Introduction
Hypertension is recognized as one of the most
frequent health concerns for the human population (1). There are numerous signaling pathways
and feedback mechanisms that contribute to the process of
hypertension and its associated diseases, including renal
dysfunction and diabetes. The renin angiotensin system (RAS) has
been demonstrated to be the main mediator in the pathogenesis of
hypertension. RAS acts not only at a circulatory level but also at
the tissue level, including the heart, kidney and brain, which are
all of marked significance for the development of hypertension
(2–4).
The principal peptide of the RAS is angiotensin II
(Ang II), which acts by binding to one of the two major Ang II
receptors AT1 and AT2 (5). In the
kidney, Ang II, through the Ang II receptor type 1 (AT1 receptor),
increases sodium retention and pressure within the glomeruli, which
accelerates the progression of hypertension. The increased sodium
retention by the proximal tubule is mediated by the
Na+/H+ exchanger (NHE) (6). NHE is a ubiquitous transport system
that is involved in the regulation of intracellular pH, cell
volume, cell growth and proliferation, and transepithelial
absorption of Na+, Cl− and
HCO3− (7).
Several isoforms of NHE have been identified in the kidney
(8). With mRNA probes, NHE1
expression was identified at the basolateral membrane of renal
epithelial cells. NHE-3, a tissue-specific isoform, is localized on
the thick ascending limbs of the loop of Henle, the luminal
membrane of proximal tubules and certain long thin descending
limbs, as detected by low-stringency screening of cDNA libraries
(9). NHE3 regulates bicarbonate
absorption, salt, volume homeostasis and tubular protein
expression. Renal NHE, particularly the NHE3 isoform, are essential
for the maintenance of acid-base homeostasis. Both the expression
and NHE activity are subject to complex regulation. In diabetic
animal models, NHE3 expression is highly stimulated in the early
phase, which is characterized by increased glomerular filtration
rate. In conclusion, NHE has an important role in renal physiology
and response to diabetes (10).
Serum and glucocorticoid-inducible kinase 1 (SGK1) is expressed
following exposure to a variety of hormones in kidney. SGK1
enhances the activity of a variety of carriers (NHE3, NHE1, SGLT1)
and ion channels (renal outer medullary potassium channel,
potassium voltage-gated channel subfamily E member 1/subfamily Q
member 1, metabotropic glutamate receptor 6 and cystic fibrosis
transmembrane conductance regulator). SGK1 contributes to
Na+ retention and K+ elimination in the
kidney. Therefore, SGK1 may participate in the pathogenesis of
hypertension (11,12).
Clinically, blockade of the RAS is the prominent
therapeutic strategy for the treatment of hypertension. The
administration of angiotensin-converting enzyme inhibitors and Ang
II receptor blockers are widely used in the treatment of
hypertension (13). Losartan is a
selective and competitive antagonist for the AT1 receptor.
Currently, losartan is a highly popular antihypertensive agent with
well-recognized clinical efficacy supported by large-scale clinical
studies. However, the molecular mechanisms underlying the
beneficial effects of losartan remain elusive.
The present study examined the expression of renal
sodium/proton exchangers, particularly NHE3, affected by activated
the RAS of renal tissue, in spontaneously hypertensive rats and
aimed to elucidate the molecular pharmacodynamic mechanism of
losartan.
Materials and methods
Subjects
A total of 12 spontaneously hypertensive rats and
six Wistar-kyoto rats (WKY; male or female) aged 10 weeks, weighing
150 g from Vital River Laboratories Co., Ltd. (Beijing, China) were
enrolled in the study. The spontaneously hypertensive rats were
randomly divided into two groups: The SHR group (saline, six
animals); the LOS group (losartan-treated, six animals). The rats
were housed under conditions of constant temperature (24°C) and
humidity (60%), exposed to a 12-h light/dark cycle, and provided
tap water to drink, as well as SPF level standard rat feed. The
experimental design was approved by the Institutional Ethics
Committee of the Institute of Zunyi Medical College (Zhuhai,
Guangdong, China).
