Introduction
Secondary iron overload and primary hemochromatosis
have received increasing attention due to worldwide epidemics
(1–3). Iron is deposited in numerous tissues,
most notably the liver, where it can lead to significant organ
damage (4). Iron overload in the
liver is observed in patients with chronic liver diseases,
including alcoholic liver disease and chronic viral hepatitis, and
is a secondary side effect of repeated blood transfusions (5–7).
Emerging evidence suggests that oxidative stress, mediated by free
radicals and reactive oxygen species (ROS), may have a role in the
pathophysiology of iron-induced liver injury and cell death
(8,9). In a previous study we found that iron
overload initiates excessive ROS formation, and that the liver is
severely damaged as a result of this oxidative stress (10). However, little is known about the
use of antioxidants as possible preventive or curative agents in
excessive iron-induced liver damage.
Taurine, a sulfur-containing amino acid, is present
at high concentrations in the liver (11). Numerous studies have found that
taurine has a protective effect against chemically-induced
hepatotoxicity (12–15). Furthermore, taurine has been
reported to act as an antioxidant in biological systems. As an
antioxidant, taurine has the ability to scavenge ROS and attenuate
lipid peroxidation and, as a consequence, stabilizes biological
membranes (16,17).
Since taurine has been shown to have potent
antioxidant properties, its role in hepatotoxicity induced by iron
overload was investigated in the present study. The status of the
physiological parameters associated with hepatotoxicity induced by
iron overload, including the levels of intracellular antioxidant
enzymes, the glutathione (GSH)/glutathione disulfide (GSSG) ratio
and the involvement of the mitochondria-dependent apoptotic
pathway, was assessed, either with or without supplementation of
taurine. The results of this may provide critical information for
the treatment of oxidative stress mediated by iron overload in the
liver.
Materials and methods
Experimental animals and iron overloading
protocols
Forty-eight male Kunming mice, weighing 14.07±0.7 g,
were obtained from Nanchang University (Nanchang, China). The mice
were randomly divided to receive either taurine (0.1 mol/l) or
vehicle (2.5% dextrose) in their drinking water 2 weeks prior to
the initial injections and throughout the course of the
experiments. The mice were continuously injected with either iron
or placebo (0.1 ml 10% dextrose) for a total of 13 weeks (total
dose, ~200 mg/25 g body weight) as previously described (18). Mice were thus divided into the
placebo plus vehicle, placebo plus taurine, iron plus vehicle and
iron plus taurine groups. All care and experiments in this study
conformed to the National Institutes of Health (NIH) Guide for Care
and Use of Laboratory Animals (NIH publication 86–23, revised in
1986). The use of animals was reviewed and approved by the Nanchang
University Animal Care Review Committee. At the end of the
experiment the mice were sacrificed by cervical dislocation and
blood was collected by cardiac puncture. The liver was immediately
excised, weighed and divided for further analysis.
Determination of iron levels in the serum
and liver
The iron concentration in the serum was determined
using an assay based on the generation of an iron-ferrozine
complex, as described by Galleano and Puntarulo (19). The iron concentration in the
digested liver sample was measured using a spectrophotometer (535
nm) following the addition of 2 mM bathophenanthroline disulfonic
acid.
Determination of aspartate transaminase
(AST) and alanine transaminase (ALT) levels
Serum levels of AST and ALT were measured using an
autoanalyzer (Roche Cobas Integra 400, Roche Diagnostics,
Holliston, MA, USA) and AST and ALT reagent kits from Roche
Diagnostics (Indianapolis, IN, USA).
Determination of taurine levels
The liver tissue was homogenized and the supernatant
was ultra-filtered and diluted with methionine sulfone (an internal
standard). The taurine concentration was determined as previously
described using a high-performance liquid chromatography system and
a specific Pico-Tag column (Waters Corp., Milford, MA, USA)
(20).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
The TUNEL assay was performed using a commercially
available kit (Promega Corp., Madison, WI, USA) in accordance with
the manufacturer’s instructions. Briefly, liver samples were fixed
by perfusion with 10% buffered formalin, and 5-μm paraffin-embedded
sections were obtained. The sections were then subjected to a
DeadEnd™ Colorimetric TUNELSystem assay. The paraffin-embedded
liver sections were re-treated to remove paraffin and fixed again
in 4% paraformaldehyde. Following permeabilization, the sections
were labeled with a terminal transferase reaction mix and bound to
streptavidin horseradish peroxidase (HRP). The sections were then
incubated with chromogen HRP and subsequently treated with
diaminobenzidine and counterstained lightly with hematoxylin. Brown
nuclei with nuclear condensation in the stained cells were
considered to be TUNEL positive. Hepatocyte apoptosis in the liver
sections was quantified by counting the number of TUNEL-positive
cells in a random microscopic low-power field (magnification,
×400).
Determination of lipid peroxidation and
antioxidant enzyme activities
Malondialdehyde (MDA)
The lipid peroxide content in the liver was
determined by quantifying the thiobarbituric acid reactive
substances as previously described (21). Briefly, 0.5 ml supernatant was
mixed with 1.5 ml thiobarbituric acid, 1.5 ml acetic acid (pH 3.5),
0.2 ml sodium dodecyl sulfate and 0.5 ml distilled water, and the
samples and standards were heated to 100°C for 1 h. The absorbance
was measured at 532 nm on a spectrophotometer. Commercially
available MDA was used as a standard.
Superoxide dismutase (SOD)
SOD activity in the tissue homogenate was measured
following the method previously described by Beauchamp and
Friedrich (22), with slight
modifications. The reaction mixture (0.8 ml), containing 100 μmol/l
xanthine, 100 μmol/l EDTA, 25 μmol/l nitroblue tetrazolium (NBT)
and 50 mmol/l Na2CO3 (pH 10.2), was added to
0.1 ml liver homogenate. After 10 min preincubation at room
temperature, the reaction was initiated by the addition of 0.1 ml
xanthine oxidase (0.05 U/ml), and the absorbance at 560 nm was
recorded every 30 sec for 5 min. A standard curve for SOD activity
was assayed spectrophotometrically as the inhibition of the
photochemical reduction of NBT at 560 nm.
