Introduction
Diabetic nephropathy (DN) is characterized
functionally by glomerular hyperfiltration and proteinuria, and
histologically by the expansion of the glomerular mesangium, which
is associated with the loss of renal function. The pathological
changes in the kidney include increased glomerular basement
membrane thickness, microaneurysm formation and mesangial nodule
formation (Kimmelsteil-Wilson bodies) (1–4). A
total of >30% of diabetes mellitus patients develop clinically
evident DN, which becomes a major cause of end-stage renal disease
(ESRD) worldwide (1,3). Its molecular pathogenesis is
therefore becoming the target of a growing number of studies.
Numerous factors have been reported to be associated with the
development of DN and a large number of investigations are involved
in therapeutic studies of the condition (4–7). One
study revealed that treatment with vildagliptin + telmisartan
controlled blood pressure, renovascular structural and biochemical
parameters in diabetic neuropathy rats (4). Endo et al (8) performed a randomized, open trial on
162 type 2 diabetic patients and found that probucol suppressed the
progression of DN and renal dysfunction events.
Tanshinone IIA (TIIA) is a major
phenanthrene-quinone isolated from Salvia miltiorrhiza
Bunge, which is a Traditional Chinese Medicine (TCM) that is
commonly used in various diseases and types of cancer (9–12).
More importantly, a study by Kim et al (13) demonstrated that TIIA has protective
effects during the progression of DN and may be a potential drug
candidate for the prevention of DN. As a multi-functional agent in
TCM, TIIA has a fundamental role in cellular responses to
extracellular stimuli. For example, TIIA protects cardiomyocytes in
part through B-cell lymphoma 2/B-cell lymphoma 2-associated X
protein and Akt-signaling pathways (14–15),
is able to produce an inhibitory effect on the sterol regulatory
element-binding transcription factor 1 pathway through the
phosphoinositide 3-kinase/Akt signaling pathway (16) and significantly inhibits the
expressions of numerous factors, including transforming growth
factor β (TGFβ), in a rat model of DN (13). However, only the primary action of
TIIA on DN has been studied, and the mechanisms underlying how TIIA
ameliorates DN require further elucidation.
There is an increasing amount of evidence that TGF-β
induces and promotes inflammatory responses via activation of
nuclear factor (NF)-κB in various diseases. In one study, TGF-β
induced p65 acetylation to enhance bacteria-induced NF-κB
activation (17). Several studies
have also indicated that TGF-β-Smad signaling mediates the
activation of NF-κB in human airway epithelial cells (18–19).
In addition, the clinical and pathological aspects of diabetic
neuropathies are associated with inflammatory phenomena (20–21).
Therefore, the underlying mechanism of the activity of TIIA on DN
may proceed via the TGFβ/p65 pathway. It was hypothesized that TIIA
may ameliorate DN by regulating TGF-β and p65. The present study
aimed to investigate the functions of TGF-β and p65 during TIIA
therapy of DN using glucose-induced HBZY-1 cells.
Materials and methods
TIIA treatment
TIIA was purchased from Nanjing Zelang Medical
Technological Co. Ltd (Jiangsu, China). The rat mesangial HBZY-1
cell line was obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The cells were cultured in RPMI-1640
(Gibco-BRL, Carlsbad, CA, USA) medium containing 10% fetal bovine
serum (FBS; HyClone, Logan, UT, USA) under standard conditions
(37°C, 5% CO2). The HBZY-1 cells were seeded in 96-well
plates at 2.0×104 cells/well, and were cultured in 100
μl Dulbecco’s modified Eagle’s medium (DMEM) with or without high
glucose (HG) and TIIA. To determine the safe dosages of TIIA to be
used in further experiments in this study, the cells were randomly
divided into six groups: i) Control; ii) high glucose (HG, 30
mmol/l); iii) HG + TIIA (10 μM); iv) HG + TIIA (20 μM); v) HG +
TIIA (40 μM) and vi) HG + TIIA (80 μM). At 48 h following treatment
with TIIA, the cell viability was examined by the MTT assay.
Small interfering (si)RNA
transfection
HBZY-1 cells were plated onto six-well plates
(1×105 cells/well), maintained in DMEM containing 10%
FBS for 12 h and transfected with a mixture containing Opti-MEM, 8
μl/well Lipofectamine™ 2000 (Invitrogen, San Diego, CA, USA) and
either 0.5 μg/well scrambled siRNA (mock) or siRNAs (smart pool;
Guangzhou RiboBio Co., Ltd., Guangzhou, Guangdong, China) for 6 h.
