Introduction
Thyroid carcinoma accounts for ~1% of all new
malignant diseases, ~0.5% of cancers in males and 1.5% in females
(1,2). Papillary thyroid carcinoma (PTC) is
the most common endocrine malignancy in thyroid carcinoma (3). Although there are numerous pathways
associated with this type of tumor, the extracellular
signal-regulated kinase/mitogen-activated protein kinase pathway
(4) and signal transducer and
activator of transcription 3 pathway (5) have been extensively studied. However,
at present, more factors need to be identified in order to predict
the genesis and development of PTC more precisely. In particular,
certain upstream regulators require further examination.
Kruppel-like factors (KLFs) consist of subfamilies
belonging to the mammalian specificity protein/KLF zinc-finger
protein family (6). Due to their
DNA-binding domains, they are important in cancer, inflammation and
heart disease (7) through their
combination with promoters of target genes in transcription
(8).
KLF17 was found to have transactivation activity in
embryonic chickens (9) and humans
(8). The decrease in the
expression of KLF17 has been associated with a poor prognosis and
pathological parameters in liver (10) and breast cancer (11). The mechanisms underlying its
function include regulation of proliferation and
epithelial-mesenchymal transition (EMT) by directly binding to the
promoters of inhibitor of DNA binding-1 (Id-1), ZO-1, E-cadherin
and Snai1 (11,12). However, the effects of KLF17 on PTC
have not been fully elucidated.
The present study explored the correlation between
KLF17 expression and malignant clinical-pathological parameters and
a poor prognosis in patients with PTC. Cells were transfected with
small interfering (si)RNA against KLF17 and their mobility,
proliferation ability and expression of Id-1, ZO-1 and Snai1 were
studied. The results indicated that KLF17 may be important in the
therapy and prognosis of PTC and thus, further investigation is
required.
Materials and methods
Patient information and samples
In total, 50 pairs of PTC samples and adjacent
normal tissues (located 1.5 cm away from the cancer margin) were
collected from patients from The General Surgery Department,
Mianyang Central Hospital, (Mianyang, Sichuan, China) between March
and October 2007. The medical records were obtained at the same
time. All samples were cut into 0.5-cm sections and stored at
−80°C. The clinical parameters, particularly the five-year survival
time and outcomes, were gathered through the present study and the
follow-up. The KLF17 expression levels between paired tumor and
healthy adjacent tissues were examined in all the samples by
western blot analysis. The samples whose KLF17 expression in
carcinoma tissue was lower than that in adjacent normal tissues
were selected as the low KLF17 expression group and vice versa. All
the patients underwent surgery to resect the tumor successfully.
Pathological data of patients were summarized according to the
Union for International Cancer Control (UICC) diagnostic criteria
(13). The maximum tumor
dimensions were calculated following surgery. Following surgery,
the patients were treated with systemic curative radioactive iodine
131 + thyroid hormone therapy treatment. During the follow-up,
serum examination and ultrasound were used to monitor recurrence
and metastases. Written informed consent from patients and ethical
approval from the Institutional Research Ethics Committee of The
Mianyang Central Hospital (Mianyang, China) was obtained prior to
initiation of the present study. All clinical information on all
patients is summarized in Table
I.
 | Table IKLF17 expression in papillary thyroid
carcinoma and the clinical-pathological parameters of 50
patients. |
Table I
KLF17 expression in papillary thyroid
carcinoma and the clinical-pathological parameters of 50
patients.
