Introduction
The most frequent malignant bone tumor in children
and adolescents, osteosarcoma (OS) (1), is a devastating disease. OS typically
exhibits marked local aggressiveness and shows a tendency to
metastasize to distant bones and the lungs. The administration of
neoadjuvant chemotherapy and improvements in surgical technology
have increased the OS survival rate to 65–75%; however, pulmonary
metastases occur in 40–50% of patients with OS and remain a
predominant cause of fatalities (2–4).
Thus, novel drugs and treatment methods for OS are urgently
required.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a promising antitumor agent since it is capable
of killing tumor cells via receptor-mediated apoptosis (5,6).
Preclinical studies using recombinant TRAIL (rhTRAIL) have provided
evidence for exogenous TRAIL efficacy in suppressing tumor growth
in vitro and in vivo (7–9).
However, resistance towards TRAIL may result in failure at any step
in the death signaling cascade. For example, TRAIL resistance may
occur at the receptor level, due to deregulated expression, or at
the death-induced signaling complex (DISC) level, mediated by
proteins counteracting DISC formation (10–12).
In addition, an inability to activate mitochondria during
apoptosis, due to high expression levels of antiapoptotic proteins,
may result in TRAIL resistance (13,14).
Furthermore, antiapoptotic signaling pathways, including the
phosphoinositol-3-kinase/Akt signaling pathway, are aberrantly
activated in numerous types of tumor cell, therefore contributing
to TRAIL resistance (15,16). Thus, identifying cancer therapeutic
agents that overcome TRAIL resistance is required.
Celecoxib (CXB) is a cyclooxygenase 2-selective
nonsteroidal anti-inflammatory drug, which has been approved for
the treatment of adult arthritis. CXB has also been shown to
exhibit therapeutic benefits in various types of cancer (17). Currently, CXB is being widely
investigated in clinical trials for therapeutic activity against
various cancer types as a single agent and in combination with
other agents (18,19).
The aim of the present study was to evaluate the
potency of CXB in combination with TRAIL in inhibiting OS cancer
cell growth and proliferation, and to reveal the underlying
molecular mechanisms involved in TRAIL-induced apoptosis. Whether
CXB, as an adjuvant agent, allows the effective dosage of TRAIL
required to treat OS cancer cells to be reduced was also
determined.
Materials and methods
Cell lines
The MG-63 human OS cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured immediately following unfreezing at a concentration
of 5×106 cells/ml in Dulbecco’s modified Eagle’s medium
(DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Hyclone), 100 U/ml penicillin and 100 μ/ml streptomycin
(Gibco Life Technologies, Carlsbad, CA, USA). The cells were
incubated in a humidified atmosphere containing 5% carbon dioxide
at 37°C.
Reagents
CXB (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) to create a 1
mM stock solution, stored at −20°C and diluted in fresh medium
immediately prior to use. TRAIL (with Enhancer applied at a
concentration of 1 μg/ml) was purchased from Alexis Biochemicals
(San Diego, CA, USA). For western blot analysis, the following
antibodies were used: Mouse monoclonal anti-β-actin
(Sigma-Aldrich); mouse monoclonal anti-B-cell lymphoma 2 (Bcl-2)
and anti-survivin, and horseradish peroxidase-conjugated anti-mouse
IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Nonidet
P-40 lysis buffer and MTT were obtained from Sigma-Aldrich. MTT
solution was prepared by dissolving 1 mg of compound in 1 ml
phosphate-buffered saline (PBS). The solution was protected from
light, stored at 4°C and used within one month.
Cell viability assay
MG-63 cells grown in monolayers were harvested and
dispensed in 96-well culture plates in 100 μl DMEM at a
concentration of 5×103 cells per well. After 24 h,
differential drug concentrations of TRAIL (0–200 ng/ml), CXB (0–250
μM) or a combination (0–100 ng/ml TRAIL plus 30 μM CXB) were added
to the cells. Cell viability after 24, 48 and 72-h incubation was
measured using the MTT colorimetric assay with an ELISA microplate
reader (Thermo Labsystems, Helsinki, Finland) at 490 nm with minor
modifications according to methods described in a previous study
(20). The inhibition rate was
calculated according to the following formula: Inhibition rate (%)
= [1−(average absorbance of experimental group/average absorbance
of blank control group)] × 100.