The sequences of these siRNAs are listed in Table I. To detect the function of TGFβ
and p65 in the HBZY-1 cells induced by HG, the cells were randomly
divided into four groups: i) Control, ii) HG + mock; iii) HG +
si-TGFβ; and iv) HG + si-p65. Following 48 h, the cell viability
was examined by the MTT assay.
 | Table IRNA oligos of sense and anti-sense
strands of small interfering RNA. |
Table I
RNA oligos of sense and anti-sense
strands of small interfering RNA.
| Gene | Strand name | RNA oligos |
|---|
| NF-κB | p65 p65 sense strand
A |
GAAGAAGAGUCCUUUCAAUtt |
| p65 anti-sense strand
A |
AUUGAAAGGACUCUUCUUCtt |
| p65 sense strand
B |
CCAUCAACUUUGAUGAGUUtt |
| p65 anti-sense strand
B |
AACUCAUCAAAGUUGAUGGtt |
| p65 sense strand
C |
GCAUUAACUUCCCUGAAGUtt |
| p65 anti-sense
strand C |
ACUUCAGGGAAGUUAAUGCtt |
| TGFβ | TGFβ sense strand
A |
CUGCUCUUGUGACAGCAAAGAtt |
| TGFβ anti-sense
strand A |
UCUUUGCUGUCACAAGAGCAGtt |
| TGFβ sense strand
B |
UUCAGCUCCACAGAGAAGAACtt |
| TGFβ anti-sense
strand B |
GUUCUUCUCUGUGGAGCUGAAtt |
MTT assay
The HBZY-1 cells treated with or without HG, siRNA
or TIIA were collected and resuspended in 100 μl DMEM. A total of
20 μl MTT (5 mg/ml) was added, followed by incubation for 4 h at
37°C. Finally, the samples were recorded at 490 nm using a
microplate reader. The wells with only MTT served as blank
controls. At least three independent experiments were
performed.
Animals
Male Sprague Dawley (SD) rats (6–8 weeks old;
weighing, 180–200 g) were obtained from the Guangdong Medical
Laboratory Animal Center (Guangdong, China). The rats were divided
into three groups: Normal control rats (n=8), DN rats (n=8) and
TIIA rats (n=8). DN and TIIA rats were injected intraperitoneally
with 45 mg/kg streptozotocin (STZ; Sigma, St. Louis, MO, USA),
while the normal control rats received physiological saline (PS).
Following seven weeks, the TIIA rats were administered an
intramuscular injection, at a dose of 8 mg/kg, of TIIA daily for
another three weeks, whereas the other rats received PS only. All
of the animals were fed with high-fat food. After the animals were
sacrificed, fresh kidney samples were stored in formaldehyde
solution for histopathological observation. The remaining kidneys
were stored at −80°C for later analysis. The experimental procedure
was approved by the Animal Experimentation Ethics Committee at The
Guangzhou University of Chinese Medicine (Guangzhou, Guangdong,
China).
Urine analyses
All of the animals were housed in metabolic cages
for 24 h to obtain urine at seven weeks (one day prior to TIIA
treatment) and 10 weeks (one day prior to sacrifice) for the
measurement of urine protein. The 24 h urinary protein excretion
was assessed by the Coomassie brilliant blue method.
Histopathology
The cell morphology was examined in formalin-fixed,
paraffin-embedded kidney sections (5 μm) stained with
hematoxylin-eosin (H&E) (22).
The histopathological scores were examined by glomerular mesangial
expansion, mesangial matrix increase, interstitial mononuclear
cells and extracellular matrix accumulation. Histological analysis
was performed using a light microscope (Leica DM400 B, Bannockburn,
IL, USA).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from the frozen kidney
samples using the TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) following the manufacturer’s instructions. The
mRNA expression of TGFβ and p65, and β-actin was detected by qPCR
utilizing a 7500 detector (ABI Prism 7500; Applied Biosystems,
Foster City, CA, USA) according to manufacturer’s instructions. PCR
was performed with 100 ng total RNA using one-step SYBR PrimeScript
RT-PCR kit (TakaRa, Tokyo, Japan) as follows: 42°C for 5 min, 94°C
for 10 sec followed by 40 cycles at 94°C for 5 sec, 60°C for 35
sec, with a final extension at 72°C for 5 min. The primers used in
the present study were as follows: TGFβ forward
5′-TGCTCTTGTGACAGCAAAGATAA-3′ and reverse
5′-CTCTGTGGAGCTGAAGCAATAGT-3′; p65 forward
5′-CACCAAAGACCCACCTCACC-3′ and reverse
5′-GGACCGCATTCAAGTCATAGTC-3′; β-actin forward
5′-GACAGGATGCAGAAGGAGATTACT-3′ and reverse
5′-TGATCCACATCTGCTGGAAGGT-3′. The gene expression of interest was
determined using the 2−ΔΔ CT method (23). The expression levels of all the
transcripts were normalized to that of the housekeeping gene
β-actin in the same tissue.
Western blot analysis
The proteins extracted from kidney tissues were
examined using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Waltham, MA, USA) and analyzed by western blot
analysis. Antibodies used in the present study included: Rabbit
polyclonal TGF-β (sc-146, diluted by 1:1,000), p65 (sc-101749,
diluted by 1:2,000) and β-actin (sc-130657, diluted by 1:5,000).
