Introduction
Despite ongoing advances in diagnosis and treatment,
colorectal cancer (CRC) remains a common fatal cancer affecting
both genders that contributes to an estimated 600,000 mortalities
worldwide annually (1). Among the
various factors affecting the chance of survival in patients with
CRC, the formation of distant metastases is the most dominant.
Various proteins have been demonstrated to be dysregulated and
function in the metastasis of CRC; however, the molecular
mechanisms and signaling pathways involved require further
study.
Y-box binding protein-1 (YB-1) is a member of the
cold-shock protein superfamily. The protein is encoded by the YBX1
gene and contains a highly conserved nucleic-acid-binding motif
that binds to both DNA and RNA. YB-1 functions within the cytoplasm
and nucleus with roles in transcriptional regulation, DNA repair
and replication and pre-mRNA splicing. Recent studies have regarded
YB-1 as an oncoprotein due to its involvement in uncontrolled
proliferation (2), apoptosis
resistance (3), sustained
angiogenesis (4), invasion and
metastasis (5). Furthermore, YB-1
has been associated with chemotherapeutic resistance (6) and high YB-1 expression is closely
linked to the poor clinical outcome in various types of cancer,
including gastric (7), ovarian
(8) and prostate (9). Other studies have suggested that the
carcinogenic effect of YB-1 may be associated with
epithelial-to-mesenchymal transition (EMT) (10,11).
EMT is a well-known regulatory program involved in
embryonic development, wound healing and tumor development
(12). Through this program,
epithelial cells lose apical-basal polarity and tight junctions,
thus acquiring a mesenchymal phenotype that is more amenable to
cell migration and invasion. Molecularly, EMT is characterized by a
downregulation of epithelial markers and an upregulation of
mesenchymal markers. E-cadherin is a well-known epithelial marker
that hinders the movement of epithelial cells by mediating adherens
junctions (13). The loss of
E-cadherin has been regarded to have an unfavorable effect on the
overall survival of patients with CRC, as described by He et
al (14). Furthermore,
emerging studies have suggested that mesenchymal markers, such as
vimentin and N-cadherin, promote the metastasis of CRC and act as
novel prognostic factors (15,16).
A close association between YB-1 and EMT was first
identified by Evdokimova et al (17), who showed that YB-1 could promote
EMT in premalignant breast epithelial cells in vivo and
in vitro. Twist-related protein 1 (Twist1) is another
well-established EMT transcription factor, and it was shown by
Shiota et al (18) that a
mutual regulation of Twist1 and YB-1 could promote the malignant
progression of bladder cancer, again suggesting an involvement of
YB-1 in EMT. However, neither the biological role of YB-1 nor its
association with EMT has been sufficiently studied in CRC. In this
study, the association between the expression of YB-1 and three EMT
markers (E-cadherin, N-cadherin and vimentin) in human CRC tissues
was analyzed. Small interfering RNA (siRNA) was employed to explore
the specific roles of YB-1 in a CRC cell line, HT-29,
well-characterized for use in EMT-associated assays in vitro
and in vivo (19,20). The aim of the study was to
investigate the hypotheses that i) YB-1 may be associated with a
dysregulated EMT phenotype in CRC and ii) YB-1 may promote the
growth, invasion and migration of CRC cells, in part through the
induction of EMT.
Materials and methods
Tissue samples and patient data
A total of 80 primary CRC tumors and corresponding
normal tissues were collected from patients with CRC undergoing
surgery at the Department of Surgery, The Sixth People’s Hospital
affiliated to Shanghai Jiao Tong University (Shanghai, China),
between 2011 and 2012. None of the patients had received
preoperative chemotherapy or radiotherapy. The study was approved
by the Ethics Committee of The Sixth People’s Hospital affiliated
to Shanghai Jiao Tong University. Written informed consent was
obtained from patients for use of their tissue specimens in the
study. The basic clinical characteristics of the patients are shown
in Table I.
 | Table IAssociation between YB-1 expression
and clinicopathological parameters. |
Table I
Association between YB-1 expression
and clinicopathological parameters.
| Variables | Number | Low YB-1 expression
(n) | High YB-1 expression
(n) | P-value |
|---|
| Total | 80 | 27 | 53 | |
| Gender |
| Male | 47 | 14 | 33 | 0.371 |
| Female | 33 | 13 | 20 | |
| Age in years |
| ≤60 | 31 | 12 | 19 | 0.456 |
| >60 | 49 | 15 | 34 | |
| Tumor location |
| Colon | 50 | 18 | 32 | 0.583 |
| Rectum | 30 | 9 | 21 | |
| Tumor
differentiation |
| Well | 28 | 15 | 13 | 0.006 |
| Moderate and
poorly | 52 | 12 | 40 | |
| Tumor invasion |
| pT1-T2 | 35 | 17 | 18 | 0.013 |
| pT3-T4 | 45 | 10 | 35 | |
| Lymph node
metastasis |
| Present | 44 | 8 | 36 | 0.001 |
| Absent | 36 | 19 | 17 | |
| Distant
metastasis |
| Present | 42 | 9 | 33 | 0.014 |
| Absent | 38 | 18 | 20 | |
Immunohistochemistry
Paraffin-embedded tissue specimens were cut at
4-μm-thickness. Following deparaffinization and dehydration, the
sections were placed in citrate buffer solution and heated using a
microwave oven for antigen retrieval. Following this, 0.3% hydrogen
peroxidase and methanol were used to block endogenous peroxidase
activity for 25 min and the sections were incubated with primary
antibodies against YB-1 (1:200, Epitomics, Burlingame, CA, USA),
E-cadherin (1:150, Epitomics), N-cadherin (1:150, Epitomics) and
vimentin (1:200, Bioworld, St. Louis Park, MN, USA) at 4°C
overnight. EnVision™ reagents (Dako, Glostrup, Denmark) were used
to detect antibody binding. The sections were then counterstained
with hematoxylin and observed by light microscopy. The
immunostaining evaluation was performed by two independent
researchers, who were blinded to the clinicopathological parameters
of the patients. The evaluation principle used was that described
in the study by Wu et al (21).
