Introduction
Stem cells possess the potential to differentiate
into specific cell phenotypes, and numerous recent studies have
focused on stem cell properties. Bone marrow mesenchymal stem cells
(BMSCs) are of interest as these cells can be easily isolated from
a small aspirate of bone marrow and have few ethical limitations
(1). Therefore, BMSCs are
considered the most suitable stem cells for research (1). A number of studies have induced BMSC
differentiation into different cell types in vitro,
including chondrocytes, osteoblasts, adipocytes and myoblasts
(2). Another area of research
concerns the replacement of impaired tissue through transplantation
of BMSCs (3,4). Studies have indicated that BMSCs
significantly enhance motor function recovery when transplanted
into animal models with cerebral infarction, subcortical capsular
infarction and traumatic brain injury (3,4).
BMSCs have shown great promise in tissue repair; however, the poor
viability of transplanted BMSCs has limited the therapeutic
potential (5,6). Therefore, enhancing the survival of
BMSCs requires further investigation. Certain studies have found
that continuous perfusion stimulates the proliferation of human
BMSCs. Furthermore, reducing the fluid flow rate while maintaining
a constant peak fluid shear stress is associated with alterations
in BMSC proliferation (7,8). These studies suggest that
proliferation of BMSCs is affected by mechanical stimulation.
Infrasound refers to inaudible noise at a frequency
<20 Hz and may be considered to consist of mechanical vibrations
that are difficult to detect with the human ear (9). The effects of infrasound are
disputed, with some researchers suggesting that infrasound is
hazardous to the human body (10,11).
Infrasound frequencies at 8 Hz, 130 dB, have been demonstrated to
impair the rat myocardium and induce elevated levels of
intracellular calcium ions in the hippocampal cells of the rat
brain (10,11). However, a previous study has shown
that infrasound with the sound pressure level <90 dB improved
the motor function of rats following middle cerebral artery
occlusion (12). Thus infrasound
with a high sound pressure level >90 dB may impair tissue, while
low pressure levels <90 dB may be beneficial. The aim of the
present study was to investigate whether infrasound with a low
sound pressure level <90 dB alters the proliferation and
apoptosis of BMSCs.
Survivin is a member of the inhibitor of apoptosis
(IAP) family and is specifically expressed at the G2/M phase of the
cell cycle. Histological examination has revealed that survivin is
expressed in growing tissues, such as the thymus, testis and the
intestine of adult mice, and numerous embryonic tissues, but not in
mature tissues (13). IAP proteins
comprise a highly conserved gene family that prevent cell death in
response to a variety of stimuli (14). Survivin has been implicated in
multiple essential functions, including cell division, programmed
cell death or apoptosis, the cellular stress response and
checkpoint mechanisms of genomic integrity (15). One study found ~83% BMSCs expressed
survivin, with more marked staining in the nucleus than in the
cytoplasm (16).
In the present study, the effects of infrasound on
the proliferation and apoptosis of BMSCs were analyzed. Evidence
indicates that survivin may mediate changes in BMSC biological
behavior following infrasound treatment (14–16).
Thus the present study was designed to investigate the association
of survivin expression levels with the effects of infrasound on
BMSCs at the molecular level.
Materials and methods
Infrasound device
The infrasound device (Chi-8™) used in the present
study was manufactured by the Chi Institute (San Juan Capistrano,
CA, USA). The device consisted of two sections containing the
transmitting probe and the mainframe. The infrasound device
consists of three options, for which option thre was used for this
study, producing a frequency of 4–20 Hz and a sound pressure level
of 79–86 dB. A previous study suggested that the this setting
exerted a protective effect on rats with cerebral
ischemia-reperfusion injury (12).
Animal experiments and ethical
approval
BMSCs were harvested from female Sprague Dawley rats
(weight, 100 g; age, 4 weeks) provided by the Animal Center of the
Southern Medical University (Guangzhou, China). All experimental
procedures on the rats were approved by the Animal Ethics Committee
of Nanfang Hospital (Permit number: NFYY20120128; Guangzhou,
China). The rats were anesthetized with 10% chloral hydrate and
then sacrificed by cervical dislocation.