Detection of apoptosis
MG-63 cells were cultured in six-well plates in DMEM
with 10% FBS medium and were treated with the respective half
maximal inhibitory concentrations (IC50) of TRAIL, CXB
or their combination for 48 h. The cover slips were washed three
times with PBS and single cell suspensions were fixed in 1% PBS.
The cells were stained with 100 μg/ml acridine orange
(Sigma-Aldrich) and 100 μg/ml ethidium bromide for 1 min.
Subsequently, the cells were observed under a fluorescent
microscope (IX73-U; Olympus, Tokyo, Japan). At least 200 cells were
counted and the percentage of apoptotic cells was determined. All
experiments were performed in triplicates conducted on five
occasions. Caspase activation, Bax-2 and Bcl-2 expression levels
were also detected by western blotting as an additional indicator
of apoptosis.
Western blot analysis
MG-63 cells (1×106 cells per 100 mm
plate) were treated with TRAIL, CXB or their combination at the
respective IC50 doses for 24 h. The cells in control
wells were treated with 0.1% DMSO for 1 h. All cells were activated
with recombinant human epidermal growth factor (25 ng/ml;
Sigma-Aldrich) for 30 min. The cells were then scraped and lysed in
Nonidet P-40 lysis buffer. Cell extracts (50 μg protein) were
separated by SDS-PAGE and transferred to nitrocellulose membranes,
which were blocked in 3% bovine serum albumin (BSA) for 2 h.
Subsequent to blocking, the membranes were incubated with primary
antibodies β-actin (1:5,000), Bcl-2 (1:1,000) and survivin
(1:3,000) overnight at 4°C and then with horseradish
peroxidase-conjugated anti-mouse IgG (1:10,000) for 2 h at room
temperature. The proteins were visualized by exposing the
chemiluminescence substrate (Sigma-Aldrich) to X-OMAT AR
autoradiography film (Eastman Kodak, Rochester, NY, USA).
Animal experiments
The tumor response to TRAIL and CXB treatment was
investigated using the MG-63 tumor-bearing female BALB mouse model
(obtained from the Experimental Animal Center of Jilin University,
Changchun, China). The mice were maintained in accordance with the
Institute of Animal Ethics Committee guidelines approved by the
University of Jilin Animal Care and Use Committee, Changchun,
China. The present study was approved by the ethics committee of
the First Hospital of Jilin University (Changchun, China) The mice
were housed and acclimated in a pathogen-free environment at the
animal facility of the First Hospital of Jilin University for one
week prior to injection with tumor cells.
Exponentially growing MG-63 cells were harvested and
a tumorigenic dose of 2.5×106 cells was injected
intraperitoneally into the 6 to 7 week-old female BALB mice. Tumors
were allowed to grow in the mice for seven days, then the animals
were randomly assigned to one of four treatment groups (10 mice per
group). The control group received 1% polysorbate resuspended in
deionized water. The other three groups were treated with CXB (3.7
mg/kg body weight), TRAIL (90 mg/kg body weight) or CXB plus TRAIL
(2 and 50 mg/kg body weight, respectively) intraperitoneally on
alternative days for two weeks. The doses were selected as
determined by previous experiments (21,22).
Tumor weight and tumor volume were measured prior to administration
of the treatment injections and on days 7 and 14 of treatment. On
day 15, the animals were sacrificed using chloroform and spleen
tissues were collected and cultured for a splenocyte surveillance
study.