All antibodies were purchased from Santa Cruz (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Statistical analysis
The presented data were analyzed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was
used to analyze the intergroup differences. The differences between
the groups were compared using a t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
TIIA inhibits HBZY-1 cell
proliferation
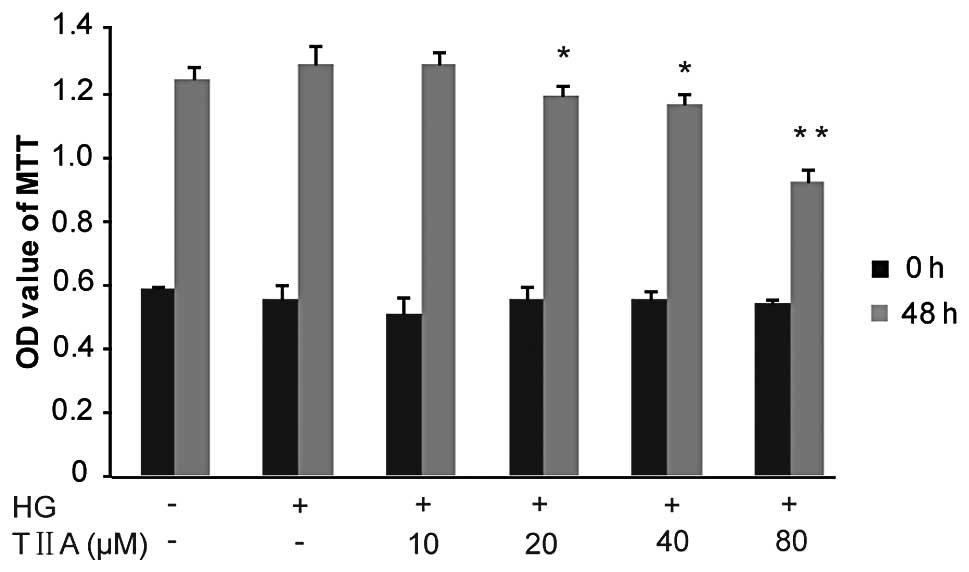
The effects of TIIA on HBZY-1 cell proliferation
were investigated using the MTT assay. Firstly, it was identified
that the proliferative ability of the HBZY-1 cells treated with HG
was slightly increased compared with that of the control group
(although P>0.05). TIIA inhibited the HG-induced HBZY-1
proliferation in a concentration-dependent manner. The data
revealed that, following 48 h, TIIA at 20 and 40 μM (P<0.05) as
well as 80 μM (P<0.01) had a significant growth-inhibitory
effect on the HBZY-1 cells (Fig.
1).
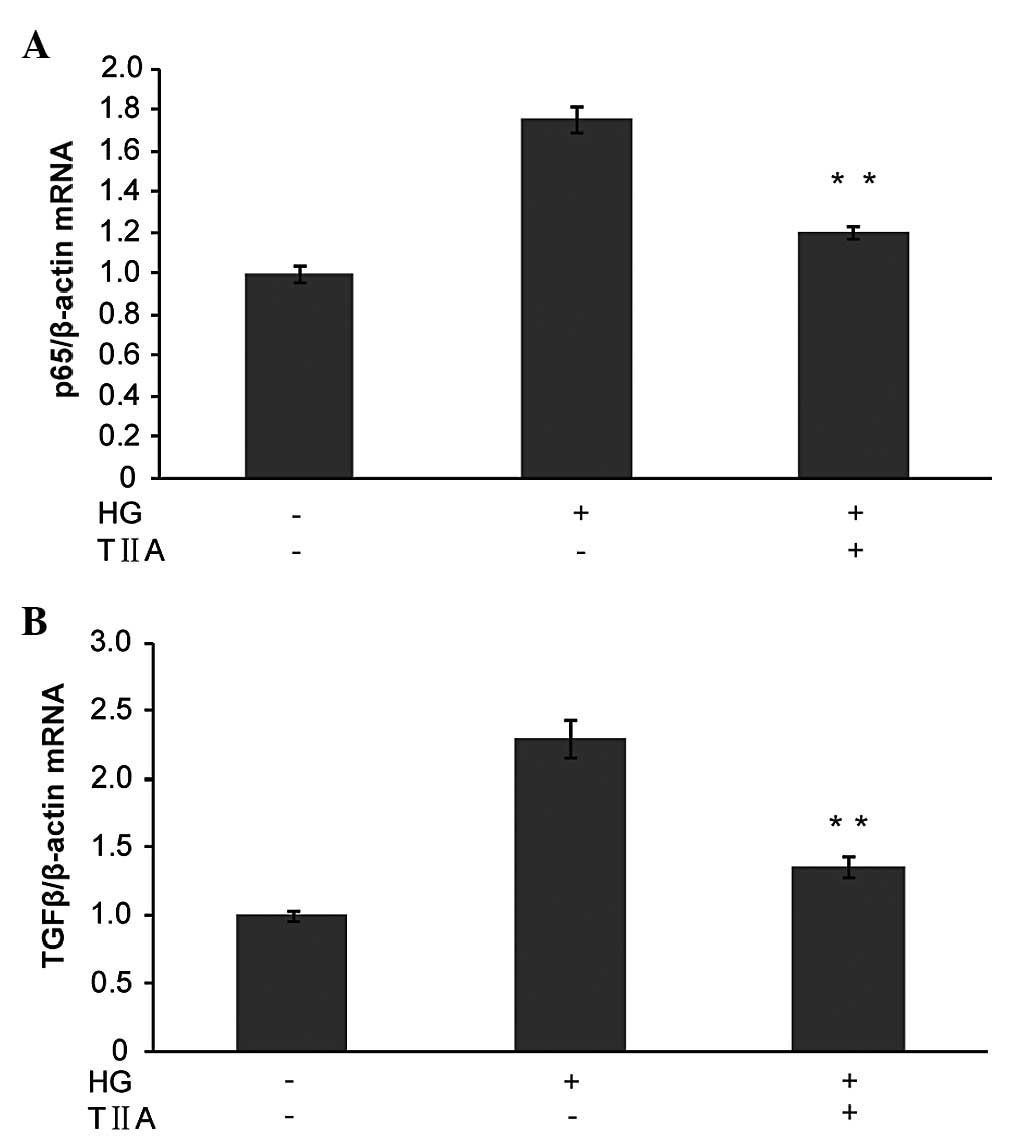
TGFβ and p65 are suppressed by TIIA in
HBZY-1 cells
Previous studies demonstrated that TGFβ and NF-κB
increased in DN and had a key role in cell proliferation (24). Therefore, next, the effects of TIIA
on TGFβ and NF-κB in HBZY-1 cells were examined using qPCR. As
demonstrated in Fig. 2, TGFβ and
p65 were significantly upregulated in the HG-induced group compared
with the control cells. However, TIIA significantly inhibited the
increase in TGFβ and p65 in the HBZY-1 cells induced by high
glucose. This result may indicate that TGFβ and p65 were involved
in the process by which TIIA inhibited HBZY-1 cell
proliferation.