BMSC harvest and culture
The rat femora and tibiae were aseptically excised
and the epiphyses of the bones were removed. The bone marrow was
flushed from the shaft with Dulbecco’s modified Eagle’s medium
(DMEM)/F12 (Hyclone, Waltham, MA, USA) using a 20-gauge needle. The
bone marrow suspension was disaggregated by pipetting several times
and the cells were collected by centrifugation (2,200 × g, 5 min)
(17). The cells were then
cultured in DMEM/F12 with 10% fetal bovine serum (Gibco-BRL,
Carlsbad, CA, USA) containing 100 μl/ml penicillin-streptomycin in
25 cm2 cell culture flasks (Corning Inc., Acton, MA,
USA). The cells were incubated at 37°C in a 5% CO2
atmosphere. After 48 h, non adherent cells were removed using
phosphate buffer saline (PBS; Boster Biological Tech Ltd, Wuhan,
China) to rinse the cells. The culture mediun was replaced every
second or third day. When the cells reached 85% confluence, the
primary culture was subcultured 1:2; third-passage BMSCs were used
in the present study.
Characterization of BMSCs by
immunophenotype
The third-passage BMSCs were dissociated with 0.25%
trypsin and suspended at a concentration of 5×105
cells/ml in PBS. A volume of 1 ml cell suspension was pipetted into
a 1.5 ml tube. CD29-PE/CY5, CD45-fluorescein isothiocyanate (FITC)
and CD90-APC/CY7 (BioLegend, Inc., San Diego, CA, USA) were added
to the respective tubes and the tubes were incubated for 30 min at
room temperature in the dark. The control group was absent of these
antibodies. Flow cytometric analysis was performed to measure the
positive rate using a BD LSRFortessa and Facsdiva 6.2 software (BD
Biosciences, San Jose, CA, USA).
Infrasound and control treatments
The third-passage BMSCs were detached with 0.25%
trypsin and resuspended in a 5 ml tube with DMEM/F12 in 10% fetal
bovine serum, then divided into infrasound and control groups. The
infrasound groups were exposed to infrasound and the control groups
were exposed to air. The durations of the two treatments were 10,
30, 60, 90 and 120 min. Following the interventions, the cells were
immediately incubated at 37°C in a CO2 atmosphere for 72
h. The BMSCs in each group were seeded in 96-well plates for the
proliferation assay or in a 6 cm culture dish for the apoptosis
assay. In addition, BMSCs from the two groups that received 60 min
infrasound or air treatment were seeded in 24-well plates
containing 1×1 mm coverslips for immunofluorescence analysis and
6-well plates for quantitative polymerase chain reaction
(qPCR).
Evaluation of cell proliferation by cell
counting kit (CCK) assay
In order to evaluate the proliferation ability of
the BMSCs, a CCK (Dojindo, Kunamoto, Japan) assay was performed. A
volume of 10 μl CCK reagent was added to the wells and the cells
were then incubated at 37°C for 3 h until the emergence of an
orange supernatant. The optical density (OD) was measured at 630
nm.
Measurement of apoptotic cells
The BMSCs were dissociated with 0.25% trypsin,
centrifuged at 2,200 × g for 5 min, then suspended at a
concentration of 5×105 cells/ml with PBS. The Annexin
V-FITC Apoptosis Assay kit (Nanjing Keygen Biotech. Co., Ltd.,
Nanjing, China) was used for the assay. The cell suspension was
rinsed with PBS and centrifuged again, and the cells were then
suspended in 500 μl buffer. Annexin V-FITC and propidium iodide
were added to the cell suspension and incubated for 10 min at room
temperature in the dark according to the manufacturer’s
instructions. Flow cytometry was conducted to analyze the
results.
Survivin immunohistochemistry
Immunohistochemistry of survivin expression was
performed on only the 60 min infrasound and control groups. The
BMSCs on coverslips were fixed with fresh 4% paraformaldehyde for
30 min. Subsequent to washing with PBS three times and
permeabilization with 0.25% TX-100 for 20 min, the cells were
blocked for 40 min at room temperature with 5% bovine serum albumin
(Boster Biological Technology, Ltd., Wuhan, China). The cells were
then incubated at 4°C overnight with primary antibody against
survivin (Bioworld Technology, Inc., St. Louis Park, MN, USA).
