al:
Antimetastatic effect of nobiletin through the down-regulation of
CXC chemokine receptor type 4 and matrix metallopeptidase-9. Pharm
Biol. 50:1210–1218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luqman S and Pezzuto JM: NFkappaB: a
promising target for natural products in cancer chemoprevention.
Phytother Res. 24:949–963. 2010.PubMed/NCBI
|
|
18
|
Sheng S, Qiao M and Pardee AB: Metastasis
and AKT activation. J Cell Physiol. 218:451–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cicenas J: The potential role of Akt
phosphorylation in human cancers. Int J Biol Markers. 23:1–9.
2008.PubMed/NCBI
|
|
20
|
Lee YC, Cheng TH, Lee JS, et al:
Nobiletin, a citrus flavonoid, suppresses invasion and migration
involving FAK/PI3K/Akt and small GTPase signals in human gastric
adenocarcinoma AGS cells. Mol Cell Biochem. 347:103–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chien CS, Shen KH, Huang JS, Ko SC and
Shih YW: Antimetastatic potential of fisetin involves inactivation
of the PI3K/Akt and JNK signaling pathways with downregulation of
MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell
Biochem. 333:169–180. 2010. View Article : Google Scholar : PubMed/NCBI
|