Assay of splenocyte proliferation
Single-cell spleen suspensions were generated and
pooled in serum-free DMEM by filtering the suspension through a
sieve mesh with the aid of a glass homogenizer, which exerted
gentle pressure on the spleen fragments. The samples were washed
twice in PBS with 0.1% (w/v) BSA Following centrifugation at 200 xg
for 10 min, the cells were seeded at 3×103 cells per
well in 96-well flat-bottomed microplates in triplicate in DMEM
supplemented with 10% FBS. The cells were incubated in a total
volume of 100 μl per well. Serum-free DMEM served as a control.
After 24 h, cell proliferation was measured using an MTT assay.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5 software (La Jolla, CA, USA). Values are presented
as mean ± standard deviation. Statistical significance was
determined using one-way analysis of variance. P<0.001 and
P<0.05 were considered to indicate statistically significant
differences.
Results
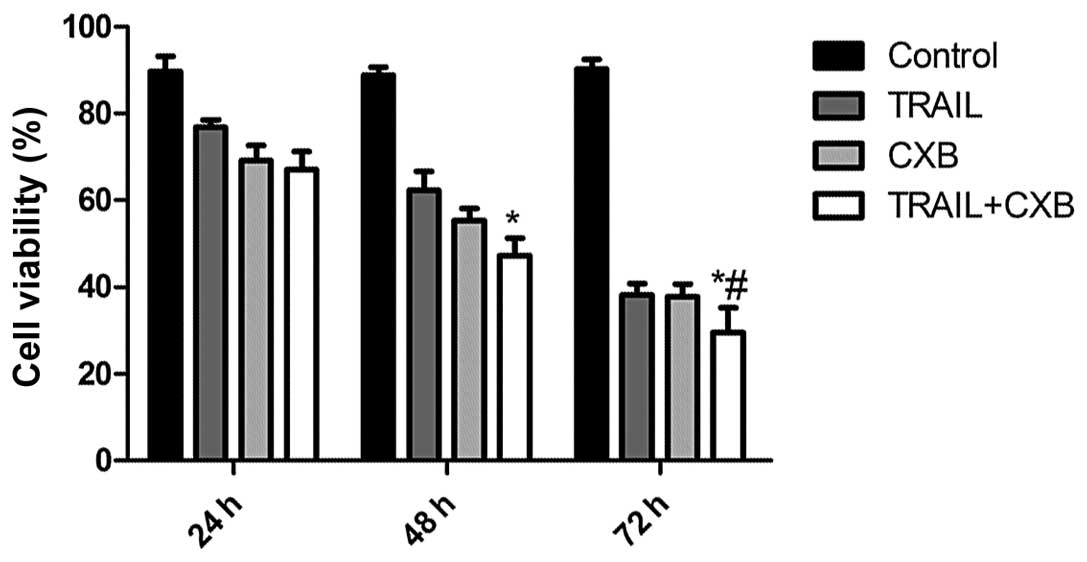
CXB enhances TRAIL-induced OS cancer cell
viability
To determine the effect of TRAIL, CXB and the
combination treatment on the viability of OS cancer cells in
vitro, MG-63 cells were treated with increasing concentrations
of CXB (0–200 μM) or TRAIL (0–200 ng/ml). The IC50 value
of TRAIL alone was determined to be 100.18±5.28 ng/ml, and the
IC50 value of CXB alone was 100.5±0.780 μM. Combination
treatment (0–100 ng/ml TRAIL in the presence of 30 μM CXB) resulted
in a shift of the cell viability curve towards lower drug
concentrations such that the IC50 value of TRAIL was
reduced to 50.76±5.14 ng/ml. These results indicated that treatment
with the two agents was more cytotoxic than either alone. According
to the results, respective drug IC50 values were
selected for further treatments throughout the study. In addition,
the inhibitory rates in the combination treatment group were found
to be higher than those of the single drug groups. No significant
difference was detected between the TRAIL and CXB single treatment
groups (Fig. 1).