Following washing, the cells were incubated at room temperature
with secondary Alexa Fluor 594 goat anti-rabbit antibodies
(Invitrogen Life Technologies, Carlsbad, CA, USA) for 1 h. The
cells were washed again and incubated with DAPI (Boster Biological
Technology, Ltd.) for 5 min. The fluorescent signals were
visualized under a confocal laser microscope (Olympus, Tokyo,
Japan).
qPCR of survivin mRNA
The BMSCs were harvested and homogenized for RNA
extraction with TRIzol™ reagent (Invitrogen Life Technologies).
Messenger RNA was reverse-transcribed to cDNA using Primescript RT
Reagent kit (Takara Bio, Inc., Shiga, Japan). qPCR was then
conducted to measure the survivin mRNA expression level. The
expression levels of the β-actin housekeeping gene were measured as
a control. The primer sequences are shown in Table I. A volume of 2 μl total cDNA from
each sample was amplified in a final volume of 25 μl reaction
mixture containing SYBR® Green I (Takara Bio Inc.,
Shiga, Japan) using the Applied Biosystems 7500 Fast Real-Time PCR
system (Applied Biosystems, Inc., Foster City, CA, USA). The
cycling conditions were as follows: 95°C for 30 sec, 40 cycles at
95°C for 5 sec and 34 sec at 60°C.
 | Table IPrimers used for quantitative
polymerase chain reaction. |
Table I
Primers used for quantitative
polymerase chain reaction.
| Gene | Primer sequence | Length (bp) | Accession no. |
|---|
| β-actin | Forward:
5′-TTGGTGGCTCTATCCTGGCCTC-3′
Reverse: 5′-AAACGCAGCTCAGTAACAGTCCG-3′ | 131 | NM_031144.2 |
| Survivin | Forward:
5′-ACTGCCCTACCGAGAATGAG-3′
Reverse: 5′-GAGTGCTTCCTATGCTCCTCT-3′ | 109 | NM_022274.1 |
Statistical analysis
All the results are presented as the means ±
standard deviation for each group. The data were analyzed using
SPSS software (SPSS, Inc., Chicago, IL, USA). Two-group comparisons
were analyzed with Student’s t-test. Comparisons among more than
two groups were analyzed with one-way analysis of variance. A
P<0.05 was considered to indicate a statistically significant
difference.
Results
Immunophenotyping of BMSCs
Flow cytometry was used to determine the phenotype
of the BMSCs through analysis of cell-surface markers. The results
revealed positive expression of CD90 and CD29 and negative
expression of CD45 marker of hemopoietic stem cells. The
characteristics of the cells was in accordance with a BMSC
phenotype.
Effects of infrasound on BMSC
proliferation
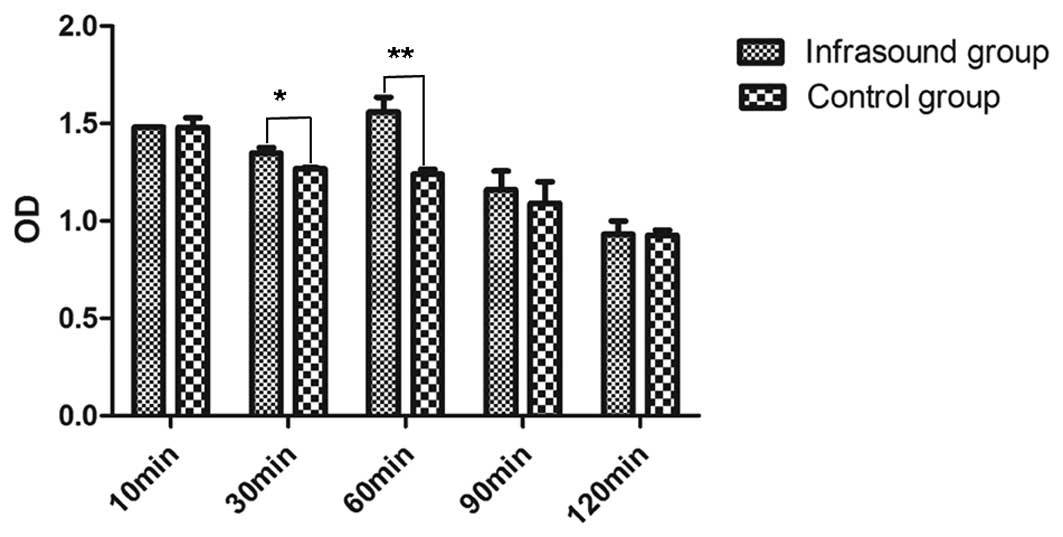
The OD of the groups receiving 10, 30, 60, 90 and
120 min infrasound or air treatment was measured by the CCK method
(Fig. 1). The results revealed
significant differences in the OD among the 10, 30, 60, 90 and 120
min infrasound groups (P<0.05). The maximum OD was observed in
the group receiving 60 min infrasound. The mean ODs for each of the
five control subgroups were observed to be sequentially reduced
with increasing treatment times. When the 60 min infrasound and
control treatments were compared, a significant elevation in the OD
was detected in the infrasound group (P<0.01). A significant
difference was also observed between the 30 min infrasound group
and the corresponding control group (P<0.05); however, the 60
min infrasound group exhibited the greatest level of proliferation
in BMSCs.