| Variable | Low KLF17 expression
(n=26) | High KLF17 expression
(n=24) |
χ2-test |
|---|
| Gender | Male (7) 26.9% | Male (6) 25% | P=0.877 |
| Female (19)
73.1% | Female (18) 75% | |
| Age, years (n) % | ≥45 (9) 34.6% | ≥45 (8) 47.1% | P=0.619 |
| <45 (17)
65.4% | <45 (16)
52.9% | |
| Volume of tumor | ≥2 cm (17) 65.4% | ≥2 cm (8) 33.3% | P=0.048* |
| <2 cm (9)
34.6% | <2 cm (16)
66.7% | |
| Differentiation | High/moderate (11)
42.3% | High/moderate (19)
79.2% | P=0.018* |
| Low (15) 57.7% | Low (5) 20.8% | |
| T-stage |
T0–T1 (11)
42.3% |
T0–T1 (16)
76.2% | P=0.041* |
|
T2–T4 (15)
57.7% |
T2–T4 (8) 23.8% | |
| N-stage | N0 (13)
50% | N0 (20)
83.3% | P=0.029* |
| N1 (13)
50% | N1 (4)
16.7% | |
| M-stage | M0 (16)
61.5% | M0 (21)
87.5% | P=0.037* |
| M1 (10)
38.5% | M1 (3)
12.5% | |
Cell lines, cell culture and transient
transfection
The 8505C and human PTC (TPC-1) cell lines were
purchased from The Cell Bank of Type Culture Collection, Chinese
Academy of Sciences (Shanghai, China). The cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum and
penicillin/streptomycin (all from HyClone, Logan, UT, USA) at 37°C
in a humidified atmosphere with 5% CO2. To inhibit the
expression of KLF17 (Abcam, Cambridge, UK), siRNA (Guangzhou
RiboBio Co., Ltd., Guangzhou, China) was used to transiently
transfect the TPC-1 cell line. The transfection was performed using
Lipofectamine™ 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions. TPC-1 cells
were seeded at a density of 5×104 per well in a six-well
plate. The mRNA expression of KLF17 was measured 48 h after
reaching confluence and, following 72 h, the protein expression was
also assessed.
Quantitative polymerase chain reaction
(qPCR)
The total RNA from paired PTC tumor samples and
adjacent normal tissues was extracted. Following application of
TRIzol reagent (Invitrogen Life Technologies), the concentration
was measured using a NanoDrop 2000 spectrophotometer (NanoDrop
Products, Wilmington, DE, USA). Total RNA (1 μg) was used in the
reverse transcription reaction. The SYBR qPCR mix TM kit (Toyobo,
Osaka, Japan) was used for qPCR. Finally, mRNA expression was
detected using an ABI Step one real-time PCR system (Applied
Biosystems, Sunnyvale, CA, USA). The process was repeated in the
TPC-1 cell line to measure the transfection efficiency of KLF17
siRNA. The following specific primers were used: KLF17, forward
5′-GCTCTGGAGTGCACACCTCTT-3′ and reverse 5′-CAGCATCTCTGCGCTGTGA-3′;
β-actin (used as control for amplification), forward
5′-TCGTCCACCGCAAATGCTTCTAG-3′ and reverse
5′-ACTGCTGTCACCTTCACCGTTCC-3′.
Western blot analysis
The proteins were extracted from tissues and cells
and their concentration was measured. Western blotting was
performed with specific primary antibodies against KLF17, Id-1,
ZO-1, Snai1, AKT, phosphorylated (p)-AKT (rabbit-anti-human
antibody; 1:1,000; Abcam, Cambridge, MA, USA) and GADPH (1:1,000;
Abcam), followed by appropriate secondary horseradish
peroxidase-conjugated antibodies (1:5,000; Pierce Biotechnology,
Inc., Rockford, IL, USA). The protein bands were visualized using
an enhanced chemiluminescence detection system (Pierce
Biotechnology, Inc.).
Cell proliferation assay
To establish cell growth curves, 5×104
cells were seeded in 6-well plates. The plates were divided into
two groups. One group was cultured with DMEM only, while the other
group was cultured with DMEM following transfection with siRNA
against KLF17. The medium was replaced every 2–3 days. The cell
numbers were counted on days three, six and nine following seeding.
The assay was repeated three times.
Transwell assay
Transfected TPC-1 cells and control cells were
seeded at a density of 5×104 on the upper chamber of a
Transwell plate (24-well insert, 8 μm pore size; Corning Costar,
Lowell, MA, USA), coated with Matrigel®. The lower
chamber was filled with 10% serum serving as a chemoattractant in
DMEM. Following 24 h, the cells that had invaded into the lower
chamber were fixed in methanol, stained with crystal violet and
counted. The area between scratched cells and the number of cells
in the transwell assay were measured by Image Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The comparison of survival times between the high
KLF17 and low KLF17 expression groups was performed by Kaplan-Meier
analysis and the log-rank test. The correlation between all sorts
of clinical-pathological parameters and the difference in
expression of KLF17 was analyzed using a χ2 test. The
mean diameter of tumors between two groups was compared by the
Wilcoxon rank sum test. In addition, for analyzing the results of
qPCR and western blot analysis as well as the Transwell assay, the
Student’s t-test was adopted. All the data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference. All experimental data were
analyzed using the SPSS statistical software (version 16.0; SPSS,
Inc., Chicago, IL, USA).