Cell culture
The HT-29 human colon adenocarcinoma cell line was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were maintained in McCoy’s 5A
culture medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco-BRL, Grand Island, NY, USA)
and 1% penicillin/streptomycin (Gibco-BRL) at 37°C in a humidified
atmosphere containing 95% air and 5% CO2. The medium was
changed every two days.
YB-1 RNA interference
Commercially generated siRNA (GenePharma, Shanghai,
China) was used to decrease YB-1 expression in HT-29 cells. A
negative random siRNA that did not target any human gene was used
as a negative control. The sequences for siRNA and control siRNA
are shown in Table II. The cells
were divided into three groups: Control (cells with transfection
reagent only), siControl (cells treated with negative control siRNA
and transfection reagent) and siYB-1 (cells treated with siRNA and
transfection reagent).
 | Table IIsiRNA sequences. |
Table II
siRNA sequences.
| Gene | Orientation | Sequence
(5′-3′) |
|---|
| YB-1-homo-375
(siRNA1) | Forward |
GGAACGGAUAUGGUUUCAUTT |
| Reverse |
AUGAAACCAUAUCCGUUCCTT |
| YB-1-homo-529
(siRNA2) | Forward |
GGAGGCAGCAAAUGUUACATT |
| Reverse |
UGUAACAUUUGCUGCCUCCTT |
| YB-1-homo-628
(siRNA3) | Forward |
GGUCCUCCACGCAAUUACCAGCAAA |
| Reverse |
UUUGCUGGUAAUUGCCUGGAGGACC |
| YB-1-homo-940
(siRNA4) | Forward |
GGACGGCAAUGAAGAAGAUTT |
| Reverse |
AUCUUCUUCAUUGCCGUCCTT |
| Control siRNA | Forward |
UUCUUCGAACGUGUCACGUTT |
| Reverse |
ACGUGACACGUUCGGAGAATT |
Transfection was performed using Lipofectamine™ 2000
transfection reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). Briefly, 3,000 cells per well were seeded in a 96-well plate
24 h before transfection. The siRNAs were then diluted in
serum-free medium and mixed with the transfection reagent that was
pre-diluted in an equal volume of serum-free medium. The mixture
was then added to each well. Following incubation at 37°C for 6 h,
the cell medium was changed to complete medium. For the following
assays, the reverse transcription polymerase chain reaction
(RT-PCR) was performed 24 h after transfection and western
blotting, MTT, apoptosis and Transwell™ assays were performed 48 h
after transfection.
Quantitative (q)PCR
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen Life Technologies) according
to the manufacturer’s instructions. The obtained RNA was used to
synthesize cDNA by Superscript III Reverse Transcriptase (Promega
Corp., Madison, WI, USA). qPCR reaction mixes were prepared using
SYBR Green (Takara, Shiga, Japan) and run on the StepOne™ Plus
Real-Time PCR system (Applied Biosystems, Foster City CA, USA) with
the following conditions: 95°C for 5 min, 95°C for 5 sec and 60°C
for 30 sec, for 40 cycles. The relative mRNA expression value was
calculated by the 2−ΔΔT method, and β-actin expression
was taken as an internal control. The sequences of the primers used
are shown in Table III.
 | Table IIIPrimer sequences for the quantitative
polymerase chain reaction. |
Table III
Primer sequences for the quantitative
polymerase chain reaction.
| Gene | Orientation | Sequence
(5′-3′) | Length (bp) |
|---|
| YB-1 | Forward |
TACCTTCGCAGTGTAGGAGAT | 96 |
| Reverse | ACCACCAGG
ACCTGTAACAT | |
| E-cadherin | Forward | AAG ACA AAG AAGGCA
AGGT | 147 |
| Reverse |
AGAGAGTGTATGTGGCAATG | |
| N-cadherin | Forward | CAAGATGGGTCA ATGGAA
ATAG | 174 |
| Reverse |
CTCAGGAATACGAGCCTTCAC | |
| Vimentin | Forward |
CTGTAAGTTGGTAGCACTGAG | 84 |
| Reverse | TTAGGGGAA
ACCGTTAGAC | |
| β-actin | Forward |
AAGGTGACAGCAGTCGGTT | 195 |
| Reverse |
TGTGTGGACTTGGGAGAGG | |
Western blot analysis
Total protein was extracted from the cells using
lysis buffer (50 mmol/L Tris-HCl, pH8.0; 150 mmol/L NaCl; 1%
TritonX-100; 100 μg/ml PMSF). The lysates containing 20 μg protein
were separated by 12% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. The membranes were blocked for 1 h at room
temperature in blocking solution [5 g skimmed milk powder and 100
ml Tris-buffered saline with 0.1% Tween 20 (TBST)]. The membranes
were then incubated overnight in primary antibody [anti-YB-1
(1:2,000), -E-cadherin (1:2,000), -N-cadherin (1:5,000) and
-vimentin (1:5,000)] at 4°C. Following three washes with TBST, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody for 2 h at room temperature. Chemiluminescence
reagent (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used
to detect the protein expression according to the manufacturer’s
instructions.
MTT assay
Cell proliferation was determined by an MTT assay.
Cells (3,000 per well) were incubated with 150 μl complete media
containing 0.5 mg/ml MTT for 3–4 h until purple precipitate was
visible. Dimethylsulfoxide was used to dissolve the precipitate and
the plate was agitated gently for 10 min. The absorbance of each
well was then measured using a microplate reader (Molecular
Devices, Sunnyvale, CA, USA) at a wavelength of 490 nm.
Apoptosis assay
Flow cytometry using propidium iodide (PI) staining
was employed to determine the effect of YB-1 on cell apoptosis. The
cells (1×106) were washed twice with phosphate-buffered
saline and resuspended in 500 μl binding buffer containing 5 μl
Annexin V-fluorescein isothiocyanate (FITC). A total of 5 μl PI was
then added to the cells and incubated at room temperature for 20
min. The apoptotic cells were subsequently counted by flow
cytometry. The Annexin V-FITC Cell Apoptosis Analysis kit was
purchased from KeyGen Biotech (Nanjing, China).