Reagents
Losartan was purchased from Merck USA (Whitehouse
Station, NJ, USA). Monoclonal antibody NHE3, monoclonal antibody
SGK1 and immunohistochemical staining avidin-biotin-peroxidase
complex (ABC) kit were purchased Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The First Strand cDNA Synthesis kit and 2×
AllinOneTM Q-PCR Mix were purchased from GeneCopoeia (Guangzhou,
China), and the Angiotensin II radioimmunoassay kit was purchased
from HTA Co. Ltd. (Beijing, China).
Blood pressure (BP) measurement
The BP was measured by the indirect tail cuff
method, using an RM-6240 multi-channel physiological signal
acquisition and processing system (Chengdu Instrument Factory,
Sichuan, China). The measurements were performed while the rats
were maintained in a temperature-regulated holder. The mean of
three consecutive readings was used for BP determination.
Immunoradiometric detected of Ang II
An immunoradiometric method was utilized for the
detection of plasma and renal Ang II by a radioimmunoassay kit
according to the manufacturer’s instructions (HTA Co. Ltd.,
Beijing, China).
Immunohistochemical demonstration of NHE3
in kidney tissue
The kidney tissues were fixed for 12–24 h in 4%
paraformaldehyde and following dehydration, were embedded in
paraffin. The tissues were cut into 5-μm thick sections, which were
then mounted onto glass slides, and following deparaffinization and
hydration, were immunostained according to the manufacturer’s
instructions. The sections were sequentially exposed to normal goat
serum for 1 h and then to NHE3 antibody at a final dilution of
1:200 overnight at 4°C. Next, samples were labeled with the ABC
complex diluted 1:200 for 30 min. The peroxidase label was revealed
by reaction with stable diaminobenzidine. Photomicrographs were
obtained with an Axiovert 200 inverted fluorescence microscope
(Zeiss, Oberkochen, Germany). Six images of six fields of the
histological sections were acquired. The samples were considered
positive when >30% of the tissue components were
immunohistochemically stained brown-yellow in the appropriate
cellular compartment.
Reverse transcription-polymerase chain
reaction
Total RNA was isolated from renal cortex using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) and the
DNA was removed by the recombinant DNaseI. For qPCR, cDNA was
prepared from 1 μg total RNA, using reverse transcriptase from the
First Strand cDNA Synthesis kit in accordance with the
manufacturer’s instructions. All PCRs were conducted using the 2×
AllinOneTM Q-PCR Mix, and cycling parameters 95°C, 15 sec; 60°C, 15
sec; and 72°C, 20 sec for 39 cycles, with primers designed against
the following mouse sequences:
| GAPDH: | (forward,
5′-GGACCAGGTTGTCTCCTGTG-3′ reverse, 5′-TGTAGGCCATGAGGTCCAC-3′) |
| NHE3: | (forward,
5′-AGGACAAATTGGACACAATTACC-3′ reverse,
5′-GCTCATGGAAAACATTCAGGA-3′) |
| SGK1: | (forward,
5′-CTGTTCTACCATCTCCAGAG-3′ reverse,
5′-CCGTAGAGCATCTCATACAG-3′) |
Quantification of NHE3 and SGK1 protein
by western blot analysis
The whole tissue lysates were separated on an 8%
polyacrylamide gel. The proteins were transferred to a
polyvinylidene difluoride membrane. The membranes were blocked with
10% skimmed milk in Tris-buffered saline-Tween-20 (TBST) for 1 h,
and then probed with primary antibodies diluted in 5% milk in TBST
overnight at 4°C. The membranes were washed three times with TBST
and then incubated with secondary antibodies at room temperature
for 1 h and developed using BeyoECL Plus kit (Beyotime Institute of
Biotechnology, Jiangsu, China).
Results
Effect of losartan on the BP
The baseline BP in the spontaneously hypertensive
rats was significantly higher than that in the WKY rats at ten
weeks (Fig. 1). Treatment with
losartan attenuated BP compared with the SHR group following two
weeks, and the tendency of the BP remained attenuate with
continuous losartan treatment in a time-dependent manner following
eight weeks. There was a significant difference between the SHR and
the LOS groups, but the blood pressure was still higher in the WKY
group.