Results
Expression of KLF-17 is decreased in
tumor tissues
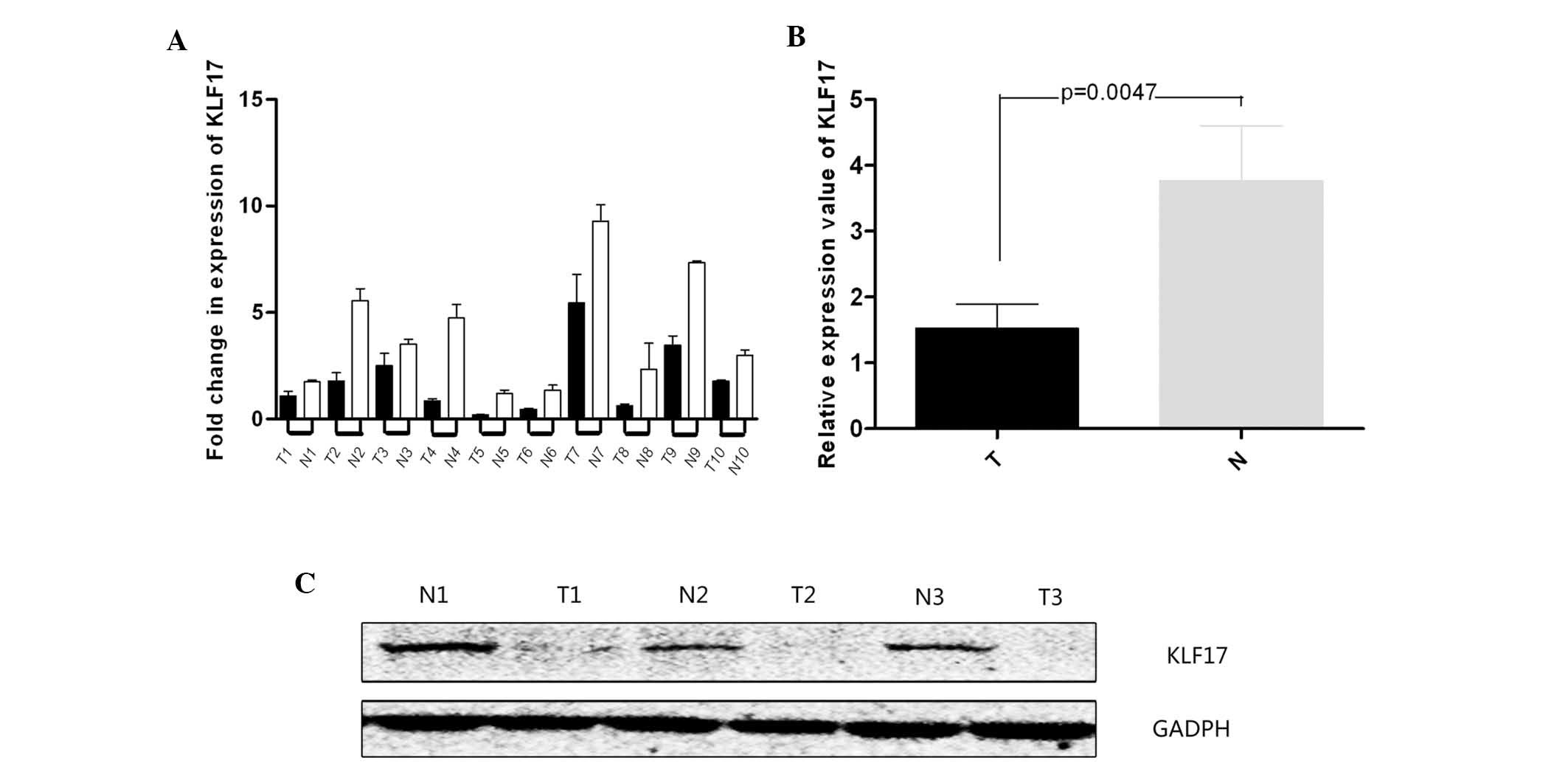
Based on qPCR results, the expression of KLF17
significantly decreased in tumor tissues compared with that in
adjacent normal tissues. The expression of KLF17 in one of the
adjacent normal tissue samples was set as the reference and the
value was 1.641±0.341 in tumor tissues. The value was 3.81±0.148 in
adjacent normal tissues (Fig. 1A and
B). The difference was statistically significant (P<0.05)
and the protein expression demonstrated a similar result (Fig. 1C). The majority of the adjacent
normal tissues exhibited a higher expression of KLF17.