Transwell assay
Transwell assays were performed to assess cell
migration and invasion using Transwell chambers (BD Biosciences,
San Jose, CA, USA). For the Transwell assay without Matrigel™,
cells resuspended in 150 μl serum-free medium were added into the
upper chamber and 600 μl McCoy’s 5A culture medium with 10% FBS was
added into the lower chamber. Following incubation for 24 h at 37°C
with 5% CO2 humidified conditions, non-migrating cells
in the upper chamber were scraped using a cotton swab. The
migrating cells were fixed with 4% polyoxymethylene for 15 min and
stained with crystal violet for 10 min. Finally, the stained cells
from six random fields were counted, and images were captured under
a light microscope.
For the Transwell assay with Matrigel, 50 μl
pre-diluted Matrigel was added into the chambers and incubated at
37°C for 2 h. The assay was then performed using the method
described for the Transwell assay without Matrigel.
Statistical analysis
All assays were repeated three times independently.
The results are presented as the mean ± standard deviation. SPSS
16.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used
to perform all the statistical analyses. The data from the cell
assays were analyzed by Student’s t test. The correlation between
YB-1 expression and clinicopathological parameters was determined
by χ2 test, while the correlation between YB-1 and
EMT-related markers was determined by nonparametric Spearman’s rank
correlation coefficient (r). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of YB-1 and EMT markers is
correlated in CRC tissues
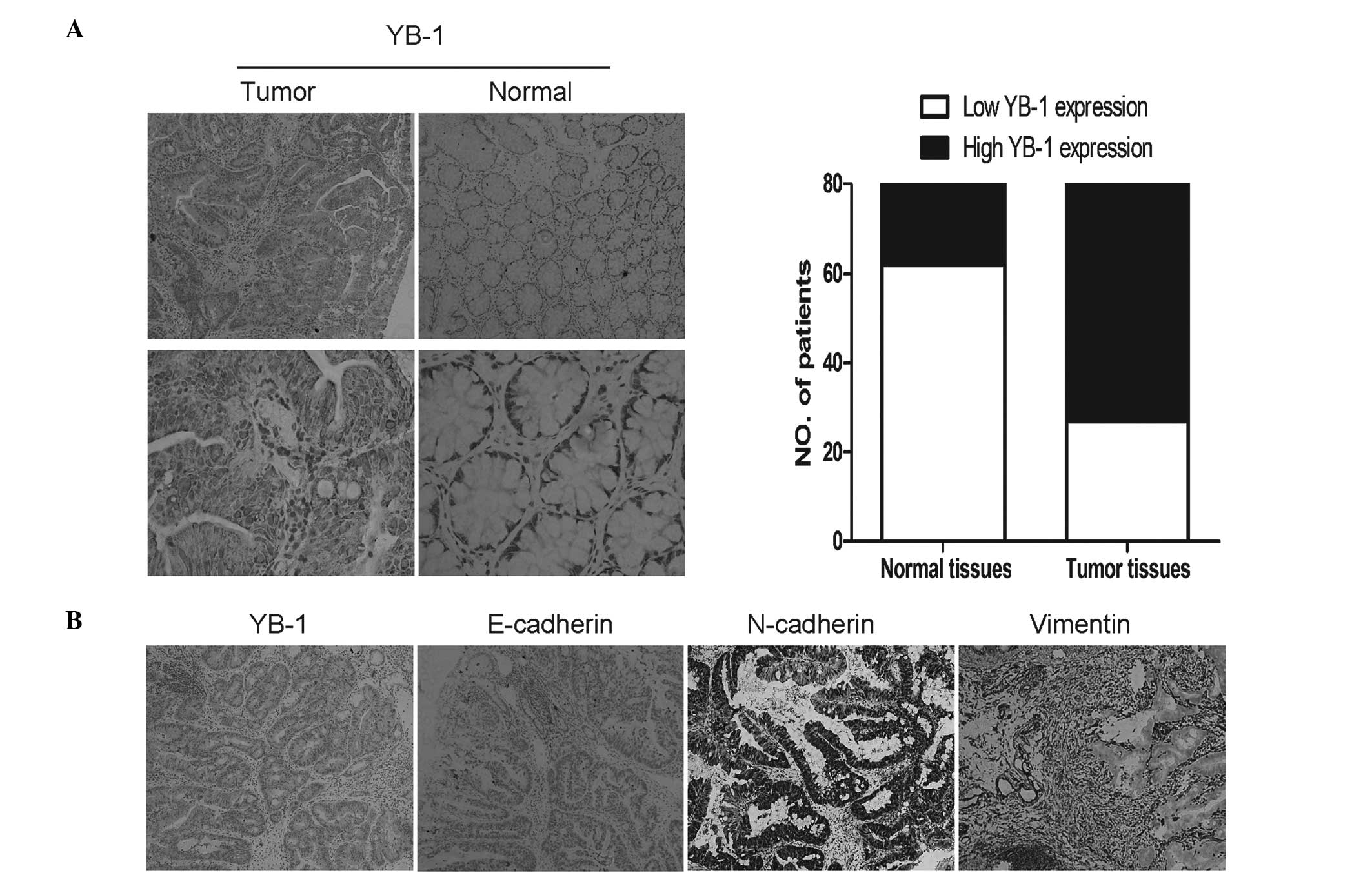
By immunohistochemical analysis, strong YB-1
expression was observed in 66.3% (53/80) of tumors, compared with
22.5% (18/80) of the matched normal tissues (Table IV). To determine whether YB-1 was
correlated with an EMT phenotype, the expression of three EMT
markers (E-cadherin, N-cadherin and vimentin) was investigated
(Fig. 1). The expression of
E-cadherin, an epithelial marker, was weak in 72.5% (58/80) of
tumors, whereas strong expression of two mesenchymal markers
(N-cadherin and vimentin) was detected in 60.0% (48/80) and 70.0%
(56/80) of the tumors, respectively (Table IV). Correlation analysis showed
that YB-1 expression was negatively correlated with E-cadherin
expression (r=−0.330, P=0.003), but positively correlated with the
expression of N-cadherin (r=0.335, P=0.002) and vimentin (r=0.456,
P<0.001).