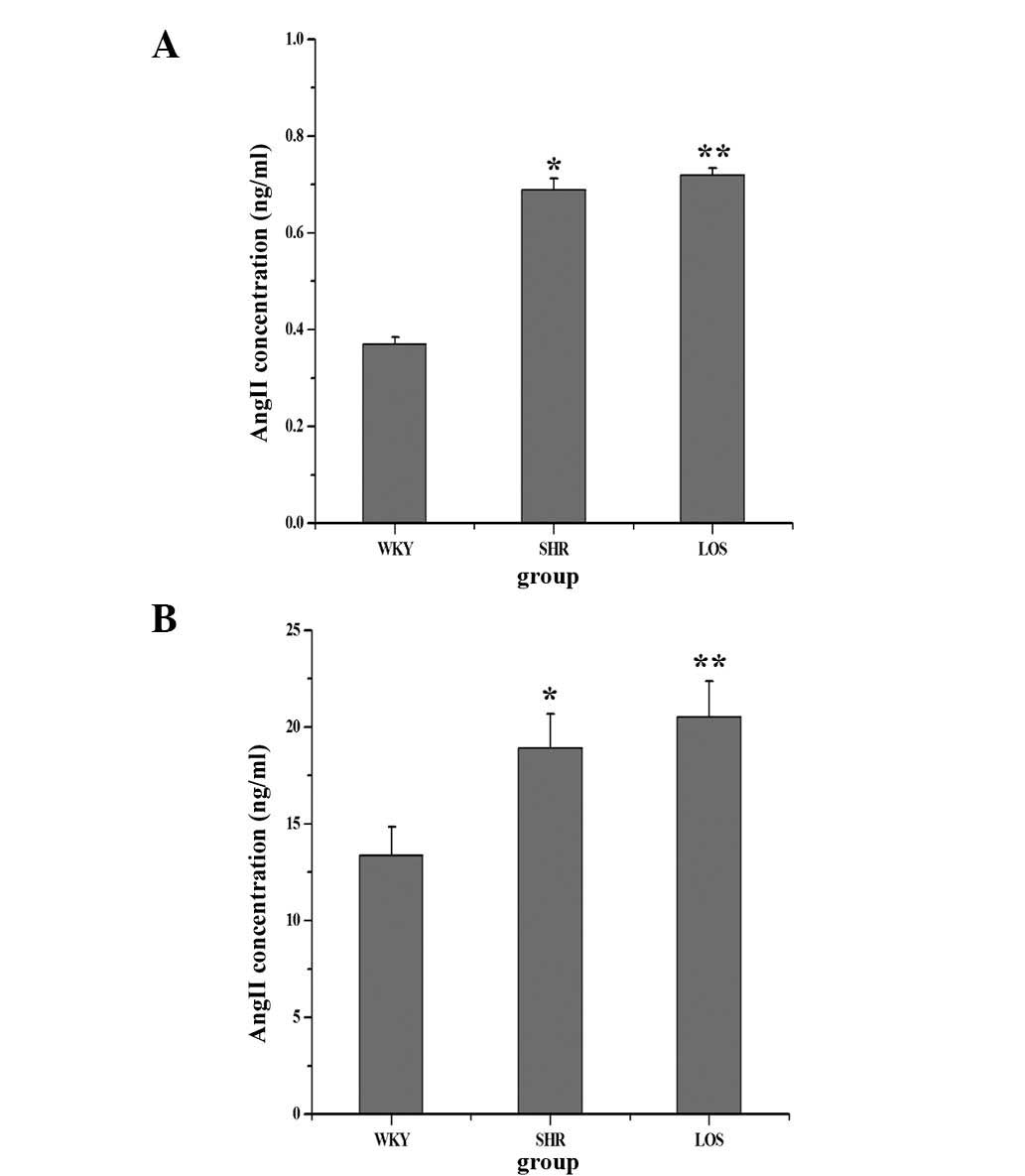
Effect of losartan on Ang II in the
plasma and renal tissue
In the present study, the concentration of Ang II in
plasma and renal tissue was examined in order to confirm that in
hypertension, both at circulatory and tissue levels, the RAS was
activated. As the results demonstrate in Fig. 2, the levels of Ang II in the SHR
and LOS groups were significantly higher than those in the WKY
group, not only in plasma (Fig.