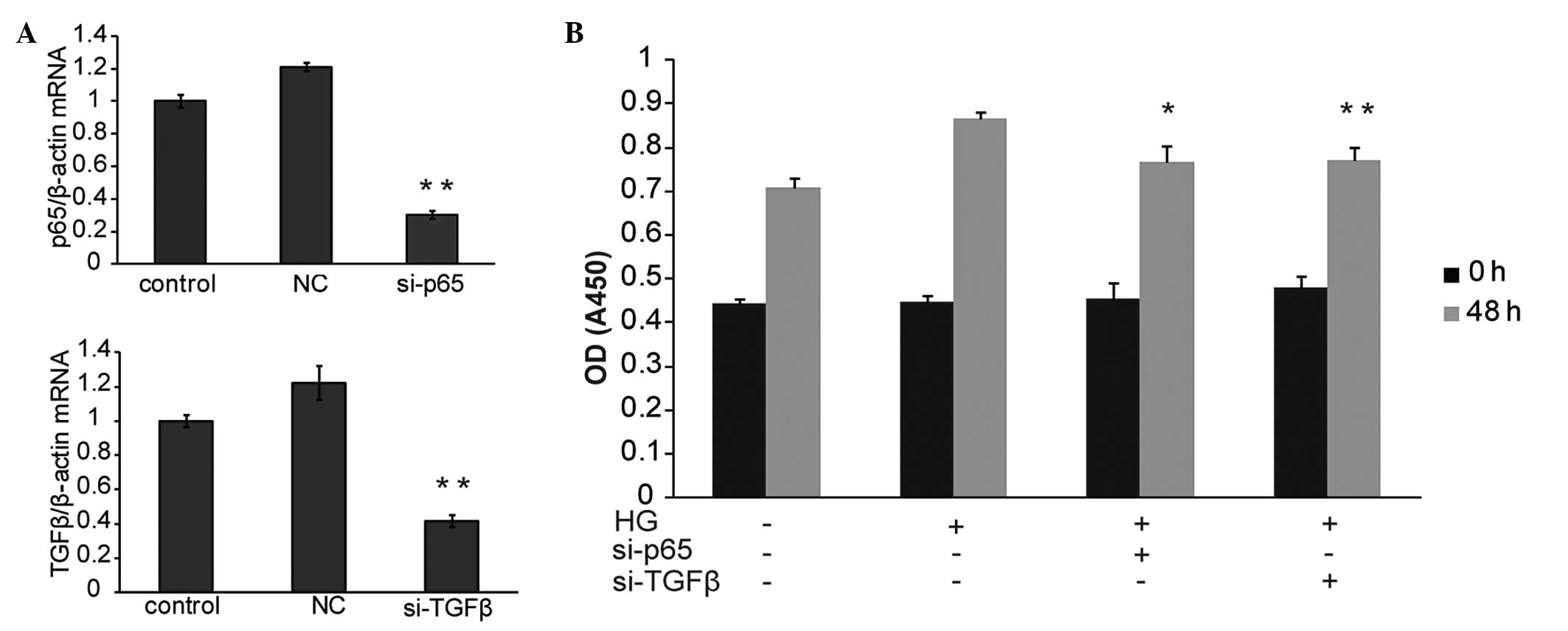
si-TGFβ and si-p65 inhibit the
proliferation of glucose induced HBZY-1 cells
To determine whether TGFβ and p65 were able to
inhibit HBZY-1 cell proliferation, their functions were
investigated using RNA interference and subsequent MTT assay. qPCR
data demonstrated that TGFβ and p65 were significantly inhibited by
the designed si-TGFβ and si-p65 separately (Fig. 3A). Of note, the MTT assay revealed
that the knockdown of either TGFβ or p65 significantly inhibited
HBZY-1 cell proliferation as compared with the HG-only treated
group (Fig. 3B). The above data
suggested that TGFβ and p65 were necessary for HBZY-1
proliferation. TGFβ and p65 may therefore be involved in the
intracellular mechanisms underlying the effect of TIIA on HBZY-1
cell proliferation.