 | Table IVCorrelation between YB-1 and EMT
markers. |
Table IV
Correlation between YB-1 and EMT
markers.
| | YB-1
expression | | |
|---|
| |
| | |
|---|
| EMT markers | N | Low (n) | High (n) | r | P-value |
|---|
| E-cadherin | | | | | |
| Low | 58 | 14 | 44 | −0.330 | 0.003 |
| High | 22 | 13 | 9 | | |
| N-cadherin | | | | | |
| Low | 32 | 17 | 15 | 0.335 | 0.002 |
| High | 48 | 10 | 38 | | |
| Vimentin | | | | | |
| Low | 24 | 16 | 8 | 0.456 | <0.001 |
| High | 56 | 11 | 45 | | |
YB-1 expression is associated with
clinicopathological parameters
The association between YB-1 and clinicopathological
parameters of patients with CRC is shown in Table I. High YB-1 expression was shown to
have a statistically significant association with tumor
differentiation (P=0.006), tumor invasion (P=0.013), lymph node
metastasis (P=0.001) and distant metastasis (P=0.014). However, no
correlation was observed between strong YB-1 expression and other
parameters, including age (P=0.456), gender (P=0.371) and tumor
location (P=0.583).
Knockdown of YB-1 inhibits proliferation
and enhances apoptosis in the HT-29 cell line
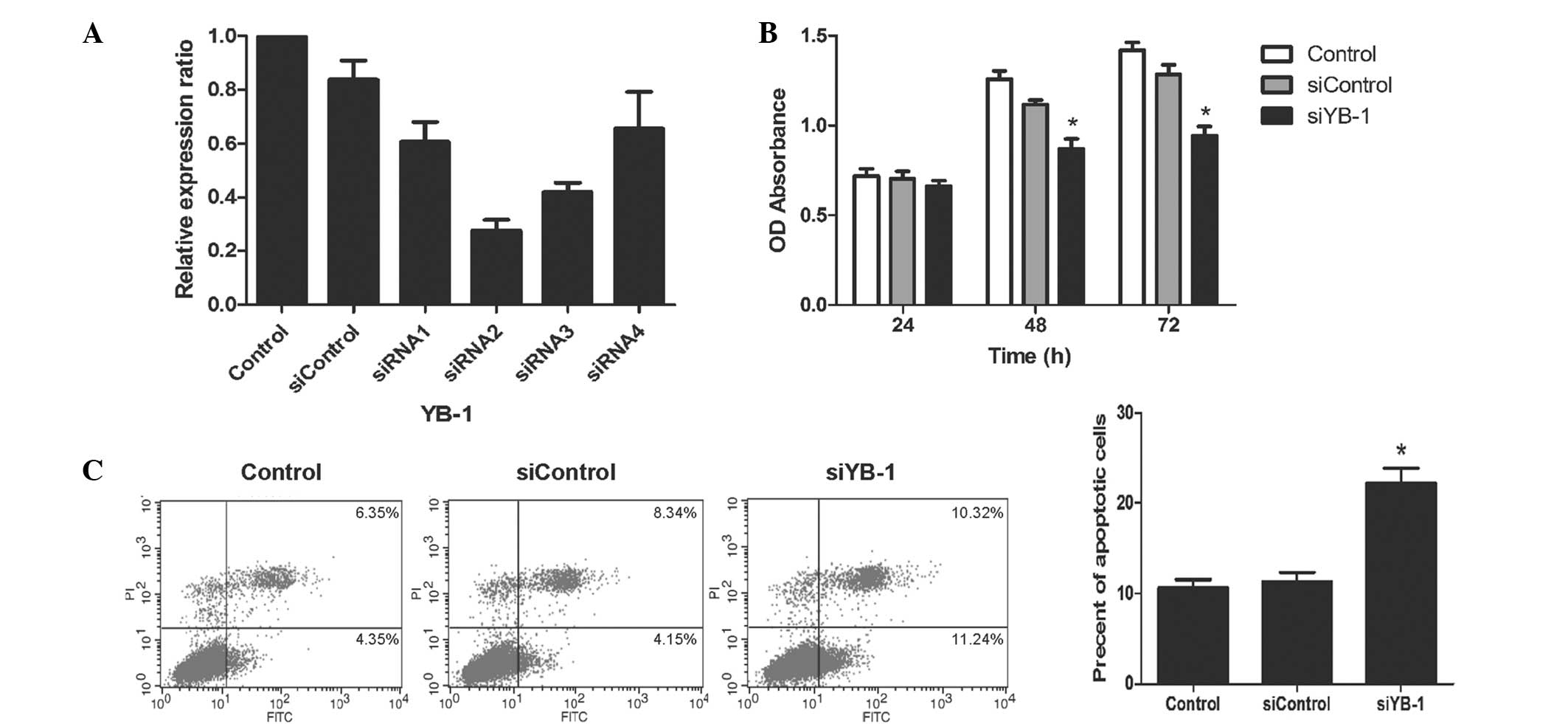
To explore the specific biological function of YB-1
in CRC, siRNA was used to knock down YB-1 in HT-29 cells. RT-PCR
was employed to determine the transfection efficiency of the siRNAs
and showed that the YB-1 mRNA expression was reduced by varying
degrees using different siRNA sequences (Fig. 2A). Compared with the siControl
group, siRNA1 reduced YB-1 mRNA expression by 27.6%, siRNA2 by
67.1% and siRNA3 by 49.8%. No statistically significant difference
was observed between the siRNA control and siRNA4 (P>0.05). As a
result, siRNA2 was selected for the subsequent in vitro
assays.