2A) but also in renal tissues (Fig. 2B). However, following losartan
treatment for eight weeks, the concentration of Ang II in plasma
and renal tissue exhibited no significant difference between the
SHR and LOS groups, but remained markedly higher than that in the
WKY group. Therefore, the RAS at the circulatory and tissue levels
was activated in the hypertension and losartan groups, while the
antagonist of the AT1 receptor did not decrease the concentration
of Ang II in the plasma and renal tissue.
NHE3 mRNA and protein expression
Previously, studies have demonstrated that Ang II
increased NHE3 in human proximal tubular cells (PTCs) (14,15).
To investigate the effect of Ang II on NHE3 in renal tissue of
hypertension-associated renal dysfunction, the mRNA and protein
expression of NHE3 was examined. The mRNA expression is revealed in
Fig. 3A. In the SHR and LOS
groups, the relative expression increased to 2.38 and 1.56,
respectively, as compared with that in the WKY group, and losartan
was able to reduce NHE3 expression. Meanwhile, the protein
expression of NHE3, as revealed in Fig. 3B, was significantly increased in
the SHR group, compared with that in the WKY group. Furthermore,
following eight weeks of losartan treatment, the mRNA expression
levels of NHE3 were decreased.
Effect of losartan on histopathology and
NHE3 expression in renal tissue
The renal tissue was stained using traditional
hematoxylin and eosin methods. Fig.
4A reveals the significant morphological changes in renal
tissue that were observed, including glomerular dilatation and
detachment of basement membrane from glomerulus, in the SHR group
as compared with the WKY group. The damage in the renal tissue was
ameliorated by treatment with losartan. Representative
immunohistochemistry (IHC) images of NHE3 in the renal tissue are
demonstrated in Fig. 4B. NHE3
staining was detected predominantly in the PTCs in the WKY group.
Intense staining was noted in the brush borders and thick ascending
limbs of the loop of Henle, except for the PTCs in the SHR group.