GSH-peroxidase (GSH-Px)
GSH-Px activity was determined as previously
described, with slight modifications (23). The liver tissue was homogenized
(1:10) in 75 mmol/l phosphate buffer (pH 7.0). The homogenate was
then centrifuged at 20,000 × g for 25 min, and the supernatant was
aspirated and assayed for total cytosolic GSH-Px activity. GSH-Px
activity was assayed in a 3-ml cuvette containing 2.0 ml 75 mmol/l
phosphate buffer (pH 7.0). The following solutions were then added:
50 μl glutathione (60 mmol/l), 100 μl glutathione reductase
solution (30 U/ml), 50 μl NaN3 (0.12 mol/l), 100 μl
Na2EDTA (15 mmol/l), 100 μl reduced nicotinamide adenine
dinucleotide phosphate (NADPH; 3.0 mmol/l) and 100 μl cytosolic
fraction. The reaction was initiated by the addition of 100 μl 7.5
mmol/l H2O2, and the conversion of NADPH to
NADP was monitored by recording the change in the absorbance at 340
nm at 1-min intervals for 5 min. The GSH-Px activity was expressed
as the quantity of reduced NADPH (in nanomoles) oxidized to NADP
per minute per milligram of protein, with a molar extinction
coefficient for NADPH at 340 nm of 6.22×106.
Catalase
Catalase activity was determined as previously
described (23). The liver tissue
was homogenized (1:10) in 50 mmol/l potassium phosphate buffer (pH
7.4), and the homogenate was centrifuged at 40,000 × g for 30 min.
Approximately 50 μl supernatant was added to a 3 ml-cuvette
containing 2.95 ml hydrogen peroxide (19 mmol/l) in 50 mmol/l
potassium phosphate buffer (pH 7.4). Changes in absorbance at 240
nm were recorded continuously for 5 min. Catalase activity was
expressed as units per milligram of protein.
Measurement of total glutathione
(GSSG+GSH) levels (reduced and oxidized)
The concentration of GSSG+GSH in the liver was
measured using the glutathione
reductase/5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) recycling
assay (24). The rate of
5′-thio-2-nitrobenzoic acid formation was measured at an absorbance
of 412 nm and was proportional to the sum of GSH+GSSG present.
Liver tissue was homogenized in 5% sulfosalicylic acid, and the
homogenate was centrifuged for 10 min at 10,000 × g. The
supernatant was stored at 4°C until assayed. GSSG alone was
measured by treating the sulfosalicylic acid supernatant with
2-vinylpyridine and triethanolamine. The solution was vigorously
mixed, and the final pH of the solution was adjusted to between six
and seven. After 60 min, the samples were assayed as described
above in the DTNB-GSSG reductase recycling assay. The GSH values
were calculated as the difference between the total (GSSG+GSH) and
GSSG concentrations. The values are reported in GSH equivalents and
expressed as micromoles per gram of tissue.
Hepatocyte preparation
Hepatocytes were isolated using a two-step
collagenase perfusion method. Following mechanical disruption of
the liver capsule, liver cells were collected in Williams’ Medium E
and serially filtered (30-, 50- and 80-mesh) through an 85-ml
Cellector (Bellco Biotechnology, Vineland, NJ, USA) tissue sieve.
Between 10×106 and 25×106 cells were obtained
from a single mouse liver.
Measurement of intracellular ROS
The fluorescent probe
2′,7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA) is converted
by intracellular esterases to DCFH, which is then oxidized by ROS
to highly fluorescent DCF. The ROS Detection Reagent assay
(Invitrogen Life Technologies, Carslbad, CA, USA) was performed in
accordance with the manufacturer’s instructions. Hepatocytes were
washed twice with cold phosphate-buffered saline (PBS) and
incubated in Dulbecco’s Modified Eagle’s Medium containing 10 μM
DCFH-DA (Invitrogen Life Technologies). Following centrifugation at
800 × g for 5 min and two washes with cold PBS, the fluorescence
intensity of each group was determined using flow cytometric
analysis (Becton-Dickinson, Franklin Lakes, NJ, USA) at excitation
and emission (ex/em) wavelengths of 485/528 nm, respectively.
Assessment of the mitochondrial
membrane potential (Δψ)
The mitochondrial membrane potential was assessed
using the fluorescent dye JC-1 (Invitrogen Life Technologies), a
lipophilic cationic dye that selectively enters mitochondria and
reversibly changes color from green to red when the Δψ increases
(25). Therefore, the ratio of the
red to green fluorescent intensity of the cells reflects the Δψ.
Suspended hepatocytes were incubated with JC-1 (200 μl) for 20 min
at 37°C followed by two washes with PBS to remove the excess dye.
Fluorescence was then measured using a flow cytometer
(Becton-Dickinson) with ex/em wavelengths of 530/580 nm (red), and
485/530 nm (green).
Preparation of mitochondrial
fraction
Mitochondria were isolated using conventional
differential centrifugation from the liver of mice that had fasted
overnight. The livers were homogenized in 250 mM sucrose, 1 mM
ethylene glycol tetraacetic acid (EGTA) and 10 mM HEPES buffer (pH
7.2). The mitochondrial suspension was washed twice in the same
medium containing 0.1 mM EGTA, and the final pellet was resuspended
in 250 mM sucrose. The final protein concentration was 80–100
mg/ml, as measured by the Biuret method, using bovine serum albumin
as the protein standard.
Mitochondrial swelling
The swelling experiments were performed as
previously described by Beavis et al (26) using a standard medium containing
125 mM sucrose, 10 mM HEPES buffer (pH 7.2), 2.5 mM succinate and
4.0 mM rotenone at 25°C. The final volume used was 1.0 ml, and the
protein concentration was ~0.5 mg/ml. Changes in absorbance at 520
nm were monitored in a thermostatically controlled Hitachi U 2000
spectrophotometer (Hitachi, Ltd., Tokyo, Japan).
Statistical analysis
Data analysis was performed using SPSS®
statistical software version 11.0 (SPSS Inc., Chicago, IL, USA).