KLF17 expression correlates with
clinical-pathological parameters and the apparent effects on the
prognosis of PTC patients
Next, the χ2 test was adopted to
investigate the correlation between KLF17 expression and
clinical-pathological data of all patients. The majority of the
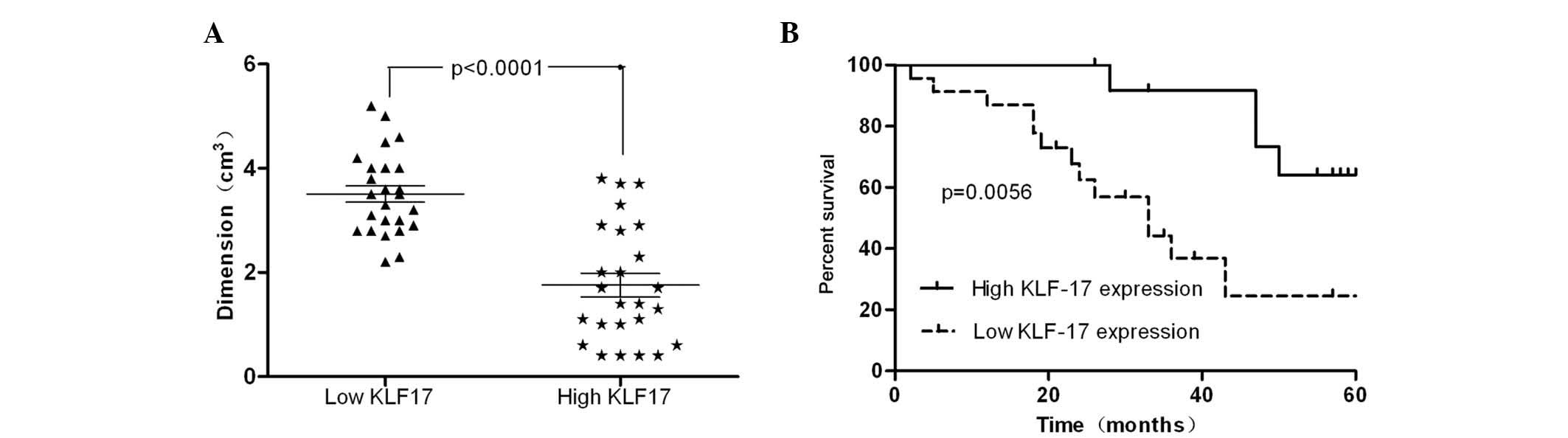
parameters had no clear association with KLF17 expression (Table I). However, tumor size, T stage, N
stage and M stage demonstrated a tight correlation with KLF17
expression. The tumors with a dimension >2 cm had lower
expression levels of KLF17 compared with the group with a tumor
dimension <2 cm (P<0.05; Fig.
2A). In addition, the development of differentiation (P=0.018),
T stage (P=0.041), N stage (P=0.029) and M stage (P=0.037) were
negatively correlated with the expression of KLF17.