These effects were weakened by losartan treatment. The quantified
immunostaining intensities of NHE3 are revealed in (Fig. 4C).
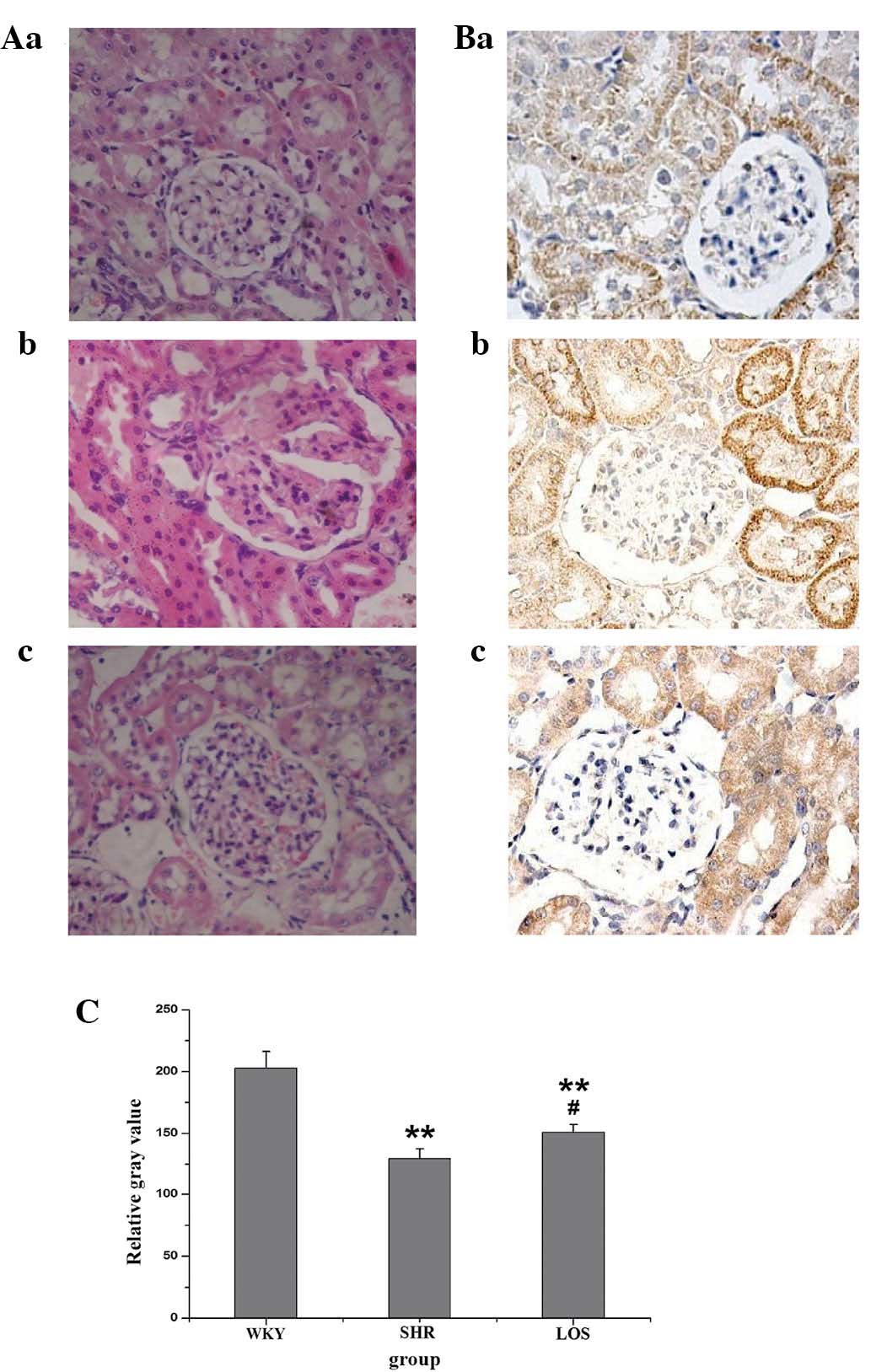 | Figure 4Effect of losartan on histological and
NHE3 changes observed by H&E and IHC staining, respectively.
(A) H&E staining demonstrated the changes of renal tissue in
the (a) WKY group, (b) SHR group and (c) LOS group. (B) IHC
staining demonstrated the changes of NHE3 in renal tissue in the
(a) WKY group, (b) SHR group and (c) LOS group. (C) The relative
gray values of the western blot analysis results of B. Values are
presented as the mean ± standard error of the mean of each group.
Compared with the WKY group, the SHR and LOS groups were
significantly lower (**P<0.01). However, the LOS
group was significantly increased compared with the SHR group
(#P<0.05). LOS group, losartan-treated rats; WKY,
Wistar-kyoto rats; SHR, spontaneously hypertensive rats; SBP,
systolic blood pressure; NHE3, Na+/H+
exchanger 3; IHC, immunohistochemical; H&E, hematoxylin and
eosin. |
SGK1 mRNA and protein expression
The effect of Ang II, via the AT1 receptor, on the
mRNA and protein expression of NHE3 was examined, but the mechanism
underlying this effect remained elusive. Previously, Stevens et
al (16) confirmed that the
Ang II-induced increase in the NHE3 expression was mediated through
SGK1 in human renal PTCs of diabetes mellitus. It was therefore
considered that Ang II may regulate NHE3 through an SGK1-dependent
mechanism in hypertension-associated renal dysfunction (17). The present study investigated the
mRNA and protein expression of SGK1 in renal tissue. The data
(Fig. 5) demonstrated that the
mRNA expression of SGK1 in the SHR and LOS groups was increased to
3.31 and 1.84 compared with that in the WKY group. Furthermore, the
protein expression in the SHR and LOS groups was higher than in the
WKY group. However, it was demonstrated that losartan treatment was
able to significantly decrease the expression of SGK1.