The data are expressed as the mean ± standard error of the mean
from ≥12 independent experiments. Each treatment was performed in
triplicate culture wells. The differences between the means of each
group were tested using a one-way analysis of variance followed by
the Student-Newman-Keuls test to compare between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Taurine improves the liver-to-body ratio,
as well as the ALT and AST levels, without affecting iron
accumulation
Serum and hepatic iron levels were significantly
increased in all iron-overloaded mice, regardless of taurine
supplementation. To investigate whether liver injury and
dysfunction were caused by iron overload, the liver-to-body weight
ratio (%) and the levels of serum ALT and AST, which are important
markers of dysfunction, were analyzed. Iron-overloaded mice showed
a 1.9-fold increase in the liver-to-body weight ratio, and a 4.5-
and 3.7-fold elevation in the serum ALT and AST levels,
respectively. However, treatment with taurine was found to suppress
these changes (Table I).
 | Table IEffect of taurine on the serum and
hepatic iron concentration, liver-to-body weight ratio, serum
levels of ALT and AST and hepatic taurine levels in iron-injected
mice. |
Table I
Effect of taurine on the serum and
hepatic iron concentration, liver-to-body weight ratio, serum
levels of ALT and AST and hepatic taurine levels in iron-injected
mice.
| Parameter | Placebo +
vehicle | Placebo +
taurine | Iron + vehicle | Iron + taurine |
|---|
| Serum iron
concentration (μmol/l) | 34.64±1.32 | 32.80±1.41a |
420.36±15.42b | 401.2±13.82b,c |
| Hepatic iron
concentration (mg/g dry weight) | 0.068±0.003 | 0.062±0.003a | 1.042±0.026b | 1.021±0.028b,c |
| Liver-to-body ratio
(mg/g) | 48.2±1.9 | 46.8±2.1a | 92.4±4.1b | 61.5±2.5b,d |
| ALT (U/l) | 50.81±1.52 | 48.83±1.65a | 228.31±9.42b | 125.06±5.33b,d |
| AST (U/l) | 106.20±3.58 | 113.42±3.91a |
395.13±14.22b | 216.42±8.23b,d |
| Taurine level
(μmol/g) | 30.03±1.51 | 49.16±2.63e | 28.52±1.62a | 47.38±2.85d,f |
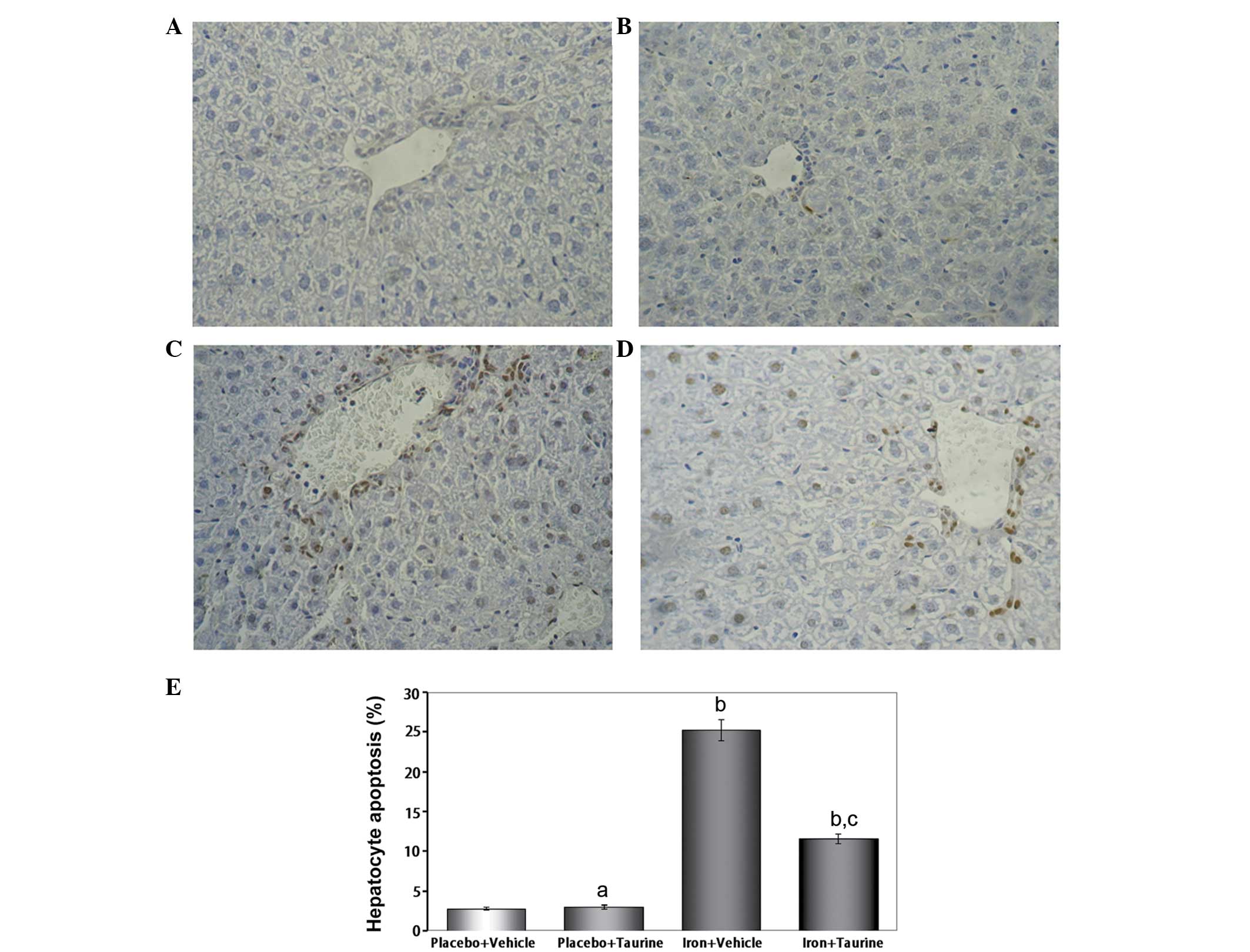
Taurine prevents apoptosis in
iron-overloaded mice
In mice that did not receive iron treatment, only a
few TUNEL-positive hepatocytes were identified; however, numerous
TUNEL-positive hepatocytes were observed in the iron-overloaded
animals. Taurine supplementation significantly reduced the total
number of TUNEL-positive hepatocytes to 11.6±0.62% of the total
cell count (Fig. 1).