Patients were then divided into two groups according
to their KLF17 expression at the protein level. The survival time
of the two groups was compared by Kaplan-Meier analysis. The
cumulative five-year survival rate was 64% in the high KLF17
expression group; however, it was 21% in the low expression group
(Fig. 2B; P=0.0073). This finding
indicated that downregulated KLF17 expression was associated with a
poor prognosis in PTC
Expression of KLF17 affects the
proliferation and motility of the PTC cell line
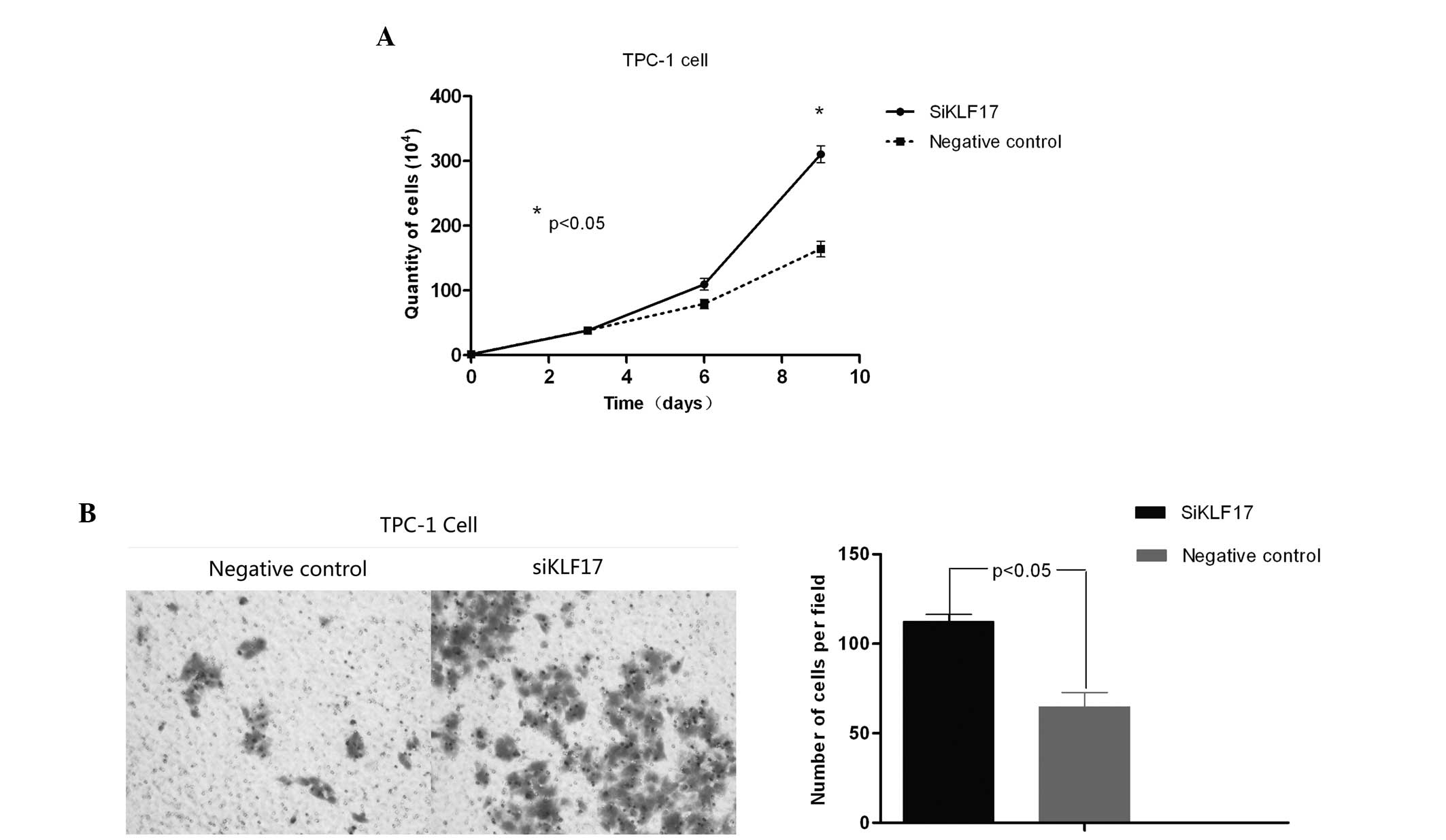
TPC-1 cells were employed as the target of RNA
interference (RNAi). siRNA was applied to transfect this cell line
to inhibit the expression of KLF17 and the KLF17 mRNA expression
levels in these cells were assessed. Following 24 h transfection,
transfected cells and control cells were seeded on 6-well plates.