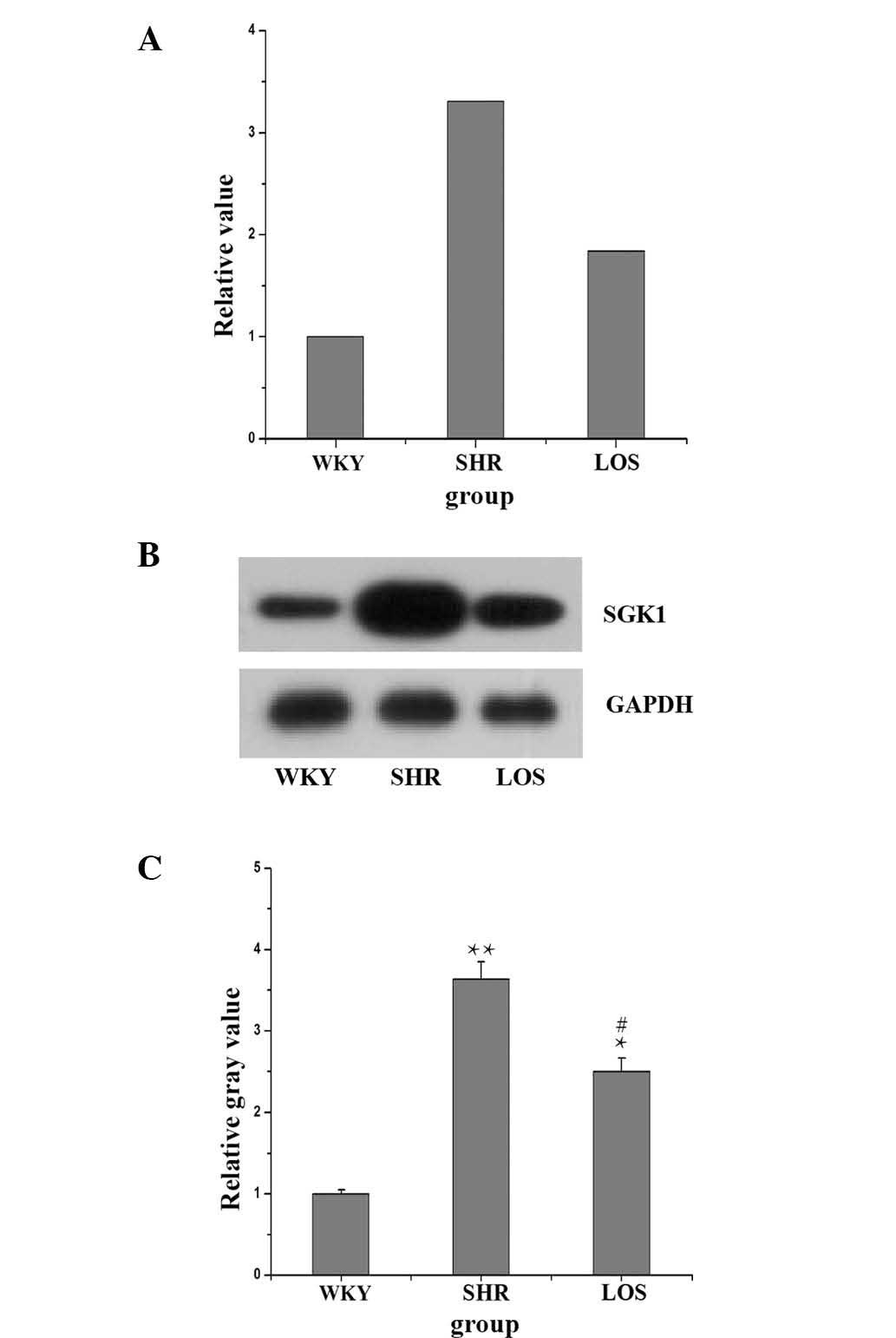 | Figure 5Effect of losartan on the SGK1 mRNA
and protein expression. (A) Quantitative polymerase chain reaction
demonstrating an increase in the SGK1 mRNA expression in the SHR
group compared the WKY group, but losartan attenuated the mRNA
expression of SGK1. (B) Western blot demonstrating an increase in
the SGK1 protein expression in the SHR group compared with that in
the WKY group, while losartan attenuated the protein expression of
SGK1. (C) Relative gray values of western blot analysis result of
B. Values are presented as the mean ± standard error of the mean of
each group. Compared with the WKY group, the SHR and LOS groups
were significantly increased (*P<0.05;
**P<0.01), whilst the LOS group was significantly
reduced compared with the SHR group (#P<0.05). LOS
group, losartan-treated rats; WKY, Wistar-kyoto rats; SHR,
spontaneously hypertensive rats; SBP, systolic blood pressure;
SGK1, serum and glucocorticoid-inducible kinase 1; IHCS,
immunohistochemical staining; H&E, hematoxylin and eosin. |
Discussion
At present, hypertension affects ~1 billion
individuals worldwide, and therefore, it is highly important to
investigate the molecular mechanisms underlying the pathogenesis of
hypertension and the activities of anti-hypertensive drugs.
