Introduction
Biotherapy is a relatively new approach against
cancer compared with conventional surgery, radiation therapy or
chemotherapy. Therapeutic cancer vaccines are one of the most
important approaches in cancer biotherapy, which aims to induce the
immune response of the host to antigens that are selectively
expressed by the tumor (1). The
optimal cancer vaccine induces an effective antitumor response as
well as avoiding unintended tissue damage; therefore,
immunogenicity, tumor-specific antigens and suitable delivery
systems are all necessary. Current vaccine strategies tend to
screen out effective antigens against specified tumors as well as
avoid unspecific autoimmunity (2),
however, several types of cancer, including lung cancer, lack
highly specific biomarkers. Therefore, owing to the complete
antigen-spectrum of tumor cells, autologous whole-cell tumor
vaccines remain indispensable in the development of therapeutic
cancer vaccines (3).
Previous studies have suggested that autologous
whole-cell tumor vaccines failed to provoke strong antitumor
responses expected in large animal models and humans due to the
poor immunogenicity of autologous whole-cell tumor vaccines in
vivo (4). In order to overcome
this, several approaches have been investigated, including genetic
engineering, adjuvant exploitation and prime-boost vaccination
(5).
Interleukin (IL)-15 has long been considered as one
of the most promising cytokines to enhance antitumor activity in
several models, as it is important in the innate and adaptive
immune system. Since 1994, IL-15 has been understood to have a
similar ability to IL-2 in increasing CD8+ T-cell
expansion and the antitumor activity of tumor-specific T cells, as
it shares the same receptor β and γ chains as IL-2 (6). However, certain studies have
demonstrated that IL-15 has another unique chain that is
responsible for the promotion of survival and maintenance of memory
T cells (7–9). In addition, the development,
homeostasis, function and survival of natural killer (NK) cells are
closely associated with IL-15 (10,11).
These differences in characteristics from IL-12 may lead to IL-15
offering more promise in cancer immunotherapy. A previous study
demonstrated that an IL-15 gene-modified autologous whole-cell
tumor vaccine improved its antitumor efficacy against melanoma
(12). This result provided
further evidence towards the potential for IL-15 to enhance the
immunogenicity of autologous whole-cell tumor vaccines.
A suitable delivery system is another important
aspect of vaccine design. Cationic liposomes remain at the
forefront of vaccine design, not only owing to their
well-documented abilities to act as delivery vehicles, but also due
to their immunostimulatory ability as an adjuvant (13). In addition, previous studies
indicated that IL-15 may be used as an immunological co-adjuvant
for cationic liposomal antigens in vivo (14). Based on these findings, rather than
modifying an autologous whole-cell tumor vaccine with the IL-15
gene using transgenic technology, the present study used a cationic
liposome as a carrier of the IL-15 gene-loaded plasmids owing to
the possibility of a co-adjuvant action.
Based on these earlier findings, the present study
aimed to investigate the efficacy of cationic liposomal IL-15 DNA
as an adjuvant for an autologous whole-cell tumor vaccine and
evaluated whether the IL-15 DNA-combined autologous whole-cell
tumor vaccine enhances the immunogenic response and antitumor
efficacy against lung cancer in mice.
Materials and methods
Cell culture
Lewis lung carcinoma (LL2) cells (purchased from the
American Type Culture Collection, Manassas, VA, USA), a cell line
passaged routinely in C57BL/6 mice, were cultivated in
Dulbecco’s modified Eagle’s medium (Gibco-BRL, Grand Island, NY,
USA) supplemented with 10% fetal bovine serum (Gibco-BRL) and 1%
penicillin-streptomycin (Gibco-BRL) at 37°C in a humidified
incubator with 5% CO2.
Animal care
C57 male mice aged ~4–6 weeks were purchased
from Sichuan University Animal Centre (Sichuan, China) and housed
in cages with free access to food and water 1 week prior to
experiments. All animal experiments were performed with approval
from the Sichuan University Animal Care and Use Committee.
Autologous whole-cell tumor vaccine and
cationic liposomal IL-15 plasmid preparation
LL2 cells in the logarithmic growth phase were
collected and fixed using 2% paraformaldehyde (Sigma-Aldrich, St.
Louis, MO, USA). Following being completely fixed, all
paraformaldehyde was removed by washing with normal saline (NS;
Chengdu Qingshan Li Kang Pharmaceutical Co., Ltd., Sichuan, China)
and the whole-cell tumor vaccines were stored at −20°C.
The IL-15-plasmid open reading frame (pORF) and
IL-15-free pORF plasmids were purchased from InvivoGen (San Diego,
CA, USA) and amplified using Escherichia coli in vitro.
Plasmids were purified using an Endofree Plasmid Giga kit (Qiagen,
Valencia, CA, USA) following verification by restriction enzyme
digestion (HindIII and NheI; Takara, Dalian, China)
and DNA agarose gel electrophoresis. Fresh positive ion lipoplexes
were mixed with plasmids within 30 min prior to immunization to
ensure no deposition was present within the mixture.
Immunization
Animals were randomly divided into six groups:
Combination group (autologous whole-cell tumor vaccine + IL-15-pORF
plasmid), tumor vaccine (TV) group (autologous whole-cell tumor
vaccine), IL-15-pORF group (cationic IL-15-pORF plasmid), pORF
group (cationic IL-15-free pORF plasmid), liposome group (positive
ion lipoplexes) and NS (normal saline) group. In the combination
group, the cationic IL-15-pORF plasmid (25 μg/mouse) was injected
at the point of the autologous whole-cell tumor vaccination
(106 cells) via a subcutaneous multipoint injection. The
other groups were immunized subcutaneously with equal doses of
autologous whole-cell tumor vaccine or cationic IL-15-pORF plasmid.
Immunizations were administered a total of four times (day 1, 14,
21 and 28).
Serum was obtained from the tail veins of all
animals for the detection of antibodies and cytokines at set time
points [pre-immune, D0 (the day of tumor inoculation) and D25 (25
days after tumor inoculation)].