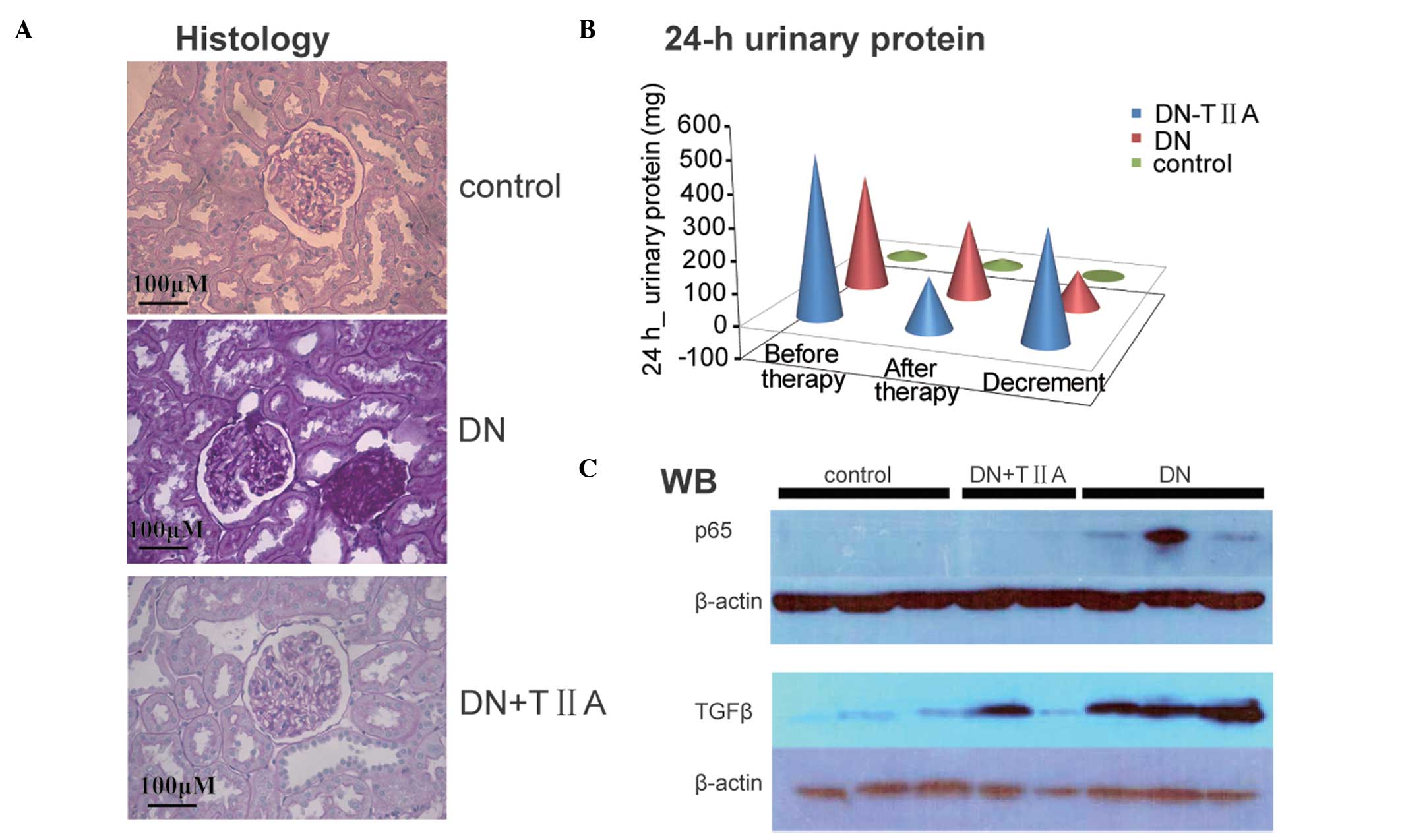
Therapeutic effect of TIIA on the
established DN rat
To determine the efficiency of TIIA therapy in
vivo, DN rat models induced by STZ were established. The 24 h
urinary protein excretion of the DN rats and control rats was
detected. In the DN rats group, the average 24 h urinary protein
excretion reached 365 mg following STZ injection for seven weeks
and then decreased to 247 mg during the subsequent physiological
saline treatment over three weeks. However, in the TIIA therapy
group, a more significant reduction in the average 24 h urinary
protein excretion was identified, from 503 mg to 161 mg during the
TIIA treatment over three weeks. This data demonstrated that TIIA
contributed to a larger decrement of average 342 mg urinary protein
compared with 118 mg in the rats that received PS (Fig. 4B). Furthermore, the morphological
changes of the kidney were also examined. The kidney sections were
stained with H&E for histochemical determination of the renal
compartments. The DN rats developed renal lesions that consisted of
an increasing glomerular mesangial matrix accumulation. In the TIIA
treatment group, a moderate decrease in mesangial cellularity and
hypertrophy of mesangial cells compared with control DN rats was
observed (Fig. 4A).