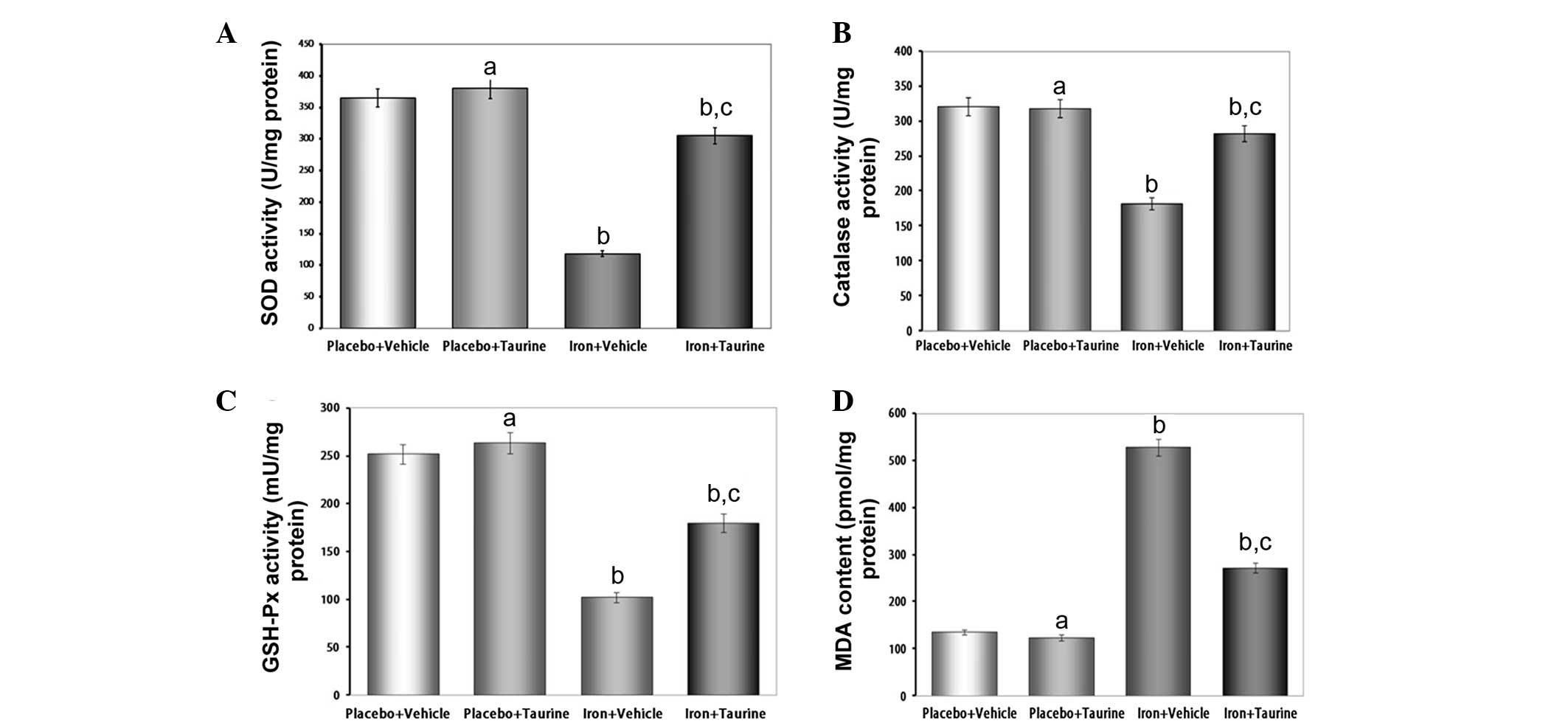
Taurine ameliorates the decreased
activities of antioxidant enzymes and increased lipid peroxidation
induced by iron overload
SOD, catalase and GSH-Px are the important
antioxidant enzymes present in the body that provide a defensive
mechanism against free radical-mediated oxidative damage. Excess
iron levels affect the activities of these enzymes as a result of
the overproduction of ROS. The antioxidant activity of SOD is
mediated by a dismutation reaction, in which SOD scavenges highly
reactive superoxide radicals and converts them to oxygen molecules
and less reactive H2O2 molecules (27). Catalase then further metabolizes
the H2O2 into water and O2. The
intracellular redox status is also maintained by the activity of
GSH-Px in the presence of glutathione. GSH-Px aids in the
decomposition of H2O2 and other organic
hydroperoxides into non-toxic products (28). It has been observed in a number of
previous studies that liver injury is associated with a reduction
in the activities of these antioxidant enzymes (29,30).
Therefore, in the present study, the effect of iron overload on the
activities of different antioxidant enzymes was investigated. It
was found that there was a significant decrease in the activity of
the enzymes analyzed; however, treatment with taurine increased the
activities of SOD, catalase and GSH-Px in the hepatic tissue of the
mice (Fig. 2).
Lipid peroxidation is believed to be one of the most
important parameters of oxidative stress and is measured by
estimating the MDA concentration, a lipid peroxidation end product.
In the present study, increased levels of MDA were observed in the
iron-overloaded livers of experimental animals; however, taurine
treatment was effective in preventing the extreme iron-induced
alterations. These results suggest that the protective effects of
taurine in the liver are correlated with the reduction in
iron-induced oxidative stress.