Preventive tumor inhibition study
All animals were subcutaneously injected with
2×105 LL2 cells to establish the lung cancer models 7
days after the fourth immunization. Tumor volumes were measured
twice a week using calipers and calculated using the following
formula: (length × width2)/2. The tumor inhibition rate
was calculated using the following formula: [(Average tumor volume
of NS group - average tumor volume of experimental group)/average
tumor volume of NS group] × 100%. Animal body weights were also
measured twice weekly in order to detect any experimental
abnormalities. Three tumor-bearing mice in each group were
sacrificed by decapitation following being anesthetized with
chloral hydrate to perform a cytotoxicity assay and obtain tumor
tissues, whilst five mice in each group were kept for determining
the survival rate.
Adoptive therapy study
For passive immunotherapy, splenic lymphocytes were
obtained by density gradient centrifugation at 800 g for 30 min
using iodixanol solution and nylon wool (Dakewe Biotech Co., Ltd.,
Shenzhen, China). The LL2 lung cancer models were established, as
described previously. Mice were injected with adoptive spleen cells
(106–107/mouse) twice a week into the tail
vein for 3 weeks. Animals were sacrificed 3 weeks after challenge
by tumor cells and the tumor weight was measured.
Cytotoxic lymphocyte (CTL) assay in
vitro
A lactate dehydrogenase (LDH)-release assay
(15,16) was performed to measure the
antigen-specific cytotoxicity of splenic lymphocytes in
vitro using a Non-radioactive Cytotoxicity assay kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer’s
instructions. Briefly, the LL2 cells were seeded in a 96-well plate
as target cells. Subsequently, the splenocytes obtained from the
immunized or the control mice, as previously described, were added
to the plate at different ratios of effector/target (E:T) cells,
followed by additional incubation in a humidified chamber at 37°C
and 5% CO2 for 4–6 h. Lysis solution and assay buffer
was added at set time points. The absorbance was recorded at 490 nm
within 1 h after the addition of 50 μl/well of stop solution. The
cytotoxicity was calculated using the following formula:
%Cytotoxicity = (experimental - effector spontaneous - target
spontaneous)/(target maximum - target spontaneous) × 100.
Measurement of antibodies and
cytokines
A cell enzyme-linked immunosorbent assay (ELISA)
(17) was performed to measure the
number of antibodies against the whole-tumor cell vaccine. LL2
cells were seeded at a density of 1×104/well into a
96-well plate. Following incubation overnight, the cells were fixed
using 4% polysaccharide for 15 min. The fixed tumor cells were
incubated with serum from the different groups at several dilutions
(for the antibodies, the dilutions were 1:500, 1:1,000, 1:2,000,
1:4,000, 1:8,000, 1:16,000, 1:32,000, 1:64,000 and for the
cytokines, the dilution was 1:5) for 2 h after blocking unspecific
antigens with 1% bovine serum albumin for 1 h. Horseradish
peroxidase-labeled goat polyclonal anti-mouse immunoglobulin G and
3,3′,5,5′-tetramethylbenzidine (Neobioscience, Shenzhen, China)
were used for coloration. The result was measured using a
microplate reader (3550-UV; Bio-Rad, Hercules, CA, USA) at 450
nm.
The levels of IL-4 and interferon-γ (IFN-γ) in the
serum from the subcutaneous tumor inhibition study were determined
using specific ELISA kits (Neobioscience, Shenzhen, China)
according to the manufacturer’s instructions.
Histopathology
The heart, liver, spleen, lungs and kidneys from the
mice were fixed in 4% neutral-buffered formalin solution and
embedded in paraffin for observation of any potential side effects