The cell number was determined following three, six and nine days,
showing that the proliferation of the transfected group was
enhanced, particularly following six days (Fig. 3A; P<0.05). Future studies may
aim to identify the correlation between N and M stage and KLF17
expression.
The invasion ability of transfected TPC-1 cells was
also examined. In the Transwell assay, following 24 h, a larger
number of cells transfected with siKLF17 penetrated into the lower
chamber (Fig. 3B) as compared with
the control group. This was statistically significant
(P<0.05).
Inhibition of KLF17 promotes the
expression of Id-1, thus activating the AKT pathway and affecting
the proliferation of PTC cells
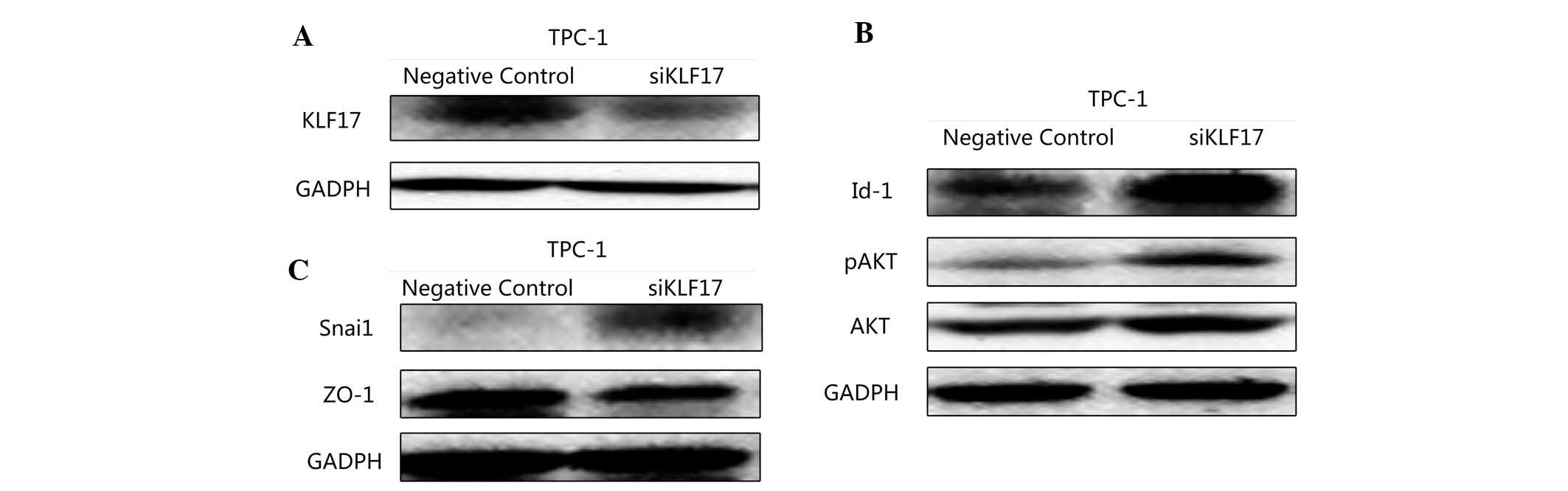
In order to further elucidate the underlying
mechanisms of the role of KLF17 in PTC its effect on proliferation,
the expression of Id-1 was detected by western blot analysis. With
the downregulation of KLF17, Id-1 expression was upregulated
(Fig. 4A). AKT and p-AKT were also
examined. Consistent with the hypothesis, with the inhibition of
KLF17, the AKT pathway was activated (Fig. 4B). This may explain the
proliferation changes following transfection.
Inhibition of KLF17 promotes the
expression of Snai1 and suppresses ZO-1, inducing EMT
Next, ZO-1 and Snai1, which have a tight association
with EMT, were assessed in transfected TPC-1 cells and control
cells by western blot analysis. ZO-1 belongs to markers of
epithelial tissues. Snai1 is regarded as a marker of mesenchymal
tissues (14). The expression of
the two proteins was markedly altered. The expression of ZO-1 was
reduced accompanied with an upregulation of Snail in transfected
TPC-1 cells (Fig. 4C).