Numerous different factors contribute to the development of
hypertension, including the RAS, which has an important role in the
regulation and progression of hypertension. The major therapeutic
strategies for the clinical treatment of hypertension are
angiotensin-converting enzyme inhibitors and Ang II receptor
blockers. Losartan is widely used in the clinic and is a typical
representative Ang II receptor blocker, but the molecular mechanism
underlying its effects have remained elusive.
The rats of the SHR and WKY groups have the same
genetic background and used in the present study. In the present
study, it was identified that the baseline BP of spontaneously
hypertensive rats was reduced significantly by losartan following
administration via intragastric injection over two weeks, and the
BP continued to decline after this time-point. This demonstrated
that losartan was effective in reducing the hallmarks of
hypertension, which is consistent with the results of other studies
(18–20). Next, it was confirmed that the
circulatory and tissue levels of Ang II in the SHR group were
higher than those in the WKY group, while Ang II levels in the LOS
group were even higher than those in the SHR group. It was
considered that losartan may exert its effects by increasing Ang II
compensation.
The intra-renal RAS and the effects of RAS blockade
on lead-induced nephropathy in hypertension were also examined. It
has been demonstrated in PTCs of diabetes that Ang II alone at
renal concentrations stimulates NHE3 mRNA and protein expression,
which is mediated by SGK1, as reflected by increased Na+
reabsorption (16,21,22).
This reveals an important role for the intra-renal Ang II-NHE3-SGK1
pathway in regulating Na+ uptake. Therefore, whether
blocking of the intra-renal Ang II by losartan in hypertension may
also affect this pathway was of particular interest in the present
study.
Therefore, using qPCR and western blot analysis, it
was possible to detect the mRNA and protein expression of NHE3 and
SGK1 in renal tissue and demonstrate that both of them were
upregulated in the SHR group compared with the WKY group. It was
also identified that losartan reduced the mRNA and protein
expression of NHE3 and SGK1. Furthermore, the histopathology and
IHC determination of NHE3 in the renal tissue revealed that
hypertension-associated nephropathy was reduced by losartan in the
SHR group. In conclusion, in the present study revealed that the
intra-renal RAS and Ang II-NHE3-SGK1 pathway were activated in
renal tissue under hypertension, and that losartan reduced the
hypertensive effect in the SHR group effectively via this pathway.
The present study provided evidence for a new pharmacodynamic
mechanism of action of losartan in the treatment of
hypertension.
Acknowledgements
This study was funded by grants from the Natural
Science Foundation (grant no. 31160214).
Abbreviations:
|
SHR
|
spontaneously hypertensive rats
|
|
WKY
|
Wistar kyoto rats
|
|
lOS
|
Losartan
|
|
Ang II
|
angiotensin II
|
|
BP
|
blood pressure
|
|
RAS
|
renin angiotensin system
|
|
NHE3
|
sodium/proton exchanger 3
|
|
SGK1
|
serum and glucocorticoid inducible
kinase 1
|
References
|
1
|
Gu D, Reynolds K, Wu X, et al: Prevalence,
awareness, treatment, and control of hypertension in China.
Hypertension. 40:920–927. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griendling KK, Murphy T and Alexander RW:
Molecular biology of the renin-angiotensin system. Circulation.