in the treated mice. Paraffin sections (3–5 mm) of the embedded
tumor tissues from each group were stained using hematoxylin and
eosin (Sigma-Aldrich).
Immunohistochemical analysis of the tumor sections
was performed to investigate the CD4+ T cells,
CD8+ T cells, B cells and NK cells in the tumor tissues
from the immunized and control mice with CD4, CD8, CD24 and CD57
antibodies, respectively.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Analysis of variance and an unpaired Student’s t-test were
performed for comparison of the individual time points. Survival
analysis was computed using the Kaplan-Meier method and compared by
the log-rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
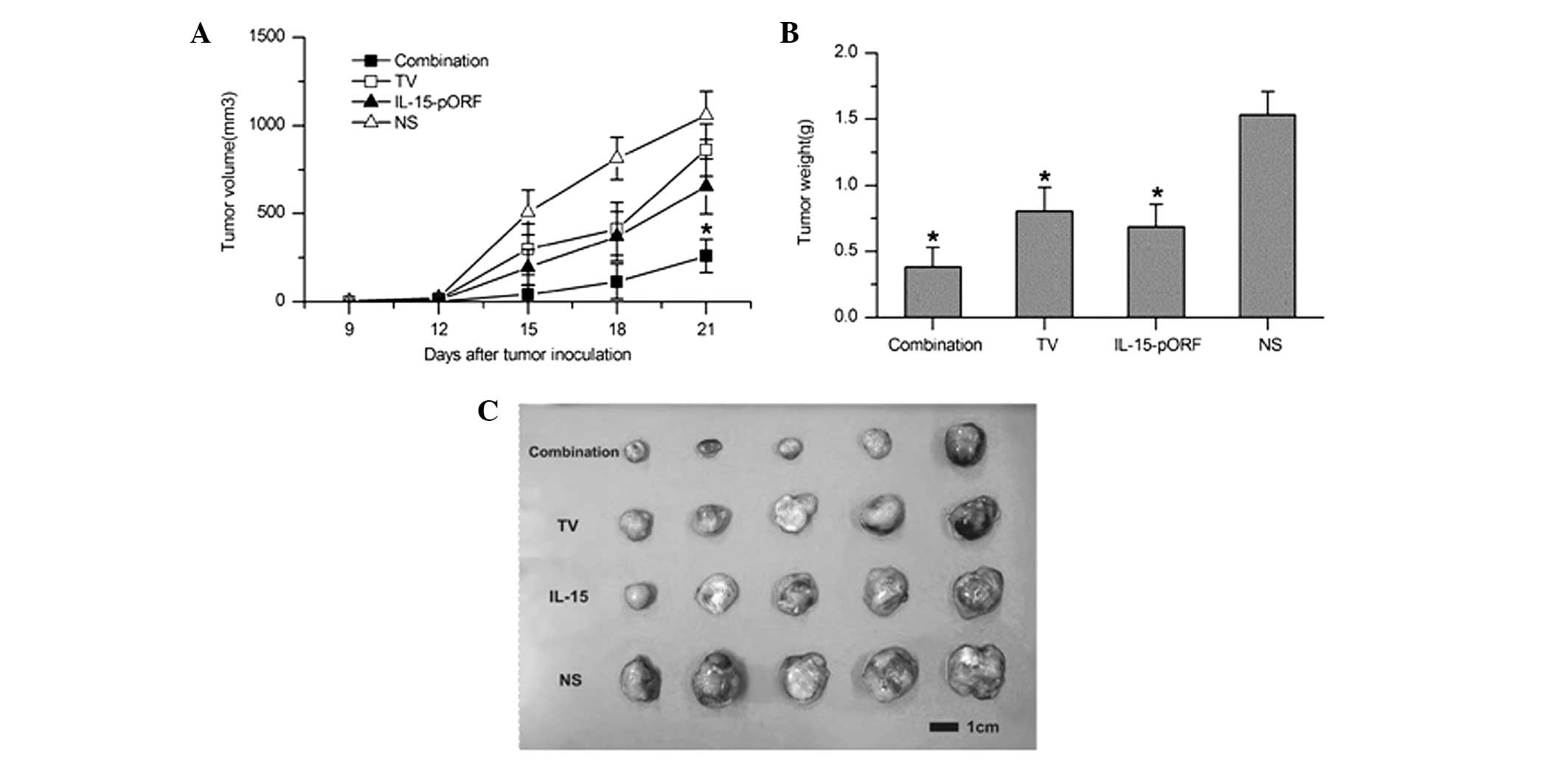
IL-15 gene enhances the efficacy of the
autologous whole-tumor cell vaccine against LL2 lung cancer in
vivo
In order to determine whether the IL-15 gene can
improve the effects of an autologous whole-cell tumor vaccine
against murine lung cancer in vivo, the effect of the
prophylactic combined treatment of the cell vaccine and the IL-15
gene on pulmonary tumorigenesis was evaluated in a subcutaneous LL2
lung cancer model. Combined immunization demonstrated a significant
inhibition of tumor growth and the maximum tumor inhibition rate of
the combined immunized mice reached 92.9% 15 days after tumor
incubation, which was higher than the TV group (70.3%) and the
IL-15-pORF group (58.9%; Fig. 1A).
Furthermore, the combination group demonstrated apparent advantages
in prolonging the median survival time compared with the other
groups (Fig. 1B). In addition, no
significant abnormal change was identified in the weight of the
mice, suggesting no overt systemic toxicity (data not shown).
 | Figure 1Induction of protective antitumor
immunity by combined immunization of the autologous whole-cell
tumor vaccine with the IL-15 gene. Mice were randomly divided into
six groups (five in each group) and immunized with the autologous
whole-cell tumor vaccine (106/100 μl) + cationic
liposomal IL-15-pORF plasmid (25 μg/100 μl), autologous whole-cell
tumor vaccine (106/100 μl), cationic liposomal
IL-15-pORF plasmid (25 μg/100 μl), cationic liposomal IL-15-free
pORF plasmid (10 μg/100 μl), cationic liposome (same dose as other
experimental groups) or NS (0.9% sodium chloride, 100 μl),
respectively. Subcutaneous tumor models were established 7 days
after the final vaccination (four in total). (A) Average tumor
volume of combined immunized mice was significantly smaller than
that of the other groups (*P<0.05, compared with the
NS group). (B) Median survival percentage of combined immunized
mice was prolonged compared with the other groups. TV, tumor
vaccine; IL, interleukin; pORF, plasmid open reading frame; NS,
normal saline. |
Combined immunization induces robust
humoral responses
ELISA was performed to assess the humoral response
in mice. The combination group and the TV group elicited vigorous
production of antibodies following vaccination compared with the
other groups and the antibodies persisted at a high level for 25
days after tumor challenge, as shown in Fig. 2A. Notably, the combination group
demonstrated a significantly slower reduction and higher titer of
antibodies compared with the TV group (Fig. 2B and C). This result indicated that
the IL-15 gene succeeded in enhancing the humoral response to the
autologous tumor cell vaccine in vivo.
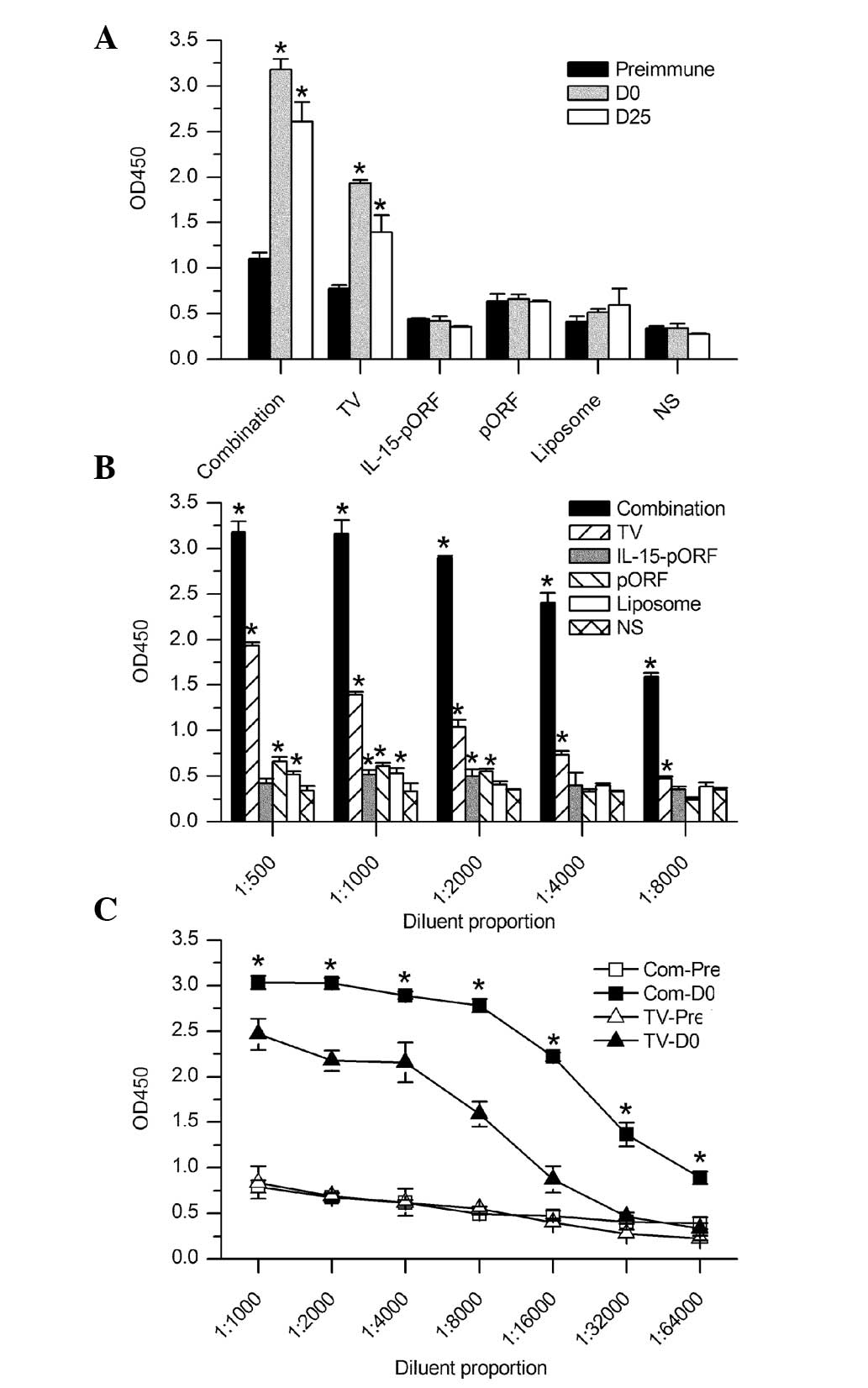 | Figure 2ELISA analysis of the humoral immune
response in mice. Serum samples from the mice were analyzed for
antibodies against Lewis lung carcinoma cells by cellular ELISA.