Effects of infrasound on BMSC
apoptosis
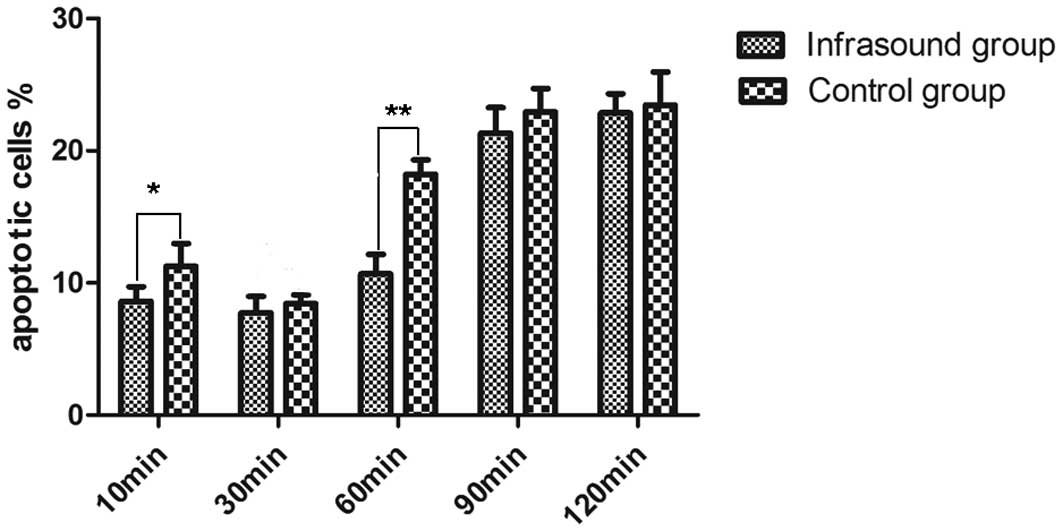
BMSC apoptosis was analyzed by flow cytometry
(Fig. 2). The apoptotic rates of
BMSCs in the infrasound groups were compared with those of the
respective control groups; the results revealed significant
reductions in apoptosis in the 10 and 60 min infrasound groups as
compared with the respective control groups. Notably, no
differences in the level of apoptosis was detected in the 30 min
infrasound group as compared with the 30 min control group
(P>0.05). A pattern of progressive increases in the percentage
of apoptotic cells with increasing treatment time was observed in
the control groups. No difference was identified between the 90 and
120 min infrasound groups and the corresponding control groups. The
evidence that the apoptotic rate of the cells receiving 60 min
infrasound exhibited a significant reduction as compared with the
60 min control group, indicated that 60 min may be a suitable
duration for infrasound treatment to inhibit BMSC apoptosis.