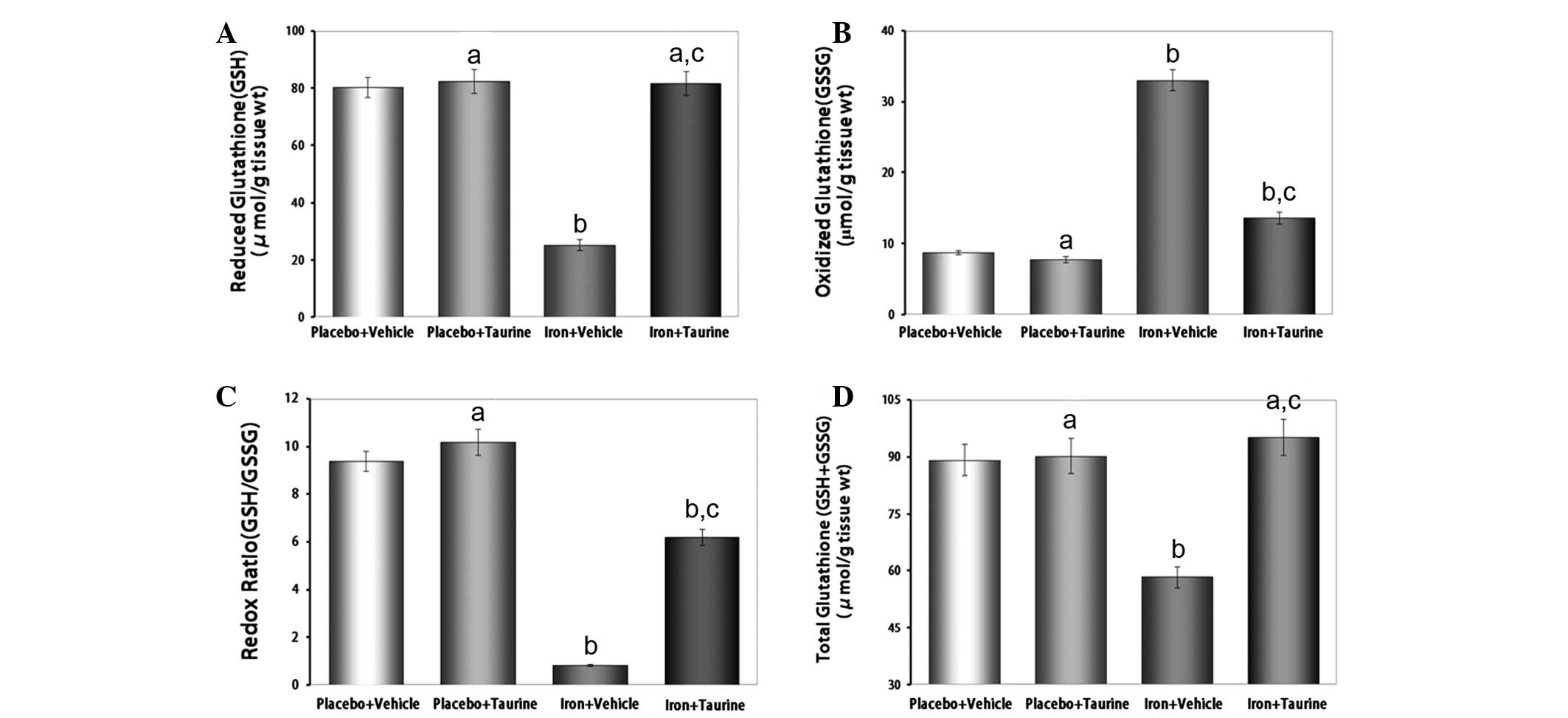
Taurine protects GSH levels in
iron-overloaded mice
Reduced glutathione, a ubiquitous tripeptide thiol,
is an important intracellular metabolite that acts as an
antioxidant and provides a secondary line of defense against
intracellular free radicals and peroxides generated by oxidative
stress (31). The reduced state of
the cell is maintained by a high GSH/GSSG ratio. Owing to the
potent antioxidant properties of taurine, it was hypothesized that
taurine had the potential to preserve this high GSH/GSSG ratio in
conditions of increased oxidative stress (32). It was observed that taurine
supplementation in iron-overloaded mice almost entirely prevented
the decrease in GSH (P<0.01) and increase in GSSG (P<0.01)
levels, thereby partially protecting the redox ratio and
normalizing the GSH+GSSG levels (Fig.
3).
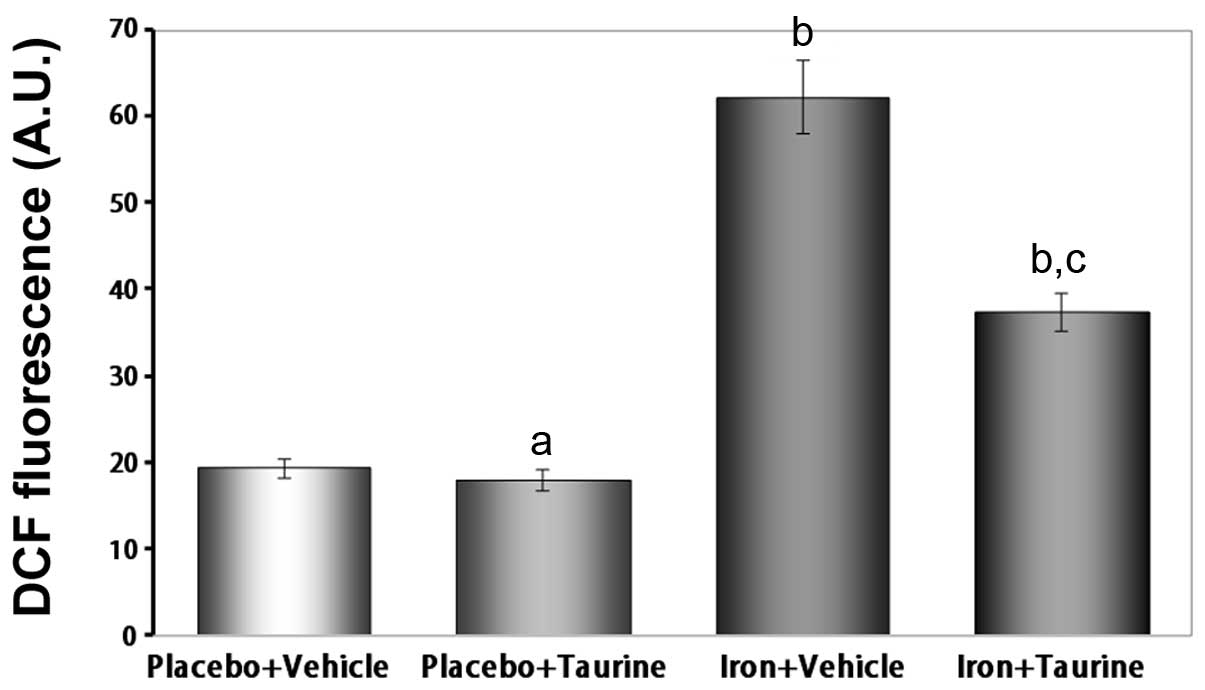
Taurine inhibits ROS generation in
iron-overloaded mice
It is well established that iron induces toxicity in
the liver, as iron is primarily stored in the liver and produces
ROS (33). Therefore, the
intracellular ROS levels were measured using a DCFH-DA assay. The
ROS levels were observed to be significantly increased in the
iron-treated animals compared with those in the untreated animals
(P<0.01). This indicates that supplementation with taurine
significantly prevents ROS formation induced by iron overload
(Fig. 4).
Taurine inhibits mitochondrial swelling
in iron-overloaded mice
Iron-induced damage to the inner mitochondrial
membrane may be assessed using classic swelling techniques, which
monitor the net influx of the osmotic support associated with a
non-specific increase in membrane permeability. It was shown that
iron induced mitochondrial swelling, indicated by the decrease in
the absorbance of the mitochondrial suspension at 520 nm. However,
taurine inhibited this swelling process (P<0.01) (Fig. 5).