(A) Antibody titers were determined at different time points,
including prior to immunization, the day of tumor inoculation (7
days after the final vaccination) and 25 days after tumor
challenge. All serum samples were diluted 500-fold. The combination
group elicited a significantly higher production of antibodies
following vaccination than the other groups (*P<0.05,
compared with those pre-immunized). (B) Antibody titers in serum
samples at different dilutions on the tumor inoculation day. A
significantly higher antibody titer was detected in the combination
group compared with the NS group (*P<0.05). (C)
Semi-quantitative comparison of antibody titer between the
combination group and the autologous whole-cell tumor vaccine group
on the tumor inoculation day. The combination group demonstrated a
significantly slower reduction at the different serum dilution
rates than the other groups (*P<0.05, compared with
the TV-Pre). D, day; Pre, pre-immunization; TV, tumor vaccine; IL,
interleukin; pORF, plasmid open reading frame; NS, normal saline;
Com, combination group; OD, optical density; ELISA, enzyme-linked
immunosorbent assay. |
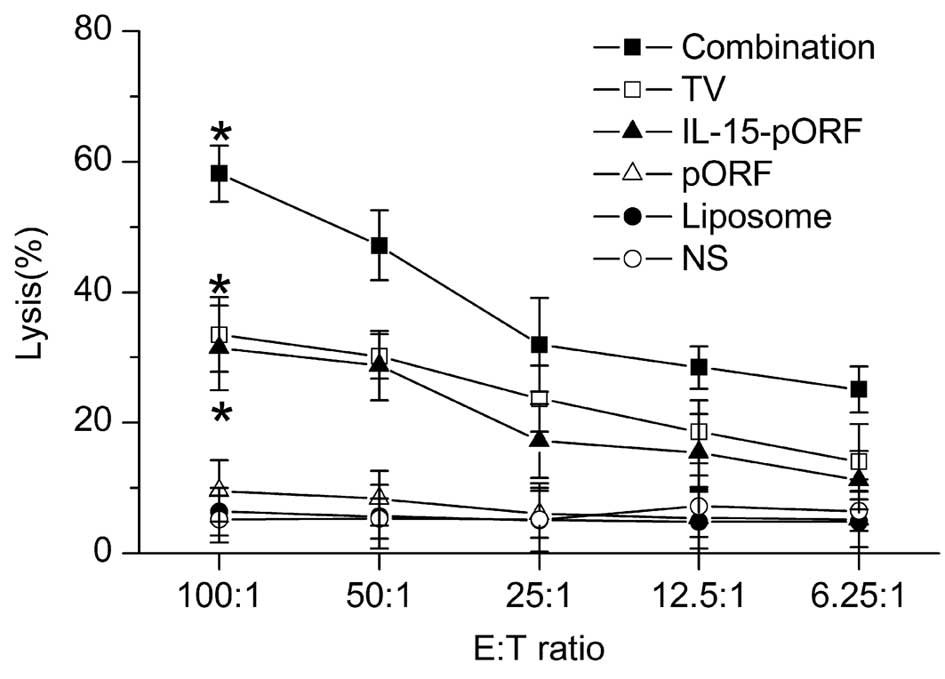
Combined immunization induces more marked
cellular responses
In order to evaluate the cellular responses, splenic
lymphocytes from the groups were harvested 25 days after tumor
inoculation. The antigen-specific cytotoxicity of the
CD8+ T cells was assessed using an LDH-release assay
in vitro, as described previously. The results demonstrated
that potent cellular responses were induced in the combination
group, reaching a peak cytotoxicity (E:T ratio=1:100) at 58.17%.
The TV group and IL-15-pORF group demonstrated similar
cytotoxicities of 33.51 and 31.47%, respectively, while the control
groups demonstrated low cytotoxicity at all E:T ratios (Fig. 3).
Combined immunization induces a higher
IFN-γ response
To further examine the type of immune response, the
secretion of cytokines in the serum was estimated using ELISA.
Significantly higher levels of IFN-γ were detected in the mice
immunized with the combined vaccine compared with the other groups
(Fig. 4A). The IL-15-pORF group
also demonstrated a considerable elevation in IFN-γ, which was
higher than in the TV group but less than in the combination group.
In addition, there was a mild increase in the secretion of IL-4 in
the combination group, TV group and IL-15-pORF group following
immunization, however, this was not significantly different between
the three groups (Fig. 4B). The
three control groups, including the pORF, liposome and NS group,
demonstrated no change in cytokine production.