It is widely accepted that uncontrolled
proliferation and resistance to apoptosis are basic features of
malignancy. In this study, cell proliferation was measured using an
MTT assay. The proliferation of the cells treated with siRNA to
downregulate YB-1 expression (siYB-1 group) was significantly
inhibited as compared with the control and siControl groups
(P<0.01, Fig. 2B). The number
of apoptotic cells was next calculated by flow cytometry. It was
observed that downregulation of YB-1 by siRNA resulted in an
increased proportion of apoptotic cells as compared with the other
two groups (apoptotic rate, 22.3±1.3 vs. 10.6±0.7 and 11.3±0.9%;
siYB-1 group versus the control and siControl groups, respectively;
P<0.01; Fig. 2C). These results
suggest that YB-1 may promote the growth of HT-29 cells.
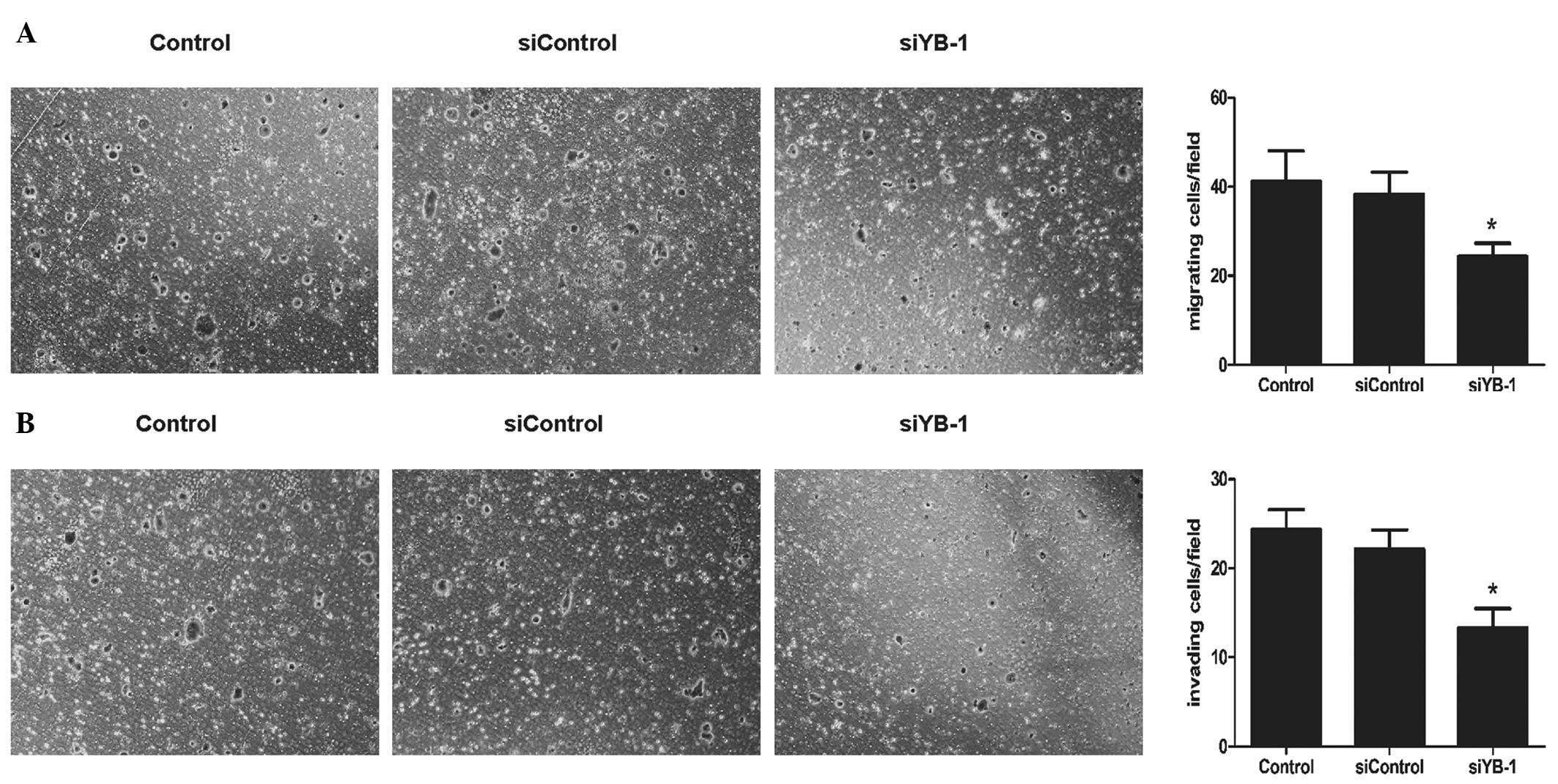
Knockdown of YB-1 inhibits the migration
and invasion of HT-29 cells
Invasion and metastasis are critical factors
associated with the high fatality rate in patients with malignant
tumors. Through the Transwell migration assay, it was calculated
that the number of migratory cells was significantly decreased in
the siYB-1 group, as compared with that in the other two groups
(24.4±2.6 vs. 42.1±6.4 and 38.9±4.6/field; siYB-1 group versus the
control and siControl groups, respectively; P<0.05; Fig. 3A). Using a Transwell invasion assay
method, it was also found that the cells in the siYB-1 group
exhibited a significant decrease in the number of cells invading
across the Matrigel (13.3±1.9 vs. 24.5±2.0 and 22.5±1.8/field;
siYB-1 group versus the control and siControl groups, respectively;
P<0.01; Fig. 3B). Collectively,
this suggested that YB-1 may contribute to the invasion and
migration of HT-29 cells.