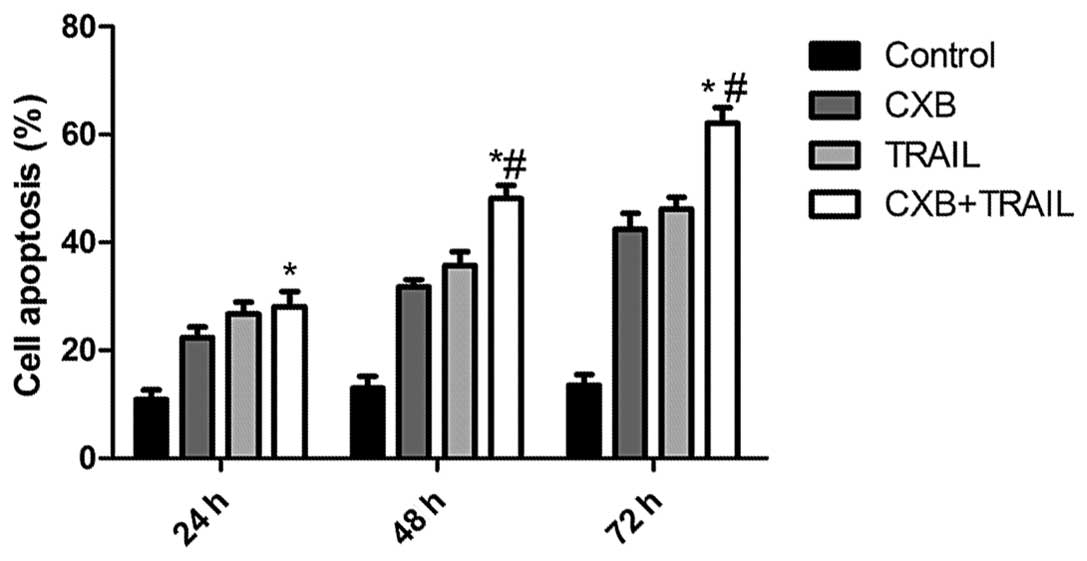
CXB enhances TRAIL-induced apoptosis
The effects of TRAIL and CXB on MG-63 cell apoptosis
were then analyzed. MG-63 cells (IC50 values: 100 μM for
CXB and 100 ng/ml for TRAIL) treated with either TRAIL or CXB
exhibited an increased percentage of apoptotic cells compared with
untreated cells (Fig. 2). However,
the low-dose combination (30 μM CXB plus 50 ng/ml TRAIL) resulted
in an even greater percentage of apoptotic cells than the higher
doses of either drug alone. These data are consistent with the
results from the MTT assay, which indicated an additive mechanism
of TRAIL and CXB in inducing cell death through apoptosis.
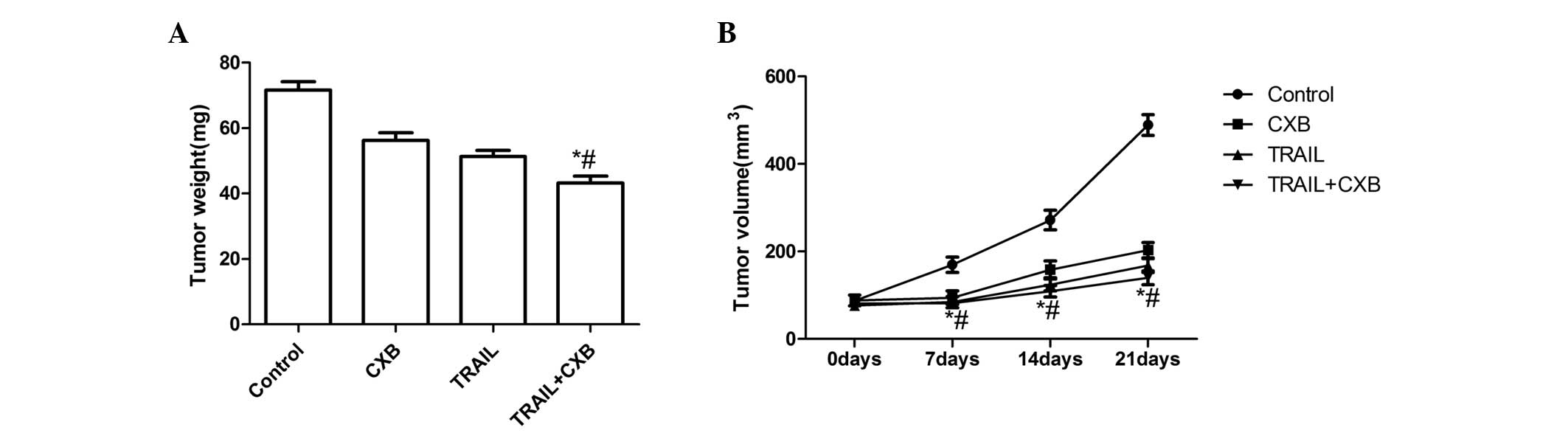
TRAIL and CXB treatment results in
significant inhibition of tumor growth
The in vivo therapeutic efficacy of TRAIL and
CXB was assessed in female BALB mice bearing MG-63 cell tumors.
TRAIL and CXB each induced tumor regression and inhibited tumor
growth in the respective treatment groups. The mice were
subsequently sacrificed on day 21 after treatment and tumor tissue
was excised. The tumor weight of the animals was subsequently
measured; the average tumor weight in the treatment groups was
lower than that in the untreated group. Furthermore, the average
tumor weight in the combination group was significantly lower than
that in the single-treatment CXB or TRAIL groups (P<0.01;
Fig. 3A). In addition, the tumor
volume was measured on days 7, 14 and 21 of treatment. The tumor
volume in the treatment groups increased at a slower rate than that
in the untreated group. The tumor volume in the combination
treatment group increased at a significantly slower rate than that
in either CXB or TRAIL single treatment groups (P<0.01; Fig. 3B). These result demonstrated that