The above findings demonstrated that the
downregulation of KLF17 was associated with increased cell motility
in TPC-1 cells. It also indicated that the reduction in KLF17
expression was able to induce the occurrence of EMT in PTC, thus,
promoting metastasis.
Discussion
The KLF family consists of several members which
each have a particular function in different types of cancer,
ranging from oncogenes to tumor suppressors (15,16).
KLF17 has been investigated in several types of cancer, including
breast cancer, liver cancer and lung adenocarcinoma (17). However, the specific functions of
KLF17 remain to be explored in depth and its role in PTC remains to
be elucidated.
The present study detected a significantly different
expression pattern of KLF17 between tumor tissues and adjacent
normal tissues in PTC patients. Furthermore, it was found that
tumor dimension and T staging were negatively correlated with the
expression of KLF17. Based on the UICC TNM Classification System
for papillary thyroid carcinoma, the majority of patients with
lower KLF17 expression were identified at stages 3–4 (P<0.05).
That is possible for the effect of KLF17 on the growth and motility
of PTC cells through its effect on Id-1 and EMT-associated factors
(10,11). In the low KLF17 expression group,
the tumor acquired a stronger metastasis tendency with the invasion
of lymph nodes and distant tissues. In addition, prognosis studies
have demonstrated that the five-year total survival rate of the
patients with low KLF17 expression (40.1%) was significantly
shorter than that of high KLF17 expression patients (60.2%). In
addition, in the high KLF17 expression group, survivors had an
improved liver function as compared with the low expression group.
These results supported the theory that the KLF17 expression levels
are a key prognostic indicator for patients with PTC. KLF17 may not
only be an indicator of survival time, but also of quality of life.
The present study was, to the best of our knowledge, the first
clinical study on PTC demonstrating an apparent clinical
correlation between KLF17 expression and poor prognosis.
To verify the results, PTC cell lines were used to
compare their KLF17 expression. Through comparison, the 8505C cell
line was found to exhibit a higher KLF17 expression, while the
TPC-1 cell line had a lower KLF17 expression. Therefore, TPC-1
cells were selected to inhibit KLF17 expression by RNAi. Following
transfection, the expression of KLF17 was verified by western blot
analysis. In addition, a cell proliferation assay was applied
between control cells and transfected cells. Following 144 h, the
proliferation of transfected cells was significantly suppressed
compared with that of the control cells (P<0.05). Based on the
Transwell assay, the enhanced potency of invasion and motility in
transfected TPC-1 cells verified the function of KLF17 in this
area. Next, western blot analysis was employed, showing that with
the decrease in KLF17 expression, the expression of Id-1, ZO-1 and
Snai1 was altered. Id-1 is a key regulator of tissue-specific gene
expression in a number of mammalian and non-mammalian organisms,
and the constitutive expression of Id proteins has been
demonstrated to inhibit the differentiation of various tissues
(18). Furthermore, Id-1 exerts
its function by promoting the AKT pathway (19). The downregulation of AKT and p-AKT
verified our hypothesis. Therefore, it was considered that KLF17
was able to suppress Id-1 expression and thus inhibit the AKT
pathway in PTC. Although this result provided a partial explanation
for the change in cell proliferation ability, the motility of cells
following transfection requires further investigation. The
expression of ZO-1 (20) and Snai1
(21), two typical markers of EMT,
were compared between two cell groups. The EMT process (22) affects cell-intrinsic and
cell-extrinsic properties of tumor cells by breaking the stability
of the cytoskeleton, loosening intercellular junctions and
promoting cancer cells to change their form and motility (23). In transfected TPC-1 cells, the
expression of ZO-1, a marker of epithelial tissues, was
downregulated significantly. In addition, Snai1, a marker for
mesenchymal tissue, was markedly upregulated (P<0.05). KLF17 was
linked to the promoter regions of these two genes and shown to
affect the expression of the associated proteins (12). Therefore, the expression changes of
the two proteins may explain the enhancement in potential for
motility and invasion of transfected cells. This may explain the
association between low KLF17 expression and poor prognosis of
patients with PTC.
In conclusion, the present study demonstrated that
KLF17 is a suppressor of PTC growth in preclinical models, although
it remains to be determined whether this finding is the case for
all histological subtypes of thyroid cancer. However, the present
study suggested that targeting this transcription factor may be
useful in estimating the prognosis of patients with PTC. As a
candidate biomarker, further examining the roles of KLF17 in the
progression of PTC and the underlying mechanisms may provide a new
therapeutic target to prolong survival time and even enhance the
quality of life of PTC patients.