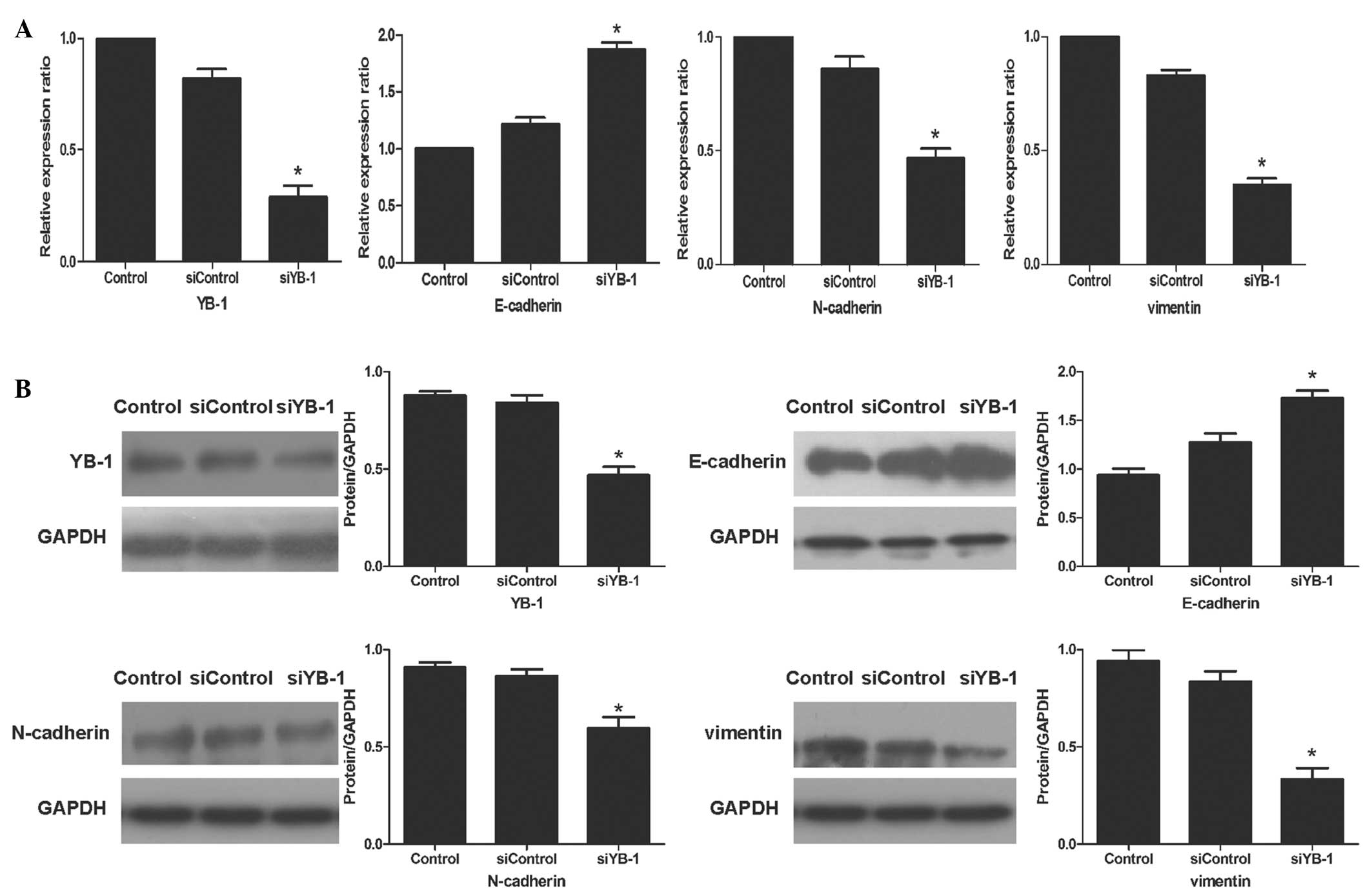
Knockdown of YB-1 reverses the EMT
phenotype
Since EMT is defined as a key molecular event
promoting tumor progression, it was investigated whether YB-1 could
promote the EMT program in HT-29 cells. Through qPCR, it was
observed that the mRNA levels of E-cadherin were increased by
53.9%, compared with those in the siControl group. Conversely, the
mRNA levels of N-cadherin and vimentin were decreased by 45.4 and
57.9%, respectively (Fig. 4A).
These results were confirmed by western blotting (Fig. 4B). Based on these findings, it can
be speculated that YB-1 can promote the invasion and metastasis of
HT-29 cells by regulating the EMT program.
Discussion
Research over the past decades has elucidated the
roles of oncogenes and epigenetic changes in the malignant
progression of CRC. Recent studies have investigated the underlying
molecular mechanisms in CRC, and particularly those associated with
the EMT, which regulate cell survival (22), drug resistance (23), invasion and metastasis (24). A further understanding of the
initiating factors that regulate EMT mechanisms is likely to be
beneficial in the development of targeted therapy for CRC.
YB-1 has been described as a versatile oncoprotein
and has been shown to facilitate numerous hallmarks of cancer
(25). However, research
investigating the specific biological role of YB-1 in CRC is still
in its infancy. Tsofack et al (26) found that overexpressing YB-1 in two
CRC cell lines (SW480 and HT-29) could induce resistance to
oxaliplatin; this process could be blocked by knockdown of non-POU
domain containing octamer-binding protein and RALY heterogeneous
nuclear ribonucleoprotein. Jürchott et al (27) demonstrated that YB-1 could activate
the mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase signaling pathway by regulating cyclin B1
in HCT116 colon cancer cells and that nuclear YB-1 expression was
associated with pulmonary metastasis. However, neither of these
studies reported the role of YB-1 in EMT. Considering the efforts
of the study by Evdokimova et al (17) in breast cancer, this study aimed to
investigate whether YB-1 is also crucial for proliferation,
apoptosis resistance, invasion and migration in CRC and whether
these malignant characteristics are caused by the regulation of
YB-1 in the EMT program.
Firstly, the expression of YB-1 in human CRC tissues
and matched normal tissues was analyzed. It was found that YB-1 was
overexpressed in tumor tissues and was significantly correlated
with tumor differentiation and invasion, lymph node metastasis and
distant metastasis. No significant correlation was observed between
YB-1 expression and other clinicopathological factors, including
gender, age and tumor location. To investigate whether YB-1
expression was associated with the characteristics of EMT,
correlation analysis between YB-1 and three widely acknowledged EMT
markers was performed. It was shown that YB-1 expression was
negatively correlated with E-cadherin expression, but positively
correlated with the expression of N-cadherin and vimentin. This
observation suggested a potential involvement of YB-1 in the EMT
program.