TRAIL and CXB, and particularly their combination, induced tumor
regression and inhibited tumor growth.
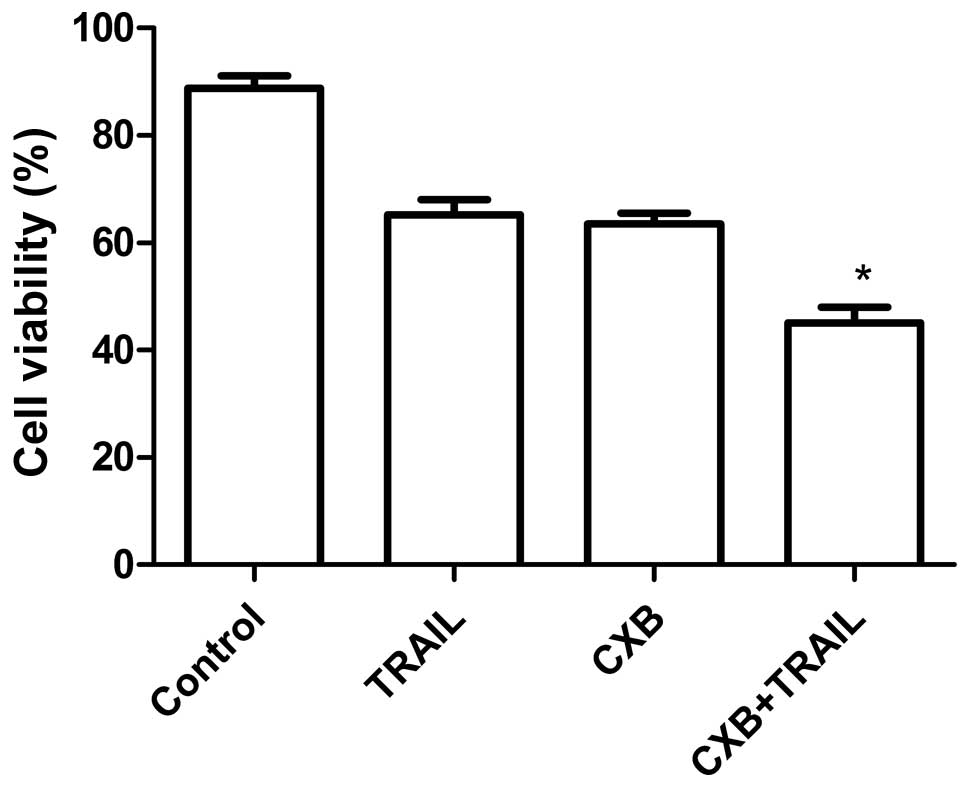
CXB increases TRAIL-induced splenocyte
proliferation inhibition
To assess the efficacy of TRAIL and CXB in
modulating splenocyte proliferation, spleen cells from treated mice
with MG-63 cell tumors were isolated and cultured in DMEM
supplemented with 10% FBS for 24 h and subjected to in vitro
proliferation assays using the MTT method. As shown in Fig. 4, the inhibitory rate in the
combination treatment group was higher than that in the single drug
treatment groups, which demonstrated that combination treatment
inhibited OS cell proliferation.
Preliminary clarification of the
mechanism of CXB- and TRAIL-induced induction of apoptosis
To clarify the molecular mechanisms of CXB-TRAIL
combination treatment on the induction of OS cell apoptosis, the
effects of CXB and TRAIL on the activation of proteins Bcl-2 and
survivin, which are involved in cell apoptosis, were analyzed. As
shown in Fig. 5, CXB administered
in combination with TRAIL significantly inhibited Bcl-2 and
survivin expression levels in OS tumor cells compared with the
single treatment and untreated groups. These results indicated that
CXB and TRAIL combination therapy inhibited apoptosis in OS tumor
cells.
Discussion
In the present study, combining TRAIL with CXB
significantly induced cell apoptosis and inhibited OS tumor growth,
whereas single treatment did not exert a significant effect.
Although the two compounds have each been extensively investigated,
to the best of our knowledge, this study is the first to reveal
that combining clinical doses of TRAIL with CXB reduces OS tumor
cell apoptosis and inhibits OS tumor growth in vivo.