TIIA inhibits the expression of TGFβ and
p65 in DN rats
Since TGFβ and p65 are functional during the
progression of DN in rats, next, whether they were involved in the
beneficial effects of TIIA therapy was investigated. The effects of
TIIA on the protein expression levels of TGFβ and p65 in the rat
kidneys were assessed by western blot analysis. As demonstrated in
Fig. 4C, the western blot results
indicated that both TGFβ and p65 proteins were firstly upregulated
in DN rats induced by STZ compared with normal control rats.
However, following treatment of the DN rats with TIIA for three
weeks, it was identified that TGFβ and p65 levels in the kidney
decreased compared with those in the DN rats treated with PS.
Discussion
DN is a progressive kidney disease characterized by
long-term damage to the kidneys as a result of long-term poorly
controlled diabetes. It is the most common diabetes mellitus in
industrialized countries and >20 million people suffer from DN
with a marked range of clinical manifestations (20).
DN is considered to result from the interactions
between a wide range of metabolic and haemodynamic factors that
induce oxidative stress, polyol pathway flux, hexosamine flux and
the accumulation of advanced glycated end-products (AGEs) (25,26).
Therefore, therapeutic strategies that target DN have various
mechanisms of action. The majority of previous treatment strategies
have focused on the control of hyperglycemia. For example,
prednisone was used at a dose of 0.75mg/kg/day, with daily control
of glycaemia in patients (20).
The major focus of newer treatments appears to be on newer targets
and is associated with glucose-dependent pathways as a result of
diabetes. For example, drugs that inhibit the formation of AGEs
include aminoguanidine, ALT-946, pyridoxamine and OPB-9195. In
other studies, prostaglandins are suggested to be involved in the
development of nephropathy. Treatment with cyclooxygenase-2
inhibitors, prostacyclin analogues and thromboxane A2 antagonists
have also been demonstrated to be beneficial (6,27–29).
The NFκB inhibitor pyrrolidine dithiocarbamate has been studied and
has been demonstrated to confer renoprotection (30,31).
Miyata et al (32)
suggested that recent approaches targeting oxygen biology may offer
novel treatments for DN. A study by Kim et al (13) demonstrated that TIIA may have
protective effects during the progression of DN and may be a
potential drug for the prevention of DN. The present study revealed
that renal hypertrophy and 24 h urinary protein excretion were
partly recovered in TIIA-treated rats, which further confirms that
TIIA ameliorated the pathological changes in DN rats.
Various theories have proposed that DN is associated
with oxygen biology, including hypoxia, oxidative stress and
dyserythropoiesis (32).
Previously, experimental studies demonstrated that a broad range of
anomalies concerning the pathogenesis of DN, including proteinuria,
genetics, race, hypoxia, ischemia and inflammation. Sustained
inflammation may be the initiator to activate tissue fibrosis
progression in DN (33,34). TGF-β has been demonstrated to have
an essential role in fibrosis. Shaker and Sadik (35) demonstrated that serum TGF-β may
also be a prognostic marker of DN. TGF-βs modulate the bodies’
overall immune response by affecting different receptors and
downstream signaling; for example, the inhibition of TGF-βs or the
TGF-β-Smad signaling pathway has been demonstrated to exhibit
anti-fibrotic effects (36–40).
In addition, Smads also interact with other signaling pathways,
including NF-κB pathways (19,41).
Liu et al (42) found that
TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal
inflammation were involved in a mouse model of obstructive
nephropathy. Ka et al (24)
demonstrated that the TGF-β/Smad and NF-κB signaling pathways were
inhibited during therapy for type II DN. Pioglitazone attenuates DN
by downregulating TGF-β and NFκB in type II diabetic rats (43). Kim et al (13) identified that TGF-β was decreased
in DN rat models treated with TIIA. In the present study, the
results revealed that TGFβ and p65 mRNA and protein expression were
upregulated in DN rats and downregulated following treatment with
TIIA. Furthermore, similar results were observed in HBZY-1 cells.
It was identified that TGFβ and p65 were suppressed by treatment
with TIIA. To further examine the interaction between TGFβ and p65
in this process, si-TGFβ or si-p65 were transfected into cells
induced by HG. The MTT assay revealed that the knockdown of either
TGFβ or p65 inhibited HBZY-1 cell proliferation, consistent with
the effect of TIIA. In conclusion, the results demonstrated that
TGFβ and of p65 were downregulated by TIIA in HBZY-1 cells. The
renoprotective effect of TIIA on DN may therefore proceed via the
suppression of TGFβ and p65. These results may provide valuable
information for the development of novel therapeutic strategies for
DN.
Acknowledgements
This study was supported by the Science and
Technology Program in the Social Development of Guangdong Province
(grant no. 20120318092). The authors thank HuanHuan Luo (Editorial
Board, Journal of New Chinese Medicine, Guangzhou, China) for
contributing to the language reviewing of this manuscript.
References
|
1
|
Breyer MD, Böttinger E, Brosius FC III, et
al: AMDCC: Mouse models of diabetic nephropathy. J Am Soc Nephrol.