Effects of infrasound on the protein and
mRNA expression levels of survivin
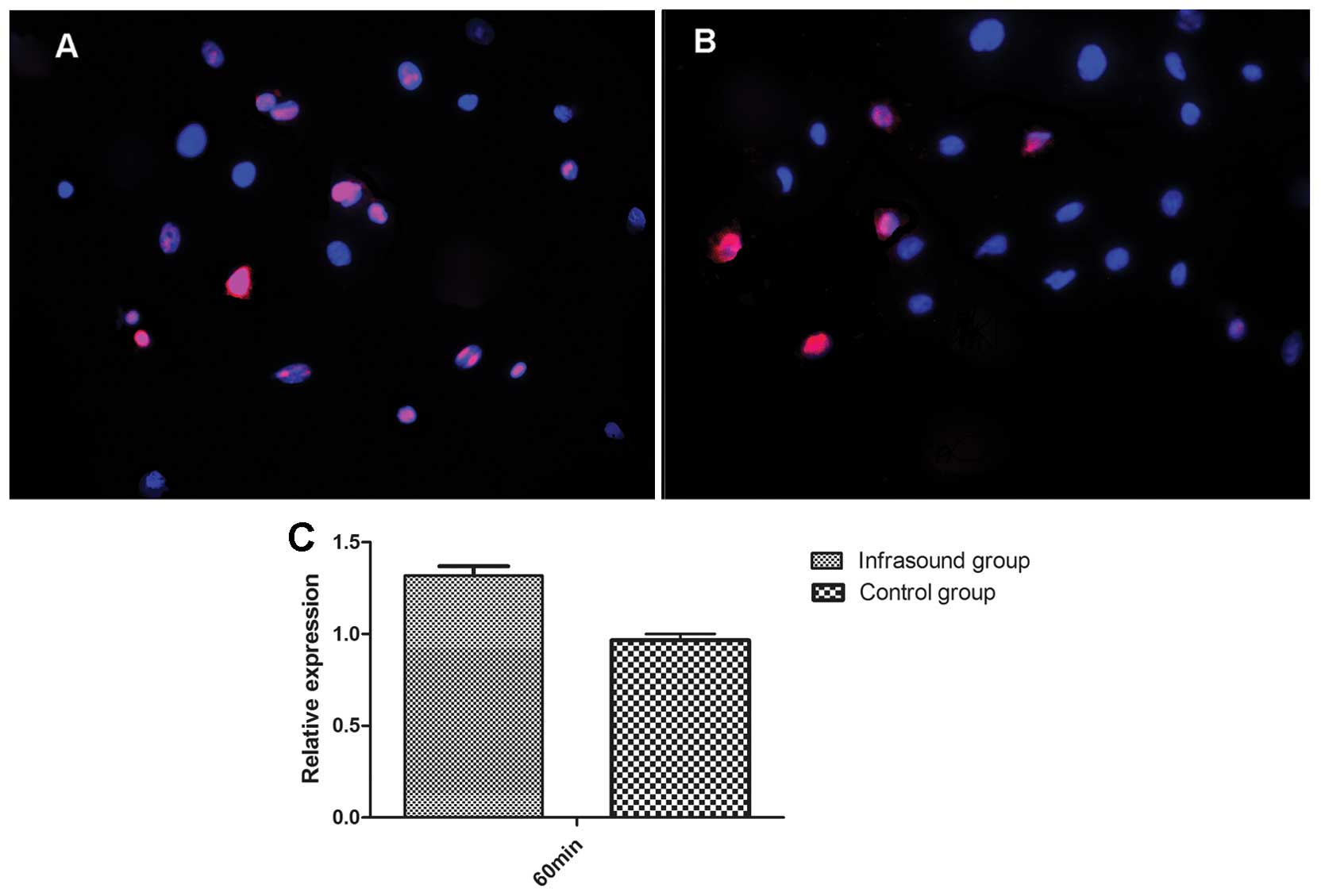
As determined by the results of the proliferation
and apoptosis assays of BMSCs, 60 min was considered the most
suitable treatment duration. Therefore, only the 60 min infrasound
and control groups were used for the measurement of survivin
expression levels. The fluorescence intensity of survivin and the
percentage of positive-staining BMSCs in the infrasound group were
significantly higher than those of the control group (P<0.05;
Fig. 3A and B). The relative
expression level of survivin mRNA in the infrasound group was
significantly increased as compared with that of the control group
(Fig. 3C). These data suggest that
infrasound enhances survivin expression levels.