Amongst the numerous methods to stimulate tumor cell
apoptosis, induction of the death receptor ligand TRAIL shows
particular promise. Previous preclinical studies have observed that
TRAIL induces tumor cell apoptosis in vivo without causing
lethal toxicity (23,24). One main issue in the clinical use
of TRAIL is its limited efficacy in monotherapeutic approaches in
different tumor entities. Thus, investigation into methods that
enhance the capacity of TRAIL to induce apoptosis is required.
Recent preclinical studies have observed that TRAIL
efficacy is increased through the use of combined chemotherapy
(25,26). Furthermore, a phase Ib trial was
conducted with rhTRAIL administered in combination with rituximab
in patients with low-grade non-Hodgkin lymphoma. Gaiser et
al (27) demonstrated that CXB
suppressed survivin levels by proteasomal degradation and thereby
induced apoptosis and enhanced TRAIL-mediated cytotoxicity in U87,
U251 and A172 glioma cells. In addition, Lu et al (28) observed that CXB and camptothecin
treatment sensitized TRAIL-resistant HepG2 and Hep3B hepatocellular
carcinoma (HCC) cell lines to TRAIL-induced apoptosis through
downregulation of cellular Fas-associated death domain-like
interleukin-1β-converting enzyme-inhibitory protein and cleavage of
caspase-8 and caspase-3. These studies demonstrated that
combination therapy with TRAIL and other anticancer drugs
significantly inhibited cell viability compared with single drug
treatments. The present study revealed that combining TRAIL with
CXB significantly induced cell apoptosis and inhibited OS tumor
growth, whereas single drug treatment did not exert statistically
significant effects. These results further demonstrated that
combination treatments in cancer therapy may be more effective than
single drug treatments.