References
|
1
|
De Jong SA: Thyroid cancer: A
comprehensive guide to clinical management. Arch Pathol Lab Med.
124:13912000.PubMed/NCBI
|
|
2
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeLellis RA: Pathology and genetics of
thyroid carcinoma. J Surg Oncol. 94:662–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fagin JA and Mitsiades N: Molecular
pathology of thyroid cancer: diagnostic and clinical implications.
Best Pract Res Clin Endocrinol Metab. 22:955–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Couto JP, Daly L, Almeida A, et al: STAT3
negatively regulates thyroid tumorigenesis. Proc Natl Acad Sci USA.
109:E2361–E2370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suske G, Bruford E and Philipsen S:
Mammalian SP/KLF transcription factors: bring in the family.
Genomics. 85:551–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McConnell BB and Yang VW: Mammalian
Krüppel-like factors in health and diseases. Physiol Rev.
90:1337–1381. 2010.
|
|
8
|
van Vliet J, Crofts LA, Quinlan KG, Czolij
R, Perkins AC and Crossley M: Human KLF17 is a new member of the
Sp/KLF family of transcription factors. Genomics. 87:474–482.
2006.PubMed/NCBI
|
|
9
|
Antin PB, Pier M, Sesepasara T,
Yatskievych TA and Darnell DK: Embryonic expression of the chicken
Krüppel-like (KLF) transcription factor gene family. Dev Dyn.
239:1879–1887. 2010.
|
|
10
|
Liu FY, Deng YL, Li Y, et al:
Down-regulated KLF17 expression is associated with tumor invasion
and poor prognosis in hepatocellular carcinoma. Med Oncol.
30:4252013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gumireddy K, Li A, Gimotty PA, et al:
KLF17 is a negative regulator of epithelial-mesenchymal transition
and metastasis in breast cancer. Nat Cell Biol. 11:1297–1304. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Z, Han Q, Zhou N, et al: MicroRNA-9
enhances migration and invasion through KLF17 in hepatocellular
carcinoma. Mol Oncol. 7:884–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobin LH, Gospodrowicz MK and Wittekind
CH: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; New York, NY: 2010
|
|
14
|
Rowe RG, Li XY, Hu Y, et al: Mesenchymal
cells reactivate Snail1 expression to drive three-dimensional
invasion programs. J Cell Biol. 184:399–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rowland BD and Peeper DS: KLF4, p21 and
context-dependent opposing forces in cancer. Nat Rev Cancer.
6:11–23. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carlson CM, Endrizzi BT, Wu J, et al:
Kruppel-like factor 2 regulates thymocyte and T-cell migration.
Nature. 442:299–302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai XD, Zhou YB, Huang LX, et al: Reduced
expression of Krüppel-like factor 17 is related to tumor growth and
poor prognosis in lung adenocarcinoma. Biochem Biophys Res Commun.
418:67–73. 2012.
|
|
18
|
Fong S, Itahana Y, Sumida T, et al: Id-1
as a molecular target in therapy for breast cancer cell invasion
and metastasis. Proc Natl Acad Sci USA. 100:13543–13548. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Wang S, Liu N and Tang X:
Correlation between the expression of Id-1 and
hyperthermia-associated molecules in oral squamous cell carcinoma.
J Clin Pathol. 66:758–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Umeda K, Ikenouchi J, Katahira-Tayama S,
et al: ZO-1 and ZO-2 independently determine where claudins are
polymerized in tight-junction strand formation. Cell. 126:741–754.
2006. View Article : Google Scholar
|
|
21
|
Rowe RG, Li XY, Hu Y, et al: Mesenchymal
cells reactivate Snail1 expression to drive three-dimensional
invasion programs. J Cell Biol. 184:399–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishiyama N, Lee SH, Liu S, et al: Dynamic
and static interactions between p120 catenin and E-cadherin
regulate the stability of cell-cell adhesion. Cell. 141:117–128.
2010. View Article : Google Scholar : PubMed/NCBI
|