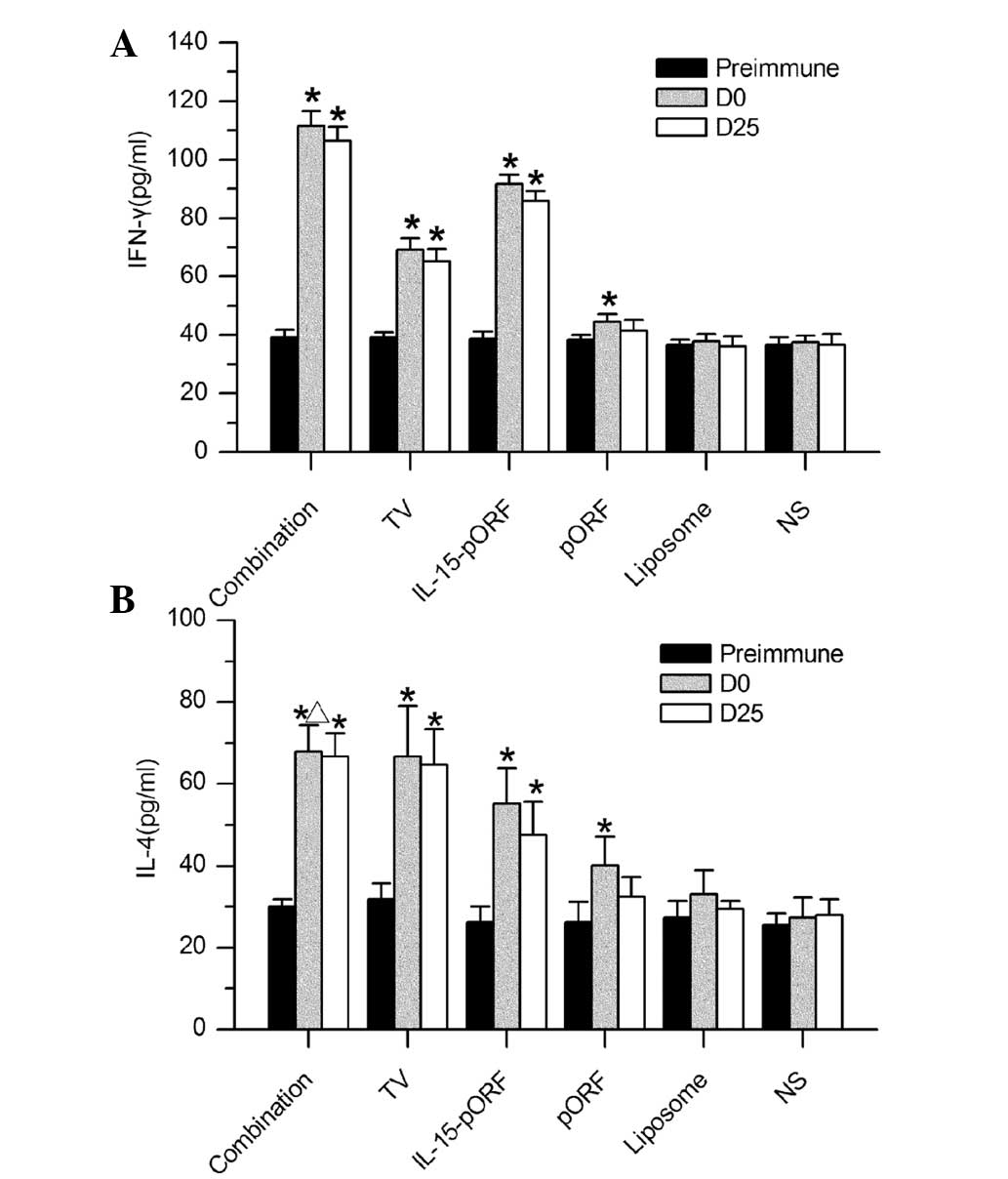 | Figure 4ELISA analysis of cytokine production
in mice. Serum samples from mice in each group were analyzed for
levels of IFN-γ and IL-4 by ELISA. (A) IFN-γ production in the
combination group was significantly higher than the other groups
(*P<0.05, compared with the pre-immune group). (B)
Levels of IL-4 were not significantly different in the combination
group, TV group or IL-15-pORF group (*P>0.05), but
remained higher than in the three control groups
(*P<0.05, compared with the pre-immune group,
ΔP>0.05, compared with the TV group). IFN-γ,
interferon-γ; TV, tumor vaccine; IL, interleukin; pORF, plasmid
open reading frame; NS, normal saline; D, day; Pre-immune,
preimmunization; ELISA, enzyme-linked immunosorbent assay. |
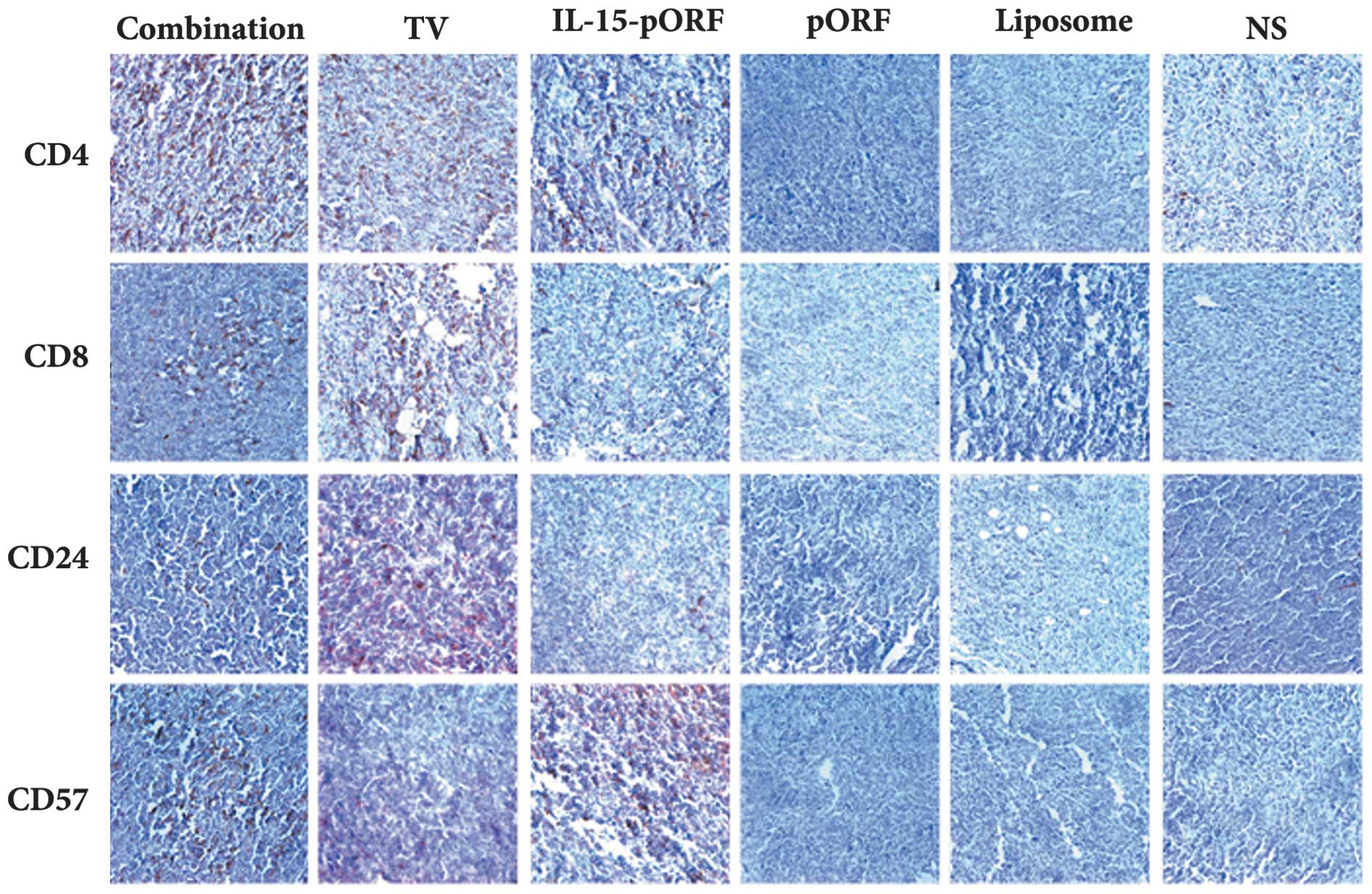
Combined immunization induces the
concentrated gathering of immune cells in tumor tissues
The number of CD4+ or CD8+ T
cells and CD24+ B cells in the tumor sections from the
TV group notably increased compared with the control groups, while
the CD57+ NK cells were limited in number in this group.
Of note, few CD4+ T cells, CD8+ T cells or