In combined chemotherapy, drugs with different
mechanisms of action are employed simultaneously, reducing the
possibility that resistant cancer cells survive and proliferate.
When drugs exerting different effects are combined, each drug may
be used at the optimal dose, without intolerable side effects
(29). Numerous studies have
demonstrated that the anticancer activity of standard
chemotherapeutic agents is enhanced by the addition of CXB
(30). The data from the present
study revealed that CXB sensitized TRAIL-resistant MG-63 OS cell
lines to TRAIL-induced apoptosis through downregulation of cellular
Bcl-2 and survivin expression. These results further confirmed that
the administration of CXB in combination with other anticancer
drugs improves the antitumor effect.
In conclusion, the present study demonstrated that
combining TRAIL with CXB significantly induced cell apoptosis and
inhibited OS tumor growth in a nude rat model. This combination
regimen requires further evaluation in clinical trials, following
further preclinical studies.
Acknowledgements
This study was supported by the Science and
Technology Research and Innovation Team funded by Jilin Province
(grant no. JL2011088).
References
|
1
|
Caronia D, Patiño-Garcia A, Peréz-Martínez
A, et al: Effect of ABCB1 and ABCC3 polymorphisms on osteosarcoma
survival after chemotherapy: a pharmacogenetic study. PLoS One.
6:e260912011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacci G, Balladelli A, Palmerini E, et al:
Neoadjuvant chemotherapy for osteosarcoma of the extremities in
preadolescent patients: the Rizzoli Institute experience. J Pediatr
Hematol Oncol. 30:908–912. 2008. View Article : Google Scholar
|
|
3
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: state of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gorlick R, Anderson P, Andrulis I, et al:
Biology of childhood osteogenic sarcoma and potential targets for
therapeutic development: meeting summary. Clin Cancer Res.
9:5442–5453. 2003.PubMed/NCBI
|
|
5
|
Mérino D, Lalaoui N, Morizot A, Solary E
and Micheau O: TRAIL in cancer therapy: present and future
challenges. Expert Opin Ther Targets. 11:1299–1314. 2007.
|
|
6
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Almasan A and Ashkenazi A: Apo2L/TRAIL:
apoptosis signaling, biology, and potential for cancer therapy.
Cytokine Growth Factor Rev. 14:337–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashkenazi A, Holland P and Eckhardt SG:
Ligand-based targeting of apoptosis in cancer: the potential of
recombinant human apoptosis ligand 2/Tumor necrosis factor-related
apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol.
26:3621–3630. 2008. View Article : Google Scholar
|
|
9
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganten TM, Haas TL, Sykora J, et al:
Enhanced caspase-8 recruitment to and activation at the DISC is
critical for sensitisation of human hepatocellular carcinoma cells
to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death
Differ. 11(Suppl 1): S86–S96. 2004. View Article : Google Scholar
|
|
11
|
Gómez-Benito M, Martinez-Lorenzo MJ, Anel
A, Marzo I and Naval J: Membrane expression of DR4, DR5 and
caspase-8 levels, but not Mcl-1, determine sensitivity of human
myeloma cells to Apo2L/TRAIL. Exp Cell Res. 313:2378–2388.
2007.PubMed/NCBI
|
|
12
|
Schuchmann M, Schulze-Bergkamen H,
Fleischer B, et al: Histone deacetylase inhibition by valproic acid
down-regulates c-FLIP/CASH and sensitizes hepatoma cells towards
CD95-and TRAIL receptor-mediated apoptosis and chemotherapy. Oncol
Rep. 15:227–230. 2006.
|
|
13
|
He SQ, Rehman H, Gong MG, et al:
Inhibiting survivin expression enhances TRAIL-induced tumoricidal
activity in human hepatocellular carcinoma via cell cycle arrest.