87:1816–1828. 1993. View Article : Google Scholar
|
|
3
|
Peach MJ: Renin-angiotensin system:
biochemistry and mechanisms of action. Physiol Rev. 57:313–370.
1977.PubMed/NCBI
|
|
4
|
Kobori H, Nangaku M, Navar LG and
Nishiyama A: The intrarenal renin-angiotensin system: from
physiology to the pathobiology of hypertension and kidney disease.
Pharmacol Rev. 59:251–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paul M, Mehr AP and Kreutz R: Physiology
of local renin-angiotensin systems. Physiological Reviews.
86:747–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biemesderfer D, Pizzonia J, Abu-Alfa A, et
al: NHE3: a Na+/H+ exchanger isoform of renal
brush border. Am J Physiol. 265:F736–F742. 1993.PubMed/NCBI
|
|
7
|
Malo ME and Fliegel L: Physiological role
and regulation of the Na+/H+ exchanger. Can J
Physiol Pharmacol. 84:1081–1095. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orlowski J and Grinstein S: Diversity of
the mammalian sodium/proton exchanger SLC9 gene family. Pfluegers
Archiv. 447:549–565. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amemiya M, Loffing J, Lötscher M, et al:
Expression of NHE-3 in the apical membrane of rat renal proximal
tubule and thick ascending limb. Kidney Int. 48:1206–1215. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan I, Batinic-Haberle I and Benov LT:
Effect of potent redox-modulating manganese porphyrin, MnTM-2-PyP,
on the Na+/H+ exchangers NHE-1 and NHE-3 in
the diabetic rat. Redox Rep. 14:236–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pao AC: SGK regulation of renal sodium
transport. Curr Opin Nephrol Hypertens. 21:534–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lang F, Huang DY and Vallon V: SGK, renal
function and hypertension. J Nephrol. 23(Suppl 16): S124–S129.
2010.PubMed/NCBI
|
|
13
|
Smith DH: Comparison of angiotensin II
type 1 receptor antagonists in the treatment of essential
hypertension. Drugs. 68:1207–1225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu L, Dixit MP, Nullmeyer KD, et al:
Regulation of Na+/H+ exchanger-NHE3 by
angiotensin-II in OKP cells. Biochimica et Biochim Biophys Acta.
1758:519–526. 2006.
|
|
15
|
Moe OW: Acute regulation of proximal
tubule apical membrane Na/H exchanger NHE-3: role of
phosphorylation, protein trafficking, and regulatory factors. J Am
Soc Nephrol. 10:2412–2425. 1999.PubMed/NCBI
|
|
16
|
Stevens VA, Saad S, Poronnik P, et al: The
role of SGK-1 in angiotensin II-mediated sodium reabsorption in
human proximal tubular cells. Nephrol Dial Transplant.
23:1834–1843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang D, Sun H, Lang F and Yun CC:
Activation of NHE3 by dexamethasone requires phosphorylation of
NHE3 at Ser663 by SGK1. Am J Physiol Cell Physiol. 289:C802–C810.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu D, Raizada MK, Iyer S, et al: Losartan
versus gene therapy chronic control of high blood pressure in
spontaneously hypertensive rats. Hypertension. 30:363–370. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baumann M, Janssen B, Rob Hermans J, et
al: Renal medullary effects of transient prehypertensive treatment
in young spontaneously hypertensive rats. Acta Physiologica (Oxf).
196:231–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koprdova R, Cebova M and Kristek F:
Long-term effect of losartan administration on blood pressure,
heart and structure of coronary artery of young spontaneously
hypertensive rats. Physiol Res. 58:327–325. 2009.PubMed/NCBI
|
|
21
|
Kaunisto KM and Rajaniemi HJ: Expression
and localization of the Na+/H+ exchanger
isoform NHE3 in the rat efferent ducts. J Androl. 23:237–241.
2002.PubMed/NCBI
|
|
22
|
Karim Z, Gérard B, Bakouh N, et al: NHERF1
mutations and responsiveness of renal parathyroid hormone. N Engl J
Med. 359:1128–1135. 2008. View Article : Google Scholar : PubMed/NCBI
|