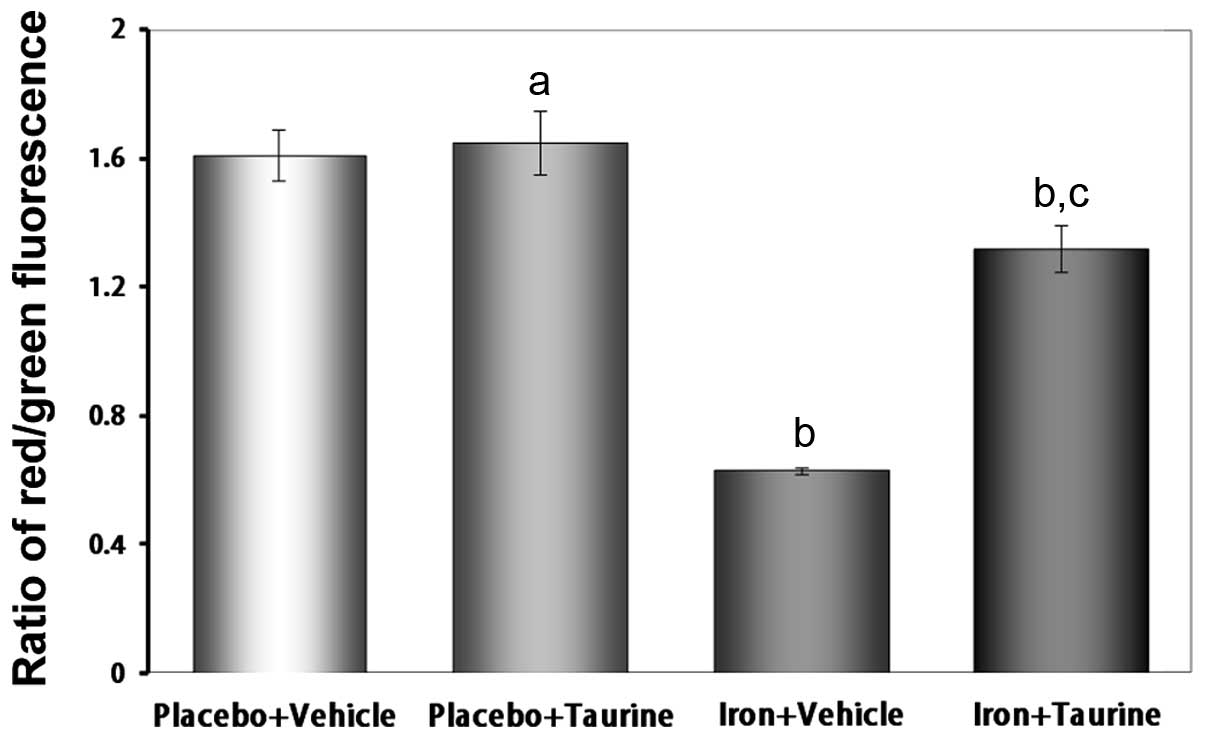
Taurine attenuates the loss of the Δψ in
iron-overloaded mice
The loss of the Δψ is an early indicator of
apoptosis. The unique fluorescent cationic dye JC-1 was used to
measure the Δψ in the mitochondria of hepatocytes. In healthy
cells, where the Δψ is normal, JC-1 accumulates as aggregates in
the mitochondrial matrix, where it emits red fluorescence. In
apoptotic and dead cells, where the Δψ is lost, JC-1 exists in a
monomeric form and emits green fluorescence. Therefore, the red to
green fluorescence intensity ratio may be used to evaluate the Δψ.
As shown in Fig. 6, the iron plus
vehicle group exhibited a decrease in the ratio of red to green
fluorescence intensity (P<0.01), indicating a loss of the Δψ.
However, treatment with taurine significantly attenuated the loss
of the Δψ (P<0.01).
Discussion
Previous studies in humans and animals have shown
that iron overload is a risk factor for liver damage (34). Although oxidative stress is
considered to be a major cause of hepatotoxicity induced by iron
overload, the underlying mechanism remains unknown. In the present
study, mice that were injected with iron exhibited marked symptoms
of iron toxicity, including an elevated liver-to-body weight ratio,
increased levels of AST and ALT in the plasma and serum and
intracellular iron deposition in the liver, concurrent with
increased apoptosis.
Free radical production and oxidative stress play
key roles in the initiation and progression of iron overload
hepatotoxicity (35). In the
livers of iron-overloaded mice, marked increases in ROS and MDA (an
end product of lipid peroxidation) levels were observed, as well as
a depletion of GSH and GSH+GSSG levels. These findings were
indicative of increased oxidative damage. Free radical-induced
lipid peroxidation leads to cellular dysfunction. Clinically, the
AST and ALT levels in the plasma represent biomarkers for liver
function (36). In the present
study, the serum AST and ALT levels in the mice were significantly
elevated following iron overload, suggesting an iron
overload-related injury to the liver.
Taurine is a conditionally essential amino acid that
contains a sulfonic acid group and has several physiological roles
(37,38). Increasing evidence has shown that
taurine exerts strong protective effects due to its antioxidant
characteristics (17,39,40).
In the present study taurine levels in the liver were increased to
investigate whether this prevented iron overload-induced hepatic
damage, despite the fact that taurine has no effect on iron
deposition. This rationale was based on the finding that there is
an increased demand for taurine in instances of oxidative stress.
The results showed that taurine supplementation was associated with
the protection of liver function in iron-overloaded mice. The
protective effects of taurine occurred concurrently with a
reduction in apoptosis, suggesting that taurine was able to
decrease the toxic effects of iron. The protective effects of
taurine have been previously associated with its antioxidant
properties. Furthermore, taurine has been shown to reduce
iron-mediated lipid peroxidation and increase the activities of
antioxidant enzymes (41).
Consistent with the antioxidant properties of
taurine observed in the present study, a marked reduction in MDA
formation was observed in the iron-overloaded mice. The potent
antioxidant properties of taurine are additionally associated with
increased antioxidant enzyme activity. SOD, catalase and GSH-Px are
the key cellular antioxidant enzymes that defend against oxidative
stress. Evidence shows that the activities of these antioxidant
enzymes are decreased when cells and tissues are subjected to
oxidative stress (42,43). The antioxidant effects attributed
to taurine may be associated with its sulfur moiety, and the
modulation of GSH and GSH levels by taurine is critical in the
cellular defense against oxidative stress (44,45).
During oxidative stress, increased cellular demand leads to the
depletion of GSH and the accumulation of GSSG. As a result, a shift
in the redox state occurs and the cell function becomes impaired.