Cancer Biol Ther. 6:1247–1257. 2007.
|
|
14
|
Han J, Goldstein LA, Gastman BR and
Rabinowich H: Interrelated roles for Mcl-1 and BIM in regulation of
TRAIL-mediated mitochondrial apoptosis. J Biol Chem.
281:10153–10163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bortul R, Tazzari PL, Cappellini A, et al:
Constitutively active Akt1 protects HL60 leukemia cells from
TRAIL-induced apoptosis through a mechanism involving NF-kappaB
activation and cFLIP(L) up-regulation. Leukemia. 17:379–389. 2003.
View Article : Google Scholar
|
|
16
|
Bosch FX, Ribes J and Borras J:
Epidemiology of primary liver cancer. Semin Liver Dis. 19:271–285.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winfield LL and Payton-Stewart F:
Celecoxib and Bcl-2: emerging possibilities for anticancer drug
design. Future Med Chem. 4:361–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei D, Wang L, He Y, et al: Celecoxib
inhibits vascular endothelial growth factor expression in and
reduces angiogenesis and metastasis of human pancreatic cancer via
suppression of Sp1 transcription factor activity. Cancer Res.
64:2030–2038. 2004. View Article : Google Scholar
|
|
19
|
Jeon YW and Suh YJ: Synergistic apoptotic
effect of celecoxib and luteolin on breast cancer cells. Oncol Rep.
29:819–825. 2013.PubMed/NCBI
|
|
20
|
Mikuła-Pietrasik J, Kuczmarska A, Kucińska
M, et al: Resveratrol and its synthetic derivatives exert opposite
effects on mesothelial cell-dependent angiogenesis via modulating
secretion of VEGF and IL-8/CXCL8. Angiogenesis. 15:361–376.
2012.PubMed/NCBI
|
|
21
|
Yoshida S, Amano H, Hayashi I, et al:
COX-2/VEGF-dependent facilitation of tumor-associated angiogenesis
and tumor growth in vivo. Lab Invest. 83:1385–1394. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Zou Y, Mao Y, et al: Preclinical
toxicity of DATR, a recombinant soluble human TRAIL mutant, in rats
and cynomolgus monkeys. Regul Toxicol Pharmacol. 61:230–235. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ashkenazi A: Targeting death and decoy
receptors of the tumour-necrosis factor superfamily. Nat Rev
Cancer. 2:420–430. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koschny R, Walczak H and Ganten TM: The
promise of TRAIL - potential and risks of a novel anticancer
therapy. J Mol Med (Berl). 85:923–935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cuello M, Ettenberg SA, Nau MM and
Lipkowitz S: Synergistic induction of apoptosis by the combination
of trail and chemotherapy in chemoresistant ovarian cancer cells.
Gynecol Oncol. 81:380–390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagane M, Pan G, Weddle JJ, et al:
Increased death receptor 5 expression by chemotherapeutic agents in
human gliomas causes synergistic cytotoxicity with tumor necrosis
factor-related apoptosis-inducing ligand in vitro and in vivo.
Cancer Res. 60:847–853. 2000.
|
|
27
|
Gaiser T, Becker MR, Habel A, et al:
TRAIL-mediated apoptosis in malignant glioma cells is augmented by
celecoxib through proteasomal degradation of survivin. Neurosci
Lett. 442:109–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu G, Liu Y, Ji B, et al: Synergistic
effect of celecoxib on TRAIL-induced apoptosis in hepatocellular
carcinoma cells. Cancer Invest. 28:629–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ullah MF: Cancer multidrug resistance
(MDR): a major impediment to effective chemotherapy. Asian Pac J
Cancer Prev. 9:1–6. 2008.PubMed/NCBI
|
|
30
|
Irie T, Tsujii M, Tsuji S, et al:
Synergistic antitumor effects of celecoxib with 5-fluorouracil
depend on IFN-gamma. Int J Cancer. 121:878–883. 2007. View Article : Google Scholar : PubMed/NCBI
|