16:27–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mauer SM, Steffes MW, Ellis EN, et al:
Structural-functional relationships in diabetic nephropathy. J Clin
Invest. 74:1143–1155. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brosius FC III, Alpers CE, Bottinger EP,
Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M,
Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K,
Takahashi N and Takahashi T: Animal Models of Diabetic
Complications Consortium: Mouse models of diabetic nephropathy. J
Am Soc Nephrol. 20:2503–2512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma AK, Kanawat DS, Mishra A, Dhakad
PK, Sharma P, Srivastava V, Joshi S, Joshi M, Raikwar SK, Kurmi MK
and Srinivasan BP: Dual therapy of vildagliptin and telmisartan on
diabetic nephropathy in experimentally induced type 2 diabetes
mellitus rats. J Renin Angiotensin Aldosterone Syst. Feb
8–2013.(Epub ahead of print).
|
|
5
|
Moresco RN, Sangoi MB, De Carvalho JA,
Tatsch E and Bochi GV: Diabetic nephropathy: traditional to
proteomic markers. Clin Chim Acta. 421:17–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goh SY, Jasik M and Cooper ME: Agents in
development for the treatment of diabetic nephropathy. Expert Opin
Emerg Drugs. 13:447–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inagi R, Yamamoto Y, Nangaku M, Usuda N,
Okamato H, Kurokawa K, van Ypersele de Strihou C, Yamamoto H and
Miyata T: A severe diabetic nephropathy model with early
development of nodule-like lesions induced by megsin overexpression
in RAGE/iNOS transgenic mice. Diabetes. 55:356–366. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Endo K, Saiki A, Yamaguchi T, Sakuma K,
Sasaki H, Ban N, Kawana H, Nagayama D, Nagumo A, Ohira M, Oyama T,
Murano T, Miyashita Y, Yamamura S, Suzuki Y, Shirai K and Tatsuno
I: Probucol suppresses initiation of chronic hemodialysis therapy
and renal dysfunction-related death in diabetic nephropathy
patients: Sakura Study. J Atheroscler Thromb. 20:494–502. 2013.
View Article : Google Scholar
|
|
9
|
Yuan SL, Wang XJ and Wei YQ: Anticancer
effect of tanshinone and its mechanisms. Ai Zheng. 22:1363–1366.
2003.(In Chinese).
|
|
10
|
Jang SI, Jeong SI, Kim KJ, Kim HJ, Yu HH,
Park R, Kim HM and You YO: Tanshinone IIA from Salvia
miltiorrhiza inhibits inducible nitric oxide synthase
expression and production of TNF-alpha, IL-1beta and IL-6 in
activated RAW 264.7 cells. Planta Med. 69:1057–1059.
2003.PubMed/NCBI
|
|
11
|
Wang JW and Wu JY: Tanshinone biosynthesis
in Salvia miltiorrhiza and production in plant tissue
cultures. Appl Microbiol Biotechnol. 88:437–449. 2010.
|
|
12
|
Kwak HB, Yang D, Ha H, Lee JH, Kim HN, Woo
ER, Lee S, Kim HH and Lee ZH: Tanshinone IIA inhibits osteoclast
differentiation through down-regulation of c-Fos and NFATc1. Exp
Mol Med. 38:256–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SK, Jung KH and Lee BC: Protective
effect of Tanshinone IIA on the early stage of experimental
diabetic nephropathy. Biol Pharm Bull. 32:220–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong HJ, Liu JC, Chen PY, Chen JJ, Chan P
and Cheng TH: Tanshinone IIA prevents doxorubicin-induced
cardiomyocyte apoptosis through Akt-dependent pathway. Int J
Cardiol. 157:174–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao J, Yang G, Pi R, Li R, Wang P, Zhang
H, Le K, Chen S and Liu P: Tanshinone IIA protects neonatal rat
cardiomyocytes from adriamycin-induced apoptosis. Transl Res.
151:79–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song DY, Huang QH, Zhou BR, Xu Y, Yin ZQ,
Permatasari F and Luo D: Tanshinone IIA inhibits the
dihydrotestosterone-induced secretion of lipids and activation of
sterol regulatory element binding protein-1 in HaCaT cells. Exp
Ther Med. 4:339–343. 2012.
|
|
17
|
Ishinaga H, Jono H, Lim JH, Kweon SM, Xu
H, Ha UH, Koga T, Yan C, Feng XH, Chen LF and Li JD: TGF-beta
induces p65 acetylation to enhance bacteria-induced NF-kappaB
activation. EMBO J. 26:1150–1162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jono H, Shuto T, Xu H, Kai H, Lim DJ, Gum
JR Jr, Kim YS, Yamaoka S, Feng XH and Li JD: Transforming growth
factor-beta-Smad signaling pathway cooperates with NF-kappa B to
mediate nontypeable Haemophilus influenzae-induced MUC2
mucin transcription. J Biol Chem. 277:45547–45557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mikami F, Lim JH, Ishinaga H, Ha UH, Gu H,
Koga T, Jono H, Kai H and Li JD: The transforming growth
factor-beta-Smad3/4 signaling pathway acts as a positive regulator
for TLR2 induction by bacteria via a dual mechanism involving
functional cooperation with NF-kappaB and MAPK phosphatase
1-dependent negative cross-talk with p38 MAPK. J Biol Chem.