Discussion
Mechanical stimulation through infrasound is a
controversial therapeutic method. Organisms may be considered as
mechanical vibration systems themselves and certain parts of the
body exhibit natural frequencies within the range of infrasound
frequency; thus infrasound may produce resonance reactions in the
body, resulting in physical changes and chemical reactions
(19). Due to marked penetration
and low attenuation properties during long distance propagation
(20–22), infrasound of BMSCs may continue to
exert effects subsequent to transplantation of the cells into the
body. Therefore the aim of the present study was to investigate the
effects of infrasound with a low sound pressure level (<90 dB)
on BMSCs in vitro.
BMSCs, also known as marrow mesenchymal cells or
mesenchymal progenitor cells, are defined as self-renewable,
multipotent progenitor cells with the capacity to differentiate
into several distinct lineages (23), such as adipocytes, osteoblasts,
vascular-smooth muscle-like cells and neuron-like cells (24). A number of studies have focused on
transplantation of BMSCs to replace impaired tissues (25–27).
Due to the changes in the microenvironments of damaged tissues,
poor BMSC survival rates have been observed following BMSC
migration into the tissues (28–30).
Therfore, improvement in the capacity of BMSCs to survive ischemic
and hypoxic conditions is required. As determined by a previous
infrasound study, infrasound was hypothesized to exert protective
effects on BMSCs (12). The
protective effects of infrasound were, to the best of our
knowledge, demonstrated for the first time in the present
study.
To determine the effects of different infrasound
durations on BMSCs, the cells were divided into infrasound and
control groups with 10, 30, 60, 90 and 120 min treatment, and
proliferation and apoptosis were measured in each group. The 10 min
infrasound treatment was found to inhibit BMSC apoptosis but
exerted no effect on BMSC proliferation. Conversely, 30 min
infrasound promoted the proliferation of BMSCs but exerted no
effect on BMSC apoptosis. Treatments for 90 and 120 min in the
infrasound and control groups induced a high apoptosis and low
proliferation rate. The data demonstrated that 60 min infrasound,
which promoted the proliferation of BMSCs and inhibited apoptosis,
appeared to be the most suitable treatment duration. Additionally,
longer durations of air treatment induced less cell proliferation
and greater apoptosis. Although the interference factor, was not
identified, there was evidence of the protective effects of
infrasound on BMSCs.
A previous study has indicated that the
apoptosis-inducing effects of higher frequencies of infrasound on
cardiac myocytes are mediated by upregulating the expression of
proapoptotic proteins and downregulating the expression of
anti-apoptotic proteins (31).
Another study suggested that survivin expression levels are cell
cycle-dependent and may inhibit cell apoptosis (16). Thus, further studies are required
to investigate the survivin response to a variety of different
frequencies of ultrasound.
In the present study, a suitable duration of
infrasound treatment, at a frequency of 4–20 Hz and sound pressure
level of 79–86 dB, was found to be 60 min. Therefore, 60 min may be
considered as a standard reference time of BMSC infrasound
treatment at this frequency. In order to elucidate the association
between the protective effects of infrasound on BMSCs and survivin
expression levels, the expression levels of survivin in the 60 min
infrasound and control groups were analyzed by immunofluorescence
and qPCR. Survivin is a member of the IAP family and is
specifically expressed at the G2/M cell cycle phase. A number of
studies have investigated the function of survivin. For example,
overexpression of survivin may augment thymocyte proliferation
(13). When MSCs were co-cultured
with U299 and H299 myeloma cell lines, the MSCs exerted an
anti-apoptotic effect on the myeloma cells, which was mediated by
survivin (32). This evidence
indicates that survivin promotes the proliferation of cells and
inhibits apoptosis. The data from the survivin immunofluorescence
assay of BMSCs in the present study revealed that the fluorescence
intensity, and thus the survivin expression levels, were
significantly greater in the infrasound group than in the control
group. In addition, the expression levels of survivin mRNA in the
infrasound group were significantly higher than those in the
control group. The results demonstrated that infrasound enhances
survivin expression levels. The change in survivin expression
levels between the two groups may be partly responsible for the
protective effect of infrasound on BMSCs. Due to the complex
process of survivin expression (33) and the effects of the Notch
signaling pathway on proliferation and apoptosis (34), it remains unclear whether the
change in survivin expression levels induced by infrasound accounts
directly for the effects of infrasound on the BMSCs. Inhibition of
the signaling pathway regulating survivin expression may be
performed to further analyze this association in future
studies.