To further investigate the role of YB-1 in the
malignant potential of CRC, YB-1 was silenced using siRNA in the
HT-29 colon cancer cell line. Silencing YB-1 induced changes in
cancer cells resembling those of reversing the EMT program.
Knockdown of YB-1 i) inhibited cell proliferation and enhanced
apoptosis; ii) inhibited cell invasion and migration; iii)
upregulated E-cadherin and downregulated N-cadherin and vimentin.
Taken together, these data indicate that YB-1 may drive malignant
progression partly by regulating EMT in HT-29 cells.
The involvement of YB-1 in EMT demonstrated in this
study has been supported by previous studies using other cancer
models and tumor types. In prostate cancer, research found that
knockdown of YB-1 caused a 50% reduction in cell invasion, and
further investigation suggested that YB-1 regulated EMT by
mammalian target of rapamycin and mitogen-activated protein kinase
signaling (9,27). In melanoma, the stimulating
function of YB-1 in proliferation, invasion, apoptosis resistance
and chemoresistance and the regulation of YB-1 in EMT-related
signaling pathways have been widely proved (29,30).
Di Constanzo et al (31)
demonstrated that YB-1 could enhance cell mobility and confer
mesenchymal characteristics in squamous cancer cells. Furthermore,
YB-1 is a transcriptional activator of the CD44 gene, which has
been identified to enhance the invasion of CRC by EMT, thus adding
further support towards a link between YB-1 and EMT in CRC
(32,33). Jürchott et al (27) suggested that CRC metastasis may be
associated with the promotion of proliferation by YB-1. However,
based on the results of the present study, it is speculated that
this may additionally be due to EMT induction by YB-1.
Of note, there are different hypotheses regarding
the function of YB-1. Although an clear pro-growth function of YB-1
has been widely proved in numerous types of tumor, such as lung
cancer and osteosarcoma (2,34),
other studies have suggested that YB-1 may enhance EMT by
inhibiting proliferation in breast cancer (15,35,36).
This difference is likely caused by the tissue specificity
exhibited by the oncogenic function of YB-1; even within the same
tumor, the role of YB-1 may be cell type-dependent (18). Other studies have found little
association between YB-1 and EMT phenotype in gastric cancer,
despite the role of YB-1 in promoting the invasion and migration of
gastric cancer (7,37). This may be due to differences in
the experimental methods and evaluation criterions. Furthermore, to
the best of our knowledge, there is no systematic and accurate
evaluation guideline for defining EMT phenotype; this is also
responsible for divergent conclusions.
In conclusion, the present study demonstrates that
the expression of YB-1 is correlated with malignant tumor
progression and an EMT phenotype in human CRC tissues. In
vitro assays suggest that YB-1 may promote the proliferation,
apoptosis resistance, invasion and migration of HT-29 cells by
inducing EMT. These data enhance the current understanding of YB-1
in CRC and suggest that YB-1 may be a promising target for
developing novel therapies for CRC. Further studies are now
required to focus on the specific signaling network by which YB-1
regulates the EMT in CRC.
Acknowledgements
The authors would like to thank Professor Yu-Ping
Gao (Department of Pathology, Renji Hospital, School of Medicine,
Shanghai Jiao Tong University) for her kind assistance in the
immunohistochemistry assay. This study was financially supported by
funding from the Science and Technology Commission of Shanghai
Municipality (11ZR1427500).
References
|
1
|
Garcia-Albeniz X and Chan AT: Aspirin for
the prevention of colorectal cancer. Best Pract Res Clin
Gastroenterol. 25:461–472. 2011. View Article : Google Scholar
|
|
2
|
Lasham A, Samuel W, Cao H, et al: YB-1,
the E2F pathway, and regulation of tumor cell growth. J Natl Cancer
Inst. 104:133–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bommert KS, Effenberger M, Leich E, et al:
The feed-forward loop between YB-1 and MYC is essential for
multiple myeloma cell survival. Leukemia. 27:441–450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi M, Shimajiri S, Izumi H, et al:
Y-box binding protein-1 is a novel molecular target for tumor
vessels. Cancer Sci. 101:1367–1373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lovett DH, Cheng S, Cape L, Pollock AS and
Mertens PR: YB-1 alters MT1-MMP trafficking and stimulates MCF-7
breast tumor invasion and metastasis. Biochem Biophys Res Commun.
398:482–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi YK, Cho SG, Choi HS, Woo SM, Yun YJ,
Shin YC and Ko SG: JNK1/2 activation by an extract from the roots
of Morus alba L. reduces the viability of
multidrug-resistant MCF-7/Dox cells by inhibiting YB-1-dependent
MDR1 expression. Evid Based Complement Alternat Med.
2013:7419852013.PubMed/NCBI
|
|
7
|
Wu Y, Yamada S, Izumi H, et al: Strong
YB-1 expression is associated with liver metastasis progression and
predicts shorter disease-free survival in advanced gastric cancer.
J Surg Oncol. 105:724–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panupinthu N, Yu S, Zhang D, et al:
Self-reinforcing loop of amphiregulin and Y-box binding protein-1
contributes to poor outcomes in ovarian cancer. Oncogene. Jul
15–2013.(Epub ahead of print).
|
|
9
|
Imada K, Shiota M, Kohashi K, et al:
Mutual regulation between Raf/MEK/ERK signaling and Y-box–binding
protein-1 promotes prostate cancer progression. Clin Cancer Res.
19:4638–4650. 2013.PubMed/NCBI
|
|
10
|
Khan MI, Adhami VM, Lall RK, et al: YB-1
expression promotes epithelial-to-mesenchymal transition in
prostate cancer that is inhibited by a small molecule fisetin.
Oncotarget. 5:2462–2474. 2014.PubMed/NCBI
|
|
11
|
Zhang H, Cheng S, Zhang M, et al:
Prostaglandin E2 promotes hepatocellular carcinoma cell invasion
through upregulation of YB-1 protein expression. Int J Oncol.