In the present study, taurine supplementation completely prevented
the iron-induced depletion in the GSH levels while protecting the
redox ratio. These findings elucidate a possible mechanism
underlying the actions of taurine and may explain its pleiotropic
and beneficial effects following an increase in oxidative stress
(42,43).
Hepatocyte damage mediated by oxidative stress is
associated with the disruption of the Δψ (46). Mitochondria are important
organelles that have a vital role in apoptosis (47). The mitochondrial permeability
transition pore (mPTP) is a multiprotein complex that can form
large, nonselective pores in the inner mitochondrial membrane
(48). ROS are one of the most
important factors stimulating the opening of the mPTPs. If the
mPTPs are constantly open, mitochondrial swelling can occur,
resulting in the rupture of the outer mitochondrial membrane and
culminating in apoptosis. The present study demonstrated that
overproduction of ROS in iron-overloaded mice increased the
mitochondrial membrane permeability, induced the opening of the
mPTPs, and resulted in mitochondrial swelling and depolarization
(49). Therefore, the collapse in
Δψ may be a consequence of the oxidative stress in hepatocytes.
Taurine treatment led to a reduction in the liver ROS levels due to
its strong ROS scavenging and antioxidant properties, as previously
mentioned. Reduced ROS levels prevented the mPTPs from opening and
the subsequent mitochondrial swelling, which blocked Δψ
depolarization.
In conclusion, the murine model of an
iron-overloaded liver used in the present study demonstrated that
taurine supplementation has beneficial effects on liver function
and apoptosis, with marked reductions in oxidative stress induced
by iron overload. Due to the benefits provided and the absence of
toxicity with taurine supplementation, increased dietary intake of
taurine represents an important nutritional modification that could
be a useful therapeutic alternative to reduce the hepatic toxicity
induced by iron overload in such diseases as hemosiderosis.
Acknowledgements
This study was supported by grants from the Natural
Scientific Foundation of China (nos. 30760075, 30860271, 81100104
and 81160309).
References
|
1
|
Santos PC, Krieger JE and Pereira AC:
Molecular diagnostic and pathogenesis of hereditary
hemochromatosis. Int J Mol Sci. 13:1497–1511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murphy CJ and Oudit GY: Iron-overload
cardiomyopathy: pathophysiology, diagnosis, and treatment. J Card
Fail. 16:888–900. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moretti D, van Doorn GM, Swinkels DW and
Melse-Boonstra A: Relevance of dietary iron intake and
bioavailability in the management of HFE hemochromatosis: a
systematic review. Am J Clin Nutr. 98:468–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alústiza Echeverría JM, Castiella A and
Emparanza JI: Quantification of iron concentration in the liver by
MRI. Insights Imaging. 3:173–180. 2012.PubMed/NCBI
|
|
5
|
Lee M and Kowdley KV: Alcohol’s effect on
other chronic liver diseases. Clin Liver Dis. 16:827–837. 2012.
|
|
6
|
Hayashi H, Piperno A, Tomosugi N, et al:
Patients with chronic hepatitis C may be more sensitive to iron
hepatotoxicity than patients with HFE-hemochromatosis. Intern Med.
49:2371–2377. 2010. View Article : Google Scholar
|
|
7
|
Abdalla MY, Fawzi M, Al-Maloul SR,
El-Banna N, Tayyem RF and Ahmad IM: Increased oxidative stress and
iron overload in Jordanian β-thalassemic children. Hemoglobin.
35:67–79. 2011.PubMed/NCBI
|
|
8
|
Li X, Li H, Lu N, Feng Y, Huang Y and Gao
Z: Iron increases liver injury through oxidative/nitrative stress
in diabetic rats: Involvement of nitrotyrosination of glucokinase.
Biochimie. 94:2620–2627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Serviddio G, Bellanti F, Sastre J,
Vendemiale G and Altomare E: Targeting mitochondria: a new
promising approach for the treatment of liver diseases. Curr Med
Chem. 17:2325–2337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu D, He H, Yin D, Que A, Tang L, Liao Z,
Huang Q and He M: Mechanism of chronic dietary iron
overload-induced liver damage in mice. Mol Med Rep. 7:1173–1179.
2013.PubMed/NCBI
|
|
11
|
Batista TM, Ribeiro RA, da Silva PM,
Camargo RL, Lollo PC, Boschero AC and Carneiro EM: Taurine
supplementation improves liver glucose control in normal protein
and malnourished mice fed a high-fat diet. Mol Nutr Food Res.
57:423–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heidari R, Babaei H and Eghbal MA:
Cytoprotective effects of taurine against toxicity induced by
isoniazid and hydrazine in isolated rat hepatocytes. Arh Hig Rada
Toksikol. 64:15–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartman JC, Brouwer K, Mandagere A, Melvin
L and Gorczynski R: Evaluation of the endothelin receptor
antagonists ambrisentan, darusentan, bosentan, and sitaxsentan as
substrates and inhibitors of hepatobiliary transporters in
sandwich-cultured human hepatocytes. Can J Physiol Pharmacol.
88:682–691. 2010. View
Article : Google Scholar
|
|
14
|
Liao Y, Lu X, Lu C, Li G, Jin Y and Tang
H: Selection of agents for prevention of cisplatin-induced
hepatotoxicity. Pharmacol Res. 57:125–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabassum H, Rehman H, Banerjee BD,
Raisuddin S and Parvez S: Attenuation of tamoxifen-induced
hepatotoxicity by taurine in mice. Clin Chim Acta. 370:129–136.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang GG, Li W, Lu XH, Zhao X and Xu L:
Taurine attenuates oxidative stress and alleviates cardiac failure
in type I diabetic rats. Croat Med J. 54:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higuchi M, Celino FT, Shimizu-Yamaguchi S,
Miura C and Miura T: Taurine plays an important role in the
protection of spermatogonia from oxidative stress. Amino Acids.
43:2359–2369. 2012. View Article : Google Scholar
|
|
18
|
Oudit GY, Sun H, Trivieri MG, Koch SE,
Dawood F, Ackerley C, Yazdanpanah M, Wilson GJ, Schwartz A, Liu PP
and Backx PH: L-type Ca2+ channels provide a major
pathway for iron entry into cardiomyocytes in iron-overload
cardiomyopathy. Nat Med. 9:1187–1194. 2003.
|
|
19
|
Galleano M and Puntarulo S: Hepatic
chemiluminescence and lipid peroxidation in mild iron overload.