In conclusion, the present study confirms the
protective effects of infrasound on BMSCs. Further studies are
required to focus on the protective effects of infrasound on BMSC
transplantation in the treatment of cerebral ischemia, as other
studies have indicated that BMSCs repair the ischemic zone
(35,36). Evidence that hypoxia induces BMSC
differentiation (37) and that
infrasound enhances the viability of BMSCs indicate that infrasound
may be a supplementary method in the transplantation of BMSCs to
treat cerebral ischemia.
Acknowledgements
The authors would like to thank colleagues at the
Department of Rehabilitation Medicine of Nanfang Hospital and Dr
Yingli Bi for guidance with the experimental methods. The
Experimental Center of Nanfang Hospital provided the facilities for
this study.
References
|
1
|
Xiao Y, Mareddy S and Crawford R: Clonal
characterization of bone marrow derived stem cells and their
application for bone regeneration. Int J Oral Sci. 2:127–135.
2006.PubMed/NCBI
|
|
2
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: mesenchymal stem cells: their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar
|
|
3
|
Bliss T, Guzman R, Daadi M and Steinberg
GK: Cell transplantation therapy for stroke. Stroke. 38:817–826.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parr AM, Tator CH and Keating A: Bone
marrow-derived mesenchymal stromal cells for the repair of central
nervous system injury. Bone Marrow Transplant. 40:609–619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng X, Yu SP, Taylor T, et al: Protective
effect of apelin on cultured rat bone marrow mesenchymal stem cells
against apoptosis. Stem Cell Res. 8:357–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaquero J, Otero L, Bonilla C, et al: Cell
therapy with bone marrow stromal cells after intracerebral
hemorrhage: impact of platelet-rich plasma scaffolds. Cytotherapy.
15:33–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jagodzinski M, Breitbart A, Wehmeier M, et
al: Influence of perfusion and cyclic compression on proliferation
and differentiation of bone marrow stromal cells in 3-dimensional
culture. J Biomech. 41:1885–1891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riddle RC, Hippe KR and Donahue HJ:
Chemotransport contributes to the effect of oscillatory fluid flow
on human bone marrow stromal cell proliferation. J Orthop Res.
26:918–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leventhall G: What is infrasound? J Prog
Biophys Mol. 93:130–137. 2007. View Article : Google Scholar
|
|
10
|
Pei Z, Sang H, Li R, et al:
Infrasound-induced hemodynamics, ultrastructure, and molecular
changes in the rat myocardium. Environ Toxicol. 22:169–175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu ZH, Chen JZ, Ye L, et al: Effects of
infrasound at 8 Hz 90 dB 130 dB on NMDAR1 expression and changes in
intracellular calcium ion concentration in the hippocampus of rats.
Mol Med Rep. 3:917–921. 2010.PubMed/NCBI
|
|
12
|
Li C, Fan JZ and Wu HY: Effects of
infrasound with low sound pressure level on rats with cerebral
ischemia-reperfusion injury. Chinese J Rehabil Med. 2009.419–412.
2009.(In Chinese).
|
|
13
|
Hikita S, Hatano M, Inoue A, et al:
Overexpression of TIAP/m-survivin in thymocytes enhances cell
proliferation. Mol Immunol. 39:289–298. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Otaki M, Hatano M, Kobayashi K, et al:
Cell cycle-dependent regulation of TIAP/m-survivin expression.
Biochim Biophys Acta. 1493:188–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altieri DC: The case for survivin as a
regulator of microtubule dynamics and cell-death decisions. Curr
Opin Cell Biol. 18:609–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yaghoobi MM and Mahani MT: NGF and BDNF
expression drop off in neurally differentiated bone marrow stromal
stem cells. Brain Res. 1203:26–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charrière K, Risold PV and Fellmann D: In
vitro interactions between bone marrow stromal cells and
hippocampal slice cultures. C R Biol. 333:582–590. 2010.PubMed/NCBI
|
|
18
|
Polisetti N, Chaitanya VG, Babu PP and
Vemuganti GK: Isolation, characterization and differentiation
potential of rat bone marrow stromal cells. Neurol India.