281:22397–22408. 2006.
|
|
20
|
Said G: Inflammatory phenomena in diabetic
neuropathies. Journ Annu Diabetol Hotel Dieu. 251–255. 2006.(In
French).
|
|
21
|
Little AA, Edwards JL and Feldman EL:
Diabetic neuropathies. Pract Neurol. 7:82–92. 2007.
|
|
22
|
Girardi JM, Farias RE, Ferreira AP and
Raposo NR: Rosuvastatin prevents proteinuria and renal inflammation
in nitric oxide-deficient rats. Clinics (Sao Paulo). 66:1457–1462.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
24
|
Ka SM, Yeh YC, Huang XR, Chao TK, Hung YJ,
Yu CP, Lin TJ, Wu CC, Lan HY and Chen A: Kidney-targeting Smad7
gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear
factor κB (NF-κB) signalling pathways, and improves diabetic
nephropathy in mice. Diabetologia. 55:509–519. 2012.PubMed/NCBI
|
|
25
|
Yamagishi S, Fukami K, Ueda S and Okuda S:
Molecular mechanisms of diabetic nephropathy and its therapeutic
intervention. Curr Drug Targets. 8:952–959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Forbes JM, Fukami K and Cooper ME:
Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin
Endocrinol Diabetes. 115:69–84. 2007. View Article : Google Scholar
|
|
27
|
Kujubu DA, Fletcher BS, Varnum BC, Lim RW
and Herschman HR: TIS10, a phorbol ester tumor promoter-inducible
mRNA from Swiss 3T3 cells, encodes a novel prostaglandin
synthase/cyclooxygenase homologue. J Biol Chem. 266:12866–12872.
1991.
|
|
28
|
Cheng HF, Wang CJ, Moeckel GW, Zhang MZ,
McKanna JA and Harris RC: Cyclooxygenase-2 inhibitor blocks
expression of mediators of renal injury in a model of diabetes and
hypertension. Kidney Int. 62:929–939. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Owada A, Suda S and Hata T: Effect of
long-term administration of prostaglandin I(2) in incipient
diabetic nephropathy. Nephron. 92:788–796. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou X, Wang B, Zhu L and Hao S: A novel
improved therapy strategy for diabetic nephropathy: targeting AGEs.
Organogenesis. 8:18–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding H, Li F, Xu M, Deng Y, Deng Q, Zhu Z,
Cheng H, Fu Z and Wang Y: The effect of inhibiting nuclear
factor-kappa B on the diabetic nephropathy. Zhonghua Nei Ke Za Zhi.
41:605–609. 2002.(In Chinese).
|
|
32
|
Miyata T, Suzuki N and van Ypersele de
Strihou C: Diabetic nephropathy: are there new and potentially
promising therapies targeting oxygen biology? Kidney Int.
84:693–702. 2013. View Article : Google Scholar
|
|
33
|
Fernández Fernández B1, Elewa U,
Sánchez-Niño MD, Rojas-Rivera JE, Martin-Cleary C, Egido J and
Ortiz A: 2012 update on diabetic kidney disease: the expanding
spectrum, novel pathogenic insights and recent clinical trials.
Minerva Med. 103:219–234. 2012.PubMed/NCBI
|
|
34
|
Kanasaki K, Taduri G and Koya D: Diabetic
nephropathy: the role of inflammation in fibroblast activation and
kidney fibrosis. Front Endocrinol (Lausanne). 4:72013.PubMed/NCBI
|
|
35
|
Shaker OG and Sadik NA: Transforming
growth factor beta 1 and monocyte chemoattractant protein-1 as
prognostic markers of diabetic nephropathy. Hum Exp Toxicol.
32:1089–1096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hills CE and Squires PE: The role of TGF-β
and epithelial-to mesenchymal transition in diabetic nephropathy.
Cytokine Growth Factor Rev. 22:131–139. 2011.
|
|
37
|
Sharma K, Ix JH, Mathew AV, Cho M,
Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA,
Donohue M, Ramachandrarao S, Xu R, Fervenza FC and Kopp JB:
Pirfenidone for diabetic nephropathy. J Am Soc Nephrol.
22:1144–1151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067.
2011.
|
|
39
|
Choi K, Lee K, Ryu SW, Im M, Kook KH and
Choi C: Pirfenidone inhibits transforming growth factor-β1-induced
fibrogenesis by blocking nuclear translocation of Smads in human
retinal pigment epithelial cell line ARPE-19. Mol Vis.
18:1010–1020. 2012.
|
|
40
|
Meng XM, Chung AC and Lan HY: Role of the
TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond).
124:243–254. 2013.
|
|
41
|
Lan HY and Chung AC: TGF-β/Smad signaling
in kidney disease. Semin Nephrol. 32:236–243. 2012.
|
|
42
|
Liu Z, Huang XR, Chen HY, Penninger JM and
Lan HY: Loss of angiotensin-converting enzyme 2 enhances
TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal
inflammation in a mouse model of obstructive nephropathy. Lab
Invest. 92:650–661. 2012.PubMed/NCBI
|
|
43
|
Ko GJ, Kang YS, Han SY, Lee MH, Song HK,
Han KH, Kim HK, Han JY and Cha DR: Pioglitazone attenuates diabetic
nephropathy through an anti-inflammatory mechanism in type 2
diabetic rats. Nephrol Dial Transplant. 23:2750–2760. 2008.
View Article : Google Scholar : PubMed/NCBI
|