Toxicology. 76:27–38. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keith ME, Ball A, Jeejeebhoy KN, Kurian R,
Butany J, Dawood F, Wen WH, Madapallimattam A and Sole MJ:
Conditioned nutritional deficiencies in the cardiomyopathic hamster
heart. Can J Cardiol. 17:449–458. 2001.PubMed/NCBI
|
|
21
|
Okhawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beauchamp C and Fridovich I: Superoxide
dismutase: improved assays and an assay applicable to acrylamide
gels. Anal Biochem. 44:276–287. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hill MF and Singal PK: Right and left
myocardial antioxidant responses during heart failure subsequent to
myocardial infarction. Circulation. 96:2414–2420. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderson ME: Determination of glutathione
and glutathione disulfide in biological samples. Methods Enzymol.
113:548–555. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalantari-Dehaghi M, Chen Y, Deng W,
Chernyavsky A, Marchenko S, Wang PH and Grando SA: Mechanisms of
mitochondrial damage in keratinocytes by Pemphigus vulgaris
antibodies. J Biol Chem. 288:16916–16925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beavis AD, Brannan RD and Garlid KD:
Swelling and contraction of the mitochondrial matrix. I. A
structural interpretation of the relationship between light
scattering and matrix volume. J Biol Chem. 260:13424–13433.
1985.PubMed/NCBI
|
|
27
|
Misra HP and Fridovich I: The role of
superoxide anion in the autoxidation of epinephrine and a simple
assay for superoxide dismutase. J Biol Chem. 247:3170–3175.
1972.PubMed/NCBI
|
|
28
|
Ketterer B: Detoxication reactions of
glutathione and glutathione transferases. Xenobiotica. 16:957–973.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu CM, Zheng GH, Ming QL, Sun JM and
Cheng C: Protective effect of quercetin on lead-induced oxidative
stress and endoplasmic reticulum stress in rat liver via the
IRE1/JNK and PI3K/Akt pathway. Free Radic Res. 47:192–201. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Banu S, Bhaskar B and Balasekar P:
Hepatoprotective and antioxidant activity of Leucas aspera
against D-galactosamine induced liver damage in rats. Pharm Biol.
50:1592–1595. 2012.
|
|
31
|
Pompella A, Visvikis A, Paolicchi A, De
Tata V and Casini AF: The changing faces of glutathione, a cellular
protagonist. Biochem Pharmacol. 66:1499–1503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaffer SW, Azuma J and Mozaffari M: Role
of antioxidant activity of taurine in diabetes. Can J Physiol
Pharmacol. 87:91–99. 2009.PubMed/NCBI
|
|
33
|
Pardo Andreu GL, Inada NM, Vercesi AE and
Curti C: Uncoupling and oxidative stress in liver mitochondria
isolated from rats with acute iron overload. Arch Toxicol.
83:47–53. 2009.PubMed/NCBI
|
|
34
|
Tirnitz-Parker JE, Glanfield A, Olynyk JK
and Ramm GA: Iron and hepatic carcinogenesis. Crit Rev Oncog.
18:391–407. 2013. View Article : Google Scholar
|
|
35
|
Sorrentino P, Terracciano L, D’Angelo S,
et al: Oxidative stress and steatosis are cofactors of liver injury
in primary biliary cirrhosis. J Gastroenterol. 45:1053–1062. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lalisang TJ: Serum bile acid: an
alternative liver function marker in the obstructive jaundice
patient. Acta Med Indones. 44:233–238. 2012.PubMed/NCBI
|
|
37
|
Manna P, Sinha M and Sil PC: Taurine plays
a beneficial role against cadmium-induced oxidative renal
dysfunction. Amino Acids. 36:417–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sinha M, Manna P and Sil PC: Taurine, a
conditionally essential amino acid, ameliorates arsenic-induced
cytotoxicity in murine hepatocytes. Toxicol In Vitro. 21:1419–1428.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aruoma OI, Halliwell B, Hoey BM and Butler
J: The antioxidant action of taurine, hypotaurine and their
metabolic precursors. Biochem J. 256:251–255. 1988.PubMed/NCBI
|
|
40
|
Budhram R, Pandya KG and Lau-Cam CA:
Protection by taurine and thiotaurine against biochemical and
cellular alterations induced by diabetes in a rat model. Adv Exp
Med Biol. 775:321–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang IS and Kim C: Taurine chloramine
administered in vivo increases NRF2-regulated antioxidant enzyme
expression in murine peritoneal macrophages. Adv Exp Med Biol.
775:259–267. 2013. View Article : Google Scholar
|
|
42
|
Ghyasi R, Sepehri G, Mohammadi M,
Badalzadeh R and Ghyasi A: Effect of mebudipine on oxidative stress
and lipid peroxidation in myocardial ischemic-reperfusion injury in
male rat. J Res Med Sci. 17:1150–1155. 2012.PubMed/NCBI
|
|
43
|
Ramesh B, Karuna R, Sreenivasa RS, Haritha
K, Sai MD, Sasi BR and Saralakumari D: Effect of Commiphora
mukul gum resin on hepatic marker enzymes, lipid peroxidation
and antioxidants status in pancreas and heart of streptozotocin
induced diabetic rats. Asian Pac J Trop Biomed. 2:895–900.
2012.
|
|
44
|
Huxtable RJ: Physiological actions of
taurine. Physiol Rev. 72:101–163. 1992.
|
|
45
|
Woo HA, Chae HZ, Hwang SC, Yang KS, Kang
SW, Kim K and Rhee SG: Reversing the inactivation of peroxiredoxins
caused by cysteine sulfinic acid formation. Science. 300:653–656.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sharma V, Anderson D and Dhawan A: Zinc
oxide nanoparticles induce oxidative DNA damage and ROS-triggered
mitochondria mediated apoptosis in human liver cells (HepG2).
Apoptosis. 17:852–870. 2012. View Article : Google Scholar
|
|
47
|
Zhao CY, Liu XY and Shen C: Molecular
mechanisms of mitochondrial damage in the development of liver
failure. Zhonghua Gan Zang Bing Za Zhi. 19:955–957. 2011.(In
Chinese).
|
|
48
|
Gottlieb RA and Gustafsson AB:
Mitochondrial turnover in the heart. Biochim Biophys Acta.
1813.1295–1301. 2011.
|
|
49
|
Bae YS, Oh H, Rhee SG and Yoo YD:
Regulation of reactive oxygen species generation in cell signaling.
Mol Cells. 32:491–509. 2011. View Article : Google Scholar : PubMed/NCBI
|