Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
clinicopathological syndrome in which the severity can range from
simple fatty liver to non-alcoholic steatohepatitis (NASH),
cirrhosis and hepatocellular carcinoma, and is observed in patients
with no history of excessive alcohol consumption (1). A previous study has indicated that
NAFLD can coexist with type 2 diabetes mellitus, obesity and
dyslipidemia. In particular, hyperlipidemia and insulin resistance
were implicated in the initiation and progression of NAFLD
(2). Currently, an increase in
energy consumption by exercise and/or a reduction in energy intake
are accepted methods for the prevention of NAFLD. No standard
treatments are currently used to reverse NAFLD, and effective
medical interventions have focused on diet control and exercise.
There remains a requirement for the development of effective
pharmacological agents, due to the increasing prevalence of
NAFLD.
Ezetimibe remains the most widely used first-line
drug for the treatment of hypercholesteremia. Ezetimibe exerts its
effect predominantly by inhibiting cholesterol absorption and it
was demonstrated to block Niemann-Pick C1 Like 1-mediated
cholesterol absorption at the brush border of the intestine and the
liver (3). In addition to
improving hypercholesterolemia in patients with dyslipidemia,
attention has recently been drawn to its potential attenuation of
liver steatosis (4,5). It has been shown that Ezetimibe may
exert these effects by reducing Srebp-1c expression in mice
fed a high-fat diet (6). Another
study demonstrated that ezetimibe was effective for reducing serum
low-density lipoprotein cholesterol levels resistant to lifestyle
intervention in patients with non-alcoholic fatty liver disease
(7). Hepatic steatosis, induced by
a high-fat diet, but not a high-fructose diet, was inhibited by
ezetimibe administration (8). It
has also been reported that hepatic iron levels in mice fed a
high-fat diet are increased following treatment with ezetimibe
(9). Despite these notable
findings, mechanisms by which ezetimibe administration ameliorates
hepatic steatosis, insulin resistance and obesity remain largely
unexplored.
NAFLD is considered as the hepatic manifestation of
the metabolic syndrome that is closely associated with obesity,
hyperlipidemia, type 2 diabetes mellitus and insulin resistance.
The initiation and progression of metabolic syndrome (MS) is mainly
associated with the consumption of high-fat diets and/or
high-carbohydrate diets. Epidemiological studies suggest that
consumption of high-fat diets (≥30% of energy from fat) is
associated with a high prevalence of being overweight, central
obesity and MS (10,11). Rodents fed a high-fat diet closely
mimic a number of the features observed in humans with NAFLD, and
present with obesity, impaired glucose tolerance, dyslipidemia and
fat accumulation in the liver (12,13).
In the current study, the effects of ezetimibe on an HF-induced
mouse model C57BL/6J (B6) for NAFLD were investigated.
Materials and methods
Animals
Male B6 mice (14 weeks old) were purchased from the
Hebei Medical University, Center for Animal Experimentation
(Shijiazhuang, China) and were maintained in the animal facilities
of Hebei Medical University with standard animal care procedures