58:201–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhuang ZQ, Pei ZH and Chen JZ: The
underlying mechanisms for infrasonic bioeffects. Chin J Dis control
Prev. 9:328–330. 2005.
|
|
20
|
Du F, Yin L, Shi M, et al: Involvement of
microglial cells in infrasonic noise-induced stress via upregulated
expression of corticotrophin releasing hormone type 1 receptor.
Neuroscience. 167:909–919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arabadzhi VI: Infrasound and biorhythms of
the human brain. Biofizika. 37:150–151. 1992.(In Russian).
|
|
22
|
Backteman O, Köhler J and Sjoberg L:
Infrasound - tutorial and review: Part 4. J Low Freq Noise Vib.
3:96–113. 1984.
|
|
23
|
Reis LA, Borges FT, Simões MJ, et al: Bone
marrow-derived mesenchymal stem cells repaired but did not prevent
gentamicin-induced acute kidney injury through paracrine effects in
rats. PLoS One. 7:e440922012. View Article : Google Scholar
|
|
24
|
Rochefort GY, Delorme B, Lopez A, et al:
Multipotential mesenchymal stem cells are mobilized into peripheral
by blood hypoxia. Stem Cells. 24:2202–2208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al Fageh H, Nor Hamdam BM, Chen HC,
Aminuddin BS and Ruszymah BH: The potential of intra-articular
injection of chondrogenic-induced bone marrow stem cells to retard
the progression of osteoarthritis in a sheep model. Exp Gerontol.
47:458–464. 2012.PubMed/NCBI
|
|
26
|
Nishida H, Nakayama M, Tanaka H, et al:
Safety of autologous bone marrow stromal cell transplantation in
dogs with acute spinal cord injury. Vet Surg. 41:437–442. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin JW, Lee JK, Lee JE, et al: Combined
effects of hematopoietic progenitor cell mobilization from bone
marrow by granulocyte colony stimulating factor and AMD3100 and
chemotaxis into the brian using stromal cell-derived factor-1α in
an Alzheimer’s disease mouse model. Stem Cells. 29:1075–1089.
2011.PubMed/NCBI
|
|
28
|
Resmanchi N, Floyd CL, Berman RF and Lyeth
BG: Cell death and long-term maintenance of neuron-like state after
differentiation of rat bone marrow stromal cells: a comparison of
protocols. Brain Res. 991:46–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li HM, Liu L, Mei X and Zhao X:
Investigation on long-term survival of transplanted bone marrow
mesenchymal stem cells in infarcted myocardium of rats. Chin J
Cardiol. 39:171–175. 2011.(In Chinese).
|
|
30
|
Boukhechba F, Balaquer T, Bouvet-Gerbettaz
S, et al: Fate of bone marrow stromal cells in a syngenic mode of
bone formation. Tissue Eng Part A. 17:2267–2278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pei ZH, Chen BY, Tie R, et al: Infrasound
exposure induces apoptosis of rat cardiac myocytes by regulating
the expression of apoptosis-related proteins. Cardiovasc Toxicol.
11:341–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Zhang Z and Yao C: Survivin is
upregulated in myeloma cell lines cocultured with mesenchymal stem
cells. Leuk Res. 34:1325–1329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang L, Ling X, Liu W, et al:
Transcriptional inhibition of p21WAF1/CIP1 gene (CDKN1) expression
by survivin is at least partially p53-dependent: evidence for
survivin acting as a transcription factor or co-factor. Biochem
Biophys Res Commun. 421:249–254. 2012. View Article : Google Scholar
|
|
34
|
Mizuguchi T, Hui T, Palm K, et al:
Enhanced proliferation and differentiation of rat hepatocytes
cultured with bone marrow stromal cells. J Cell Physiol.
189:106–119. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen LH, Li Y, Chen J, et al: Therapeutic
benefit of bone marrow stromal cells administered 1 month after
stroke. J Cereb Blood Flow Metab. 27:6–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kawabori M, Kuroda S, Ito M, et al: Timing
and cell dose determine therapeutic effects of bone marrow stromal
cell transplantation in rat model of cerebral infarct.
Neuropathology. 33:140–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin JM, Kim J, Kim HE, et al: Enhancement
of differentiation efficiency of hESCs into vascular lineage cells
in hypoxia via a paracrine mechanism. Stem Cell Res. 7:173–185.
2011. View Article : Google Scholar : PubMed/NCBI
|