44:769–780. 2014.PubMed/NCBI
|
|
12
|
Franco-Chuaire ML, Magda Carolina SC and
Chuaire-Noack L: Epithelial-mesenchymal transition (EMT):
principles and clinical impact in cancer therapy. Invest Clin.
54:186–205. 2013.PubMed/NCBI
|
|
13
|
David JM and Rajasekaran AK: Dishonorable
discharge: the oncogenic roles of cleaved E-cadherin fragments.
Cancer Res. 72:2917–2923. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, Chen Z, Jia M and Zhao X:
Downregulated E-cadherin expression indicates worse prognosis in
Asian patients with colorectal cancer: evidence from meta-analysis.
PLoS One. 8:e708582013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Todosi AM, Gavrilescu MM, Aniţei GM, Filip
B and Scripcariu V: Colon cancer at molecular level - usefulness of
epithelial-mesenchymal transition analysis. Rev Med Chir Soc Med
Nat Iasi. 116:1106–1111. 2012.PubMed/NCBI
|
|
17
|
Evdokimova V, Tognon C, Ng T, et al:
Translational activation of snail1 and other developmentally
regulated transcription factors by YB-1 promotes an
epithelial-mesenchymal transition. Cancer Cell. 15:402–415. 2009.
View Article : Google Scholar
|
|
18
|
Shiota M, Yokomizo A, Itsumi M, et al:
Twist1 and Y-box-binding protein-1 promote malignant potential in
bladder cancer cells. BJU Int. 108:E142–E149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsushita K, Toiyama Y, Tanaka K, et al:
Soluble CXCL16 in preoperative serum is a novel prognostic marker
and predicts recurrence of liver metastases in colorectal cancer
patients. Ann Surg Oncol. 19(Suppl 3): S518–S527. 2012. View Article : Google Scholar
|
|
20
|
Tang FY, Pai MH and Chiang EP: Consumption
of high-fat diet induces tumor progression and
epithelial–mesenchymal transition of colorectal cancer in a mouse
xenograft model. J Nutr Biochem. 23:1302–1313. 2012.
|
|
21
|
Wu HM, Cao W, Ye D, Ren GX, Wu YN and Guo
W: Contactin 1 (CNTN1) expression associates with regional lymph
node metastasis and is a novel predictor of prognosis in patients
with oral squamous cell carcinoma. Mol Med Rep. 6:265–270.
2012.PubMed/NCBI
|
|
22
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhangu A, Wood G, Mirnezami A, Darzi A,
Tekkis P and Goldin R: Epithelial mesenchymal transition in
colorectal cancer: Seminal role in promoting disease progression
and resistance to neoadjuvant therapy. Surg Oncol. 21:316–323.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu QC, Gao RY, Wu W and Qin HL:
Epithelial-mesenchymal transition and its role in the pathogenesis
of colorectal cancer. Asian Pac J Cancer Prev. 14:2689–2698. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lasham A, Print CG, Woolley AG, Dunn SE
and Braithwaite AW: YB-1: oncoprotein, prognostic marker and
therapeutic target? Biochem J. 449:11–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsofack SP, Garand C, Sereduk C, et al:
NONO and RALY proteins are required for YB-1 oxaliplatin induced
resistance in colon adenocarcinoma cell lines. Mol Cancer.
10:1452011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jürchott K, Kuban RJ, Krech T, et al:
Identification of Y-box binding protein 1 as a core regulator of
MEK/ERK pathway-dependent gene signatures in colorectal cancer
cells. PLoS Genet. 6:e10012312010.PubMed/NCBI
|
|
28
|
Hsieh AC, Liu Y, Edlind MP, et al: The
translational landscape of mTOR signalling steers cancer initiation
and metastasis. Nature. 485:55–61. 2012. View Article : Google Scholar
|
|
29
|
Schittek B, Psenner K, Sauer B, et al: The
increased expression of Y box-binding protein 1 in melanoma
stimulates proliferation and tumor invasion, antagonizes apoptosis
and enhances chemoresistance. Int J Cancer. 120:2110–2118. 2007.
View Article : Google Scholar
|
|
30
|
Sinnberg T, Sauer B, Holm P, Spangler B,
Kuphal S, Bosserfhoff A and Schittek B: MAPK and PI3K/AKT mediated
YB-1 activation promotes melanoma cell proliferation which is
counteracted by an autoregulatory loop. Exp Dermatol. 21:265–270.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Costanzo A, Troiano A, di Martino O, et
al: The p63 protein isoform ΔNp63α modulates Y-box binding protein
1 in its subcellular distribution and regulation of cell survival
and motility genes. J Biol Chem. 287:30170–30180. 2012.
|
|
32
|
To K, Fotovati A, Reipas KM, et al: Y-box
binding protein-1 induces the expression of CD44 and CD49f leading
to enhanced self-renewal, mammosphere growth, and drug resistance.
Cancer Res. 70:2840–2851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho SH, Park YS, Kim HJ, et al: CD44
enhances the epithelial-mesenchymal transition in association with
colon cancer invasion. Int J Oncol. 41:211–218. 2012.PubMed/NCBI
|
|
34
|
Fujiwara-Okada Y, Matsumoto Y, Fukushi J,
et al: Y-box binding protein-1 regulates cell proliferation and is
associated with clinical outcomes of osteosarcoma. Br J Cancer.
108:836–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Evdokimova V, Tognon C, Ng T and Sorensen
PH: Reduced proliferation and enhanced migration: two sides of the
same coin? Molecular mechanisms of metastatic progression by YB-1.
Cell Cycle. 8:2901–2906. 2009. View Article : Google Scholar
|
|
36
|
Mouneimne G and Brugge JS: YB-1
translational control of epithelial-mesenchyme transition. Cancer
Cell. 15:357–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo TT, Yu YN, Yip GW, Matsumoto K and Bay
BH: Silencing the YB-1 gene inhibits cell migration in gastric
cancer in vitro. Anat Rec (Hoboken). 296:891–898. 2013. View Article : Google Scholar : PubMed/NCBI
|