CD24+ B cells were observed in the tumor sections from
the IL-15-pORF group compared with the control groups. In addition,
the number of CD57+ NK cells increased significantly
compared with the TV group (Fig.
5). Taken together, the sections from the combination group
demonstrated the highest density of all types of cells mentioned
and the number of immune cells observed in the control groups was
low.
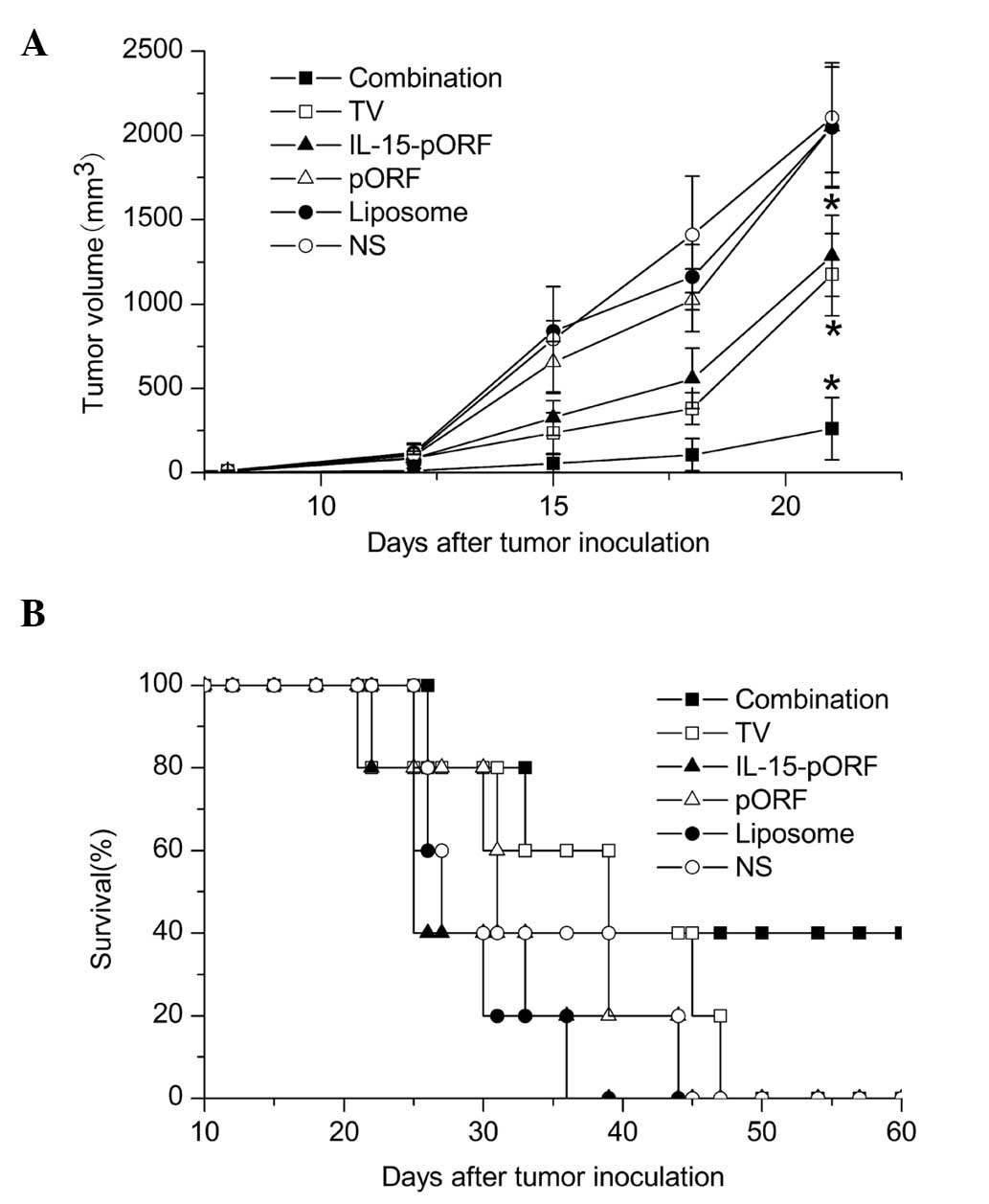
Adoptive therapy with lymphocytes from
combined immunized mice induces significant inhibition of tumor
growth in vivo
Subcutaneous LL2 lung cancer models were established
to evaluate whether the combination of the IL-15 gene with an
autologous whole-tumor cell vaccine enhances antitumor efficacy
with adoptive lymphocytes in vivo. As shown in Fig. 6, the maximum tumor inhibition rate
of the combined group reached 94.7% 9 days after tumor incubation,
which was markedly higher than that observed in the IL-15-pORF
group (78.9%) or the TV group (42.1%). This result suggested that
the adoptive therapy of lymphocytes from the combined immunized
mice induced more marked inhibition of the established tumor growth
in vivo.
Discussion
Autologous whole-cell tumor vaccines and IL-15 have
been used extensively in the treatment of various types of tumor in
pre-clinical studies. Based on previous studies, the present study
hypothesized that these two agents could co-stimulate the host
immune response against cancer. In the present study, the IL-15
gene was used more widely than the IL-15 protein, as the IL-15 gene
can persistently induce the protein expression of IL-15 in
vivo. It can also be directly co-administered with an
autologous whole-cell tumor vaccine for preventive anti-tumor
investigation. The present study demonstrated that combined
vaccination improved the inhibitory efficacy against subcutaneous
lung cancer models and prolonged the median survival time of the
tumor-bearing mice.
The mechanisms underlying the robust antitumor
efficacy of the combined immunization appears to be complex. Using
ELISA, the present study observed that combined immunized mice
produced markedly more IFN-γ than those immunized with the tumor
cell vaccine or the IL-15 gene alone, however, no significant
difference in IL-4 generation was observed between the three
experimental groups. This result suggested that the liposomal
packaging IL-15 gene combined with a whole-tumor cell vaccine
induced a predominantly cellular immune response, as IFN-γ is
mainly associated with Th1 and CD8+ T cells (18).
Previous studies have reported that IL-15
demonstrates potent antitumor potential, not only by eliciting the
priming and proliferation of CD8+ T cells, but also by
improving the maintenance of memory CD8+ T cells
(18). In addition, it has been
reported that IL-15 was able to reverse the host tolerance of tumor
antigens (18), which is commonly
observed in patients treated with a tumor vaccine, by increasing
the sensitivity of CD8+ T cells to tumor antigens. This
evidences suggests that CD8+ T cells may be important in
the antitumor efficacy of IL-15. Since autologous whole-cell tumor
vaccines exhibit a considerable efficacy against various types of
tumor, mainly through the activation of T cells observed in
previous studies, it has been hypothesized that IL-15 may
principally coordinate with the whole-tumor cell vaccine through
enhancing the function of CD8+ T cells. Evidence from a
previous study on murine melanoma supported this hypothesis
(18). In the present study, data
from the CTL assay in vitro revealed that the combined
immunization induced substantially higher activity against LL2
cancer than the other groups in splenic lymphocytes 25 days after
tumor inoculation. Since CD8+ T cells are principally
involved in CTL activity and, as the subset of memory CTLs
generated in response to a vaccination may determine the ultimate
effectiveness of the vaccine in eliciting immune protection against
cancer, the present study confirmed that combined immunization
primarily improved antitumor efficacy by promoting the function of
CD8+ T cells.
While the major role of CD8+ T cells in
the antitumor activity of IL-15 and autologous whole-cell tumor
vaccines has been established, the antitumor effect of
CD4+ T cells cannot be excluded. In the present study,
IL-15 was also found to regulate CD4+ T cells, which
were activated by CD3+/CD28+ and increase the
production of IFN-γ, TNF-α and IL-10 (19). Despite a previous report suggesting
that IL-15 promotes the apoptosis of CD4+ T cells
activated by CD3+/CD28+ in vitro
(19), autologous whole-cell tumor
vaccines may compensate for this disadvantage of IL-15 by inducing
a marked proliferation of CD4+ T cells (19). Therefore, it was hypothesized that,
although the efficacy of combined vaccination was mainly attributed
to CD8+ T cells, CD4+ T cells were also
involved. This was confirmed in the present study, which observed a
larger number of CD4+ T cells in tumor sections from
prophylactic immunized mice through immunohistochemical analysis.
However, more evidence is required to further elucidate the
function of the CD4+ T cells affected by combined
immunization.
An adoptive therapy study was also performed in the
present study to confirm the role of the cellular response against
the tumor in the combined vaccination in vivo. The results
suggested that splenic lymphocytes from the combined immunized mice
induced significant inhibition of established tumor growth when
compared with the other groups.
Although no significant difference was identified in
the generation of IL-4 between the three experimental groups, the
ELISA result exhibited a notably higher humoral response in the
combined immunized mice. Previous studies have reported that IL-15
is markedly correlated with NK cell proliferation, differentiation
and maturation (10,11). NK cells are considered as one of
the most indispensable aspects of host antitumor activity owing to
the marked upregulation of antibody-dependent cell-mediated
cytotoxicity (ADCC) when IL-15 was used in tumor-bearing animals as
an antineoplastic drug alone. In addition, whole-tumor cell vaccine
and IL-15 can induce B cell proliferation and induce the production
of antibodies (20). Therefore,
another possible explanation for the antitumor efficacy enhancement
of combined immunization is that the increasing number of
tumor-specific antibodies and functional NK cells may co-act on the
upregulation of ADCC. Immunohistochemical analysis of tumor
sections in the present study confirmed that CD24+ B
cells and CD57+ NK cells were substantially concentrated
in the combined immunized tumor tissues.
In the present study, preventive vaccination with
the liposomal IL-15 gene alone was invaluable in improving the
median survival time of tumor-bearing mice, while the average tumor
volume and immune reaction suggested a significant antitumor
activity in vivo. A possible explanation for this is that
IL-15 has an additional potential for the promotion of
angiogenesis, as vascular endothelial cells express soluble
receptor-α, which has high affinity to IL-15 (21). Thus, an increasing rate of lung
metastasis may be observed in IL-15-treated subcutaneous lung
cancer models due to the growing number of tumor angiogenic blood
vessels. This hypothesis may also explain the previous evidence of
a correlation between poor outcome and a high concentration of
IL-15 in lung cancer patients (22). However, it has been hypothesized
that tumor angiogenic blood vessels may assist antitumor immune
cells, including CTLs, in reaching the target cells, which may be
the reason for certain studies demonstrating the potential for
improving the outcome of tumor-bearing animals using IL-15 as the
only therapeutic agent. Further investigation is required to
confirm the correlation between angiogenesis caused by IL-15 and
the final outcome in lung cancer models.
Through use of an LL2 subcutaneous tumor model, the
present study demonstrated that the cationic liposome-carrying
IL-15 gene combined with an autologous whole-cell tumor vaccine may
stimulate the host innate and adaptive immune system to develop
robust antitumor immunity. This antitumor immunity protected the
mice from challenge with a parental tumor. Furthermore, the
adoptive transferring lymphocytes in the combined immunized mice
inhibited the growth of the pre-challenged tumor. In addition, the
development of tumor immunity in response to combined vaccination
was principally dependent upon the function of CD8+ T
cells and the activation of ADCC.
Acknowledgements
Yang Wu and Yan Luo participated in designing the
experiments, analyzing the data and revising the manuscript. Xi
Chen participated in performing the cell culture, animal studies,
analyzing the data and writing the manuscript. Jie Ni participated
in performing the animal studies and in collecting samples from
tumor-bearing mice. Hui Meng and Dandan Li participated in
conducting the animal studies and the CTL assay. All authors read
and approved the final manuscript. This study was supported by the
Special Projects of the National Natural Sciences Foundation of
China (no. 81123003) and the Project of the National Natural
Sciences Foundation of China (no. 30901773).
References
|
1
|
Pardoll DM: Cancer vaccines. Nat Med.
4:525–531. 1998. View Article : Google Scholar
|
|
2
|
Van Der Bruggen P, Zhang Y, Chaux P, et
al: Tumor-specific shared antigenic peptides recognized by human T
cells. Immunol Rev. 188:51–64. 2002.PubMed/NCBI
|
|
3
|
Ward S, Casey D, Labarthe MC, et al:
Immunotherapeutic potential of whole tumour cells. Cancer Immunol
Immunother. 51:351–357. 2002. View Article : Google Scholar
|
|
4
|
Le DT, Pardoll DM and Jaffee EM: Cellular
vaccine approaches. Cancer J. 16:304–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belardelli F, Ferrantini M, Parmiani G,
Schlom J and Garaci E: International meeting on cancer vaccines:
how can we enhance efficacy of therapeutic vaccines? Cancer Res.
64:6827–6830. 2004. View Article : Google Scholar
|
|
6
|
Grabstein KH, Eisenman J, Shanebeck K, et
al: Cloning of a T ell growth factor that interacts with the beta
chain of the interleukin-2 receptor. Science. 264:965–968. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ku CC, Murakami M, Sakamoto A, Kappler J
and Marrack P: Control of homeostasis of CD8+ memory T
cells by opposing cytokines. Science. 288:675–678. 2000.PubMed/NCBI
|
|
8
|
Becker TC, Wherry EJ, Boone D, et al:
Interleukin 15 is required for proliferative renewal of
virus-specific memory CD8 T cells. J Exp Med. 195:1541–1548. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Sun S, Hwang I, Tough DF and
Sprent J: Potent and selective stimulation of memory-phenotype
CD8+ T cells in vivo by IL-15. Immunity. 8:591–599.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prlic M, Blazar BR, Farrar MA and Jameson
SC: In vivo survival and homeostatic proliferation of natural
killer cells. J Exp Med. 197:967–976. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carson WE, Giri JG, Lindemann MJ, et al:
Interleukin (IL) 15 is a novel cytokine that activates human
natural killer cells via components of the IL-2 receptor. J Exp
Med. 180:1395–1403. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Basak GW, Zapala L, Wysocki PJ, Mackiewicz
A, Jakobisiak M and Lasek W: Interleukin 15 augments antitumor
activity of cytokine gene-modified melanoma cell vaccines in a
murine model. Oncology Rep. 19:1173–1179. 2008.PubMed/NCBI
|
|
13
|
Christensen D, Korsholm KS, Andersen P and
Agger EM: Cationic liposomes as vaccine adjuvants. Expert Rev
Vaccine. 10:513–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gursel M and Gregoriadis G: Interleukin-15
acts as an immunological co-adjuvant for liposomal antigen in vivo.
Immunol Lett. 55:161–165. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge W, Li Y, Li ZS, et al: The antitumor
immune responses induced by nanoemulsion-encapsulated
MAGE1-HSP70/SEA complex protein vaccine following peroral
administration route. Cancer Immunol Immunother. 58:201–208. 2009.
View Article : Google Scholar
|
|
16
|
Yang YW, Wu CA and Morrow WJ: Cell death
induced by vaccine adjuvants containing surfactants. Vaccine.
22:1524–1536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JC, Cevallos AM, Naeem A,
Lennard-Jones JE and Farthing MJ: Detection of anti-colon
antibodies in inflammatory bowel disease using human cultured
colonic cells. Gut. 44:196–202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelso A, Troutt AB, Maraskovsky E, et al:
Heterogeneity in lymphokine profiles of CD4+ and
CD8+ T cells and clones activated in vivo and in vitro.
Immunological Rev. 123:85–114. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin SJ, Cheng PJ and Hsiao SS: Effect of
interleukin-15 on effector and regulatory function of
anti-CD3/anti-CD28-stimulated CD4(+) T cells. Bone Marrow
Transplant. 37:881–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Armitage RJ, Macduff BM, Eisenman J,
Paxton R and Grabstein KH: IL-15 has stimulatory activity for the
induction of B cell proliferation and differentiation. J Immunol.
154:483–490. 1995.PubMed/NCBI
|
|
21
|
Angiolillo AL, Kanegane H, Sgadari C,
Reaman GH and Tosato G: Interleukin-15 promotes angiogenesis in
vivo. Biochem Biophys Research Commun. 233:231–237. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seike M, Yanaihara N, Bowman ED, et al:
Use of a cytokine gene expression signature in lung adenocarcinoma
and the surrounding tissue as a prognostic classifier. J Natl
Cancer Inst. 99:1257–1269. 2007. View Article : Google Scholar : PubMed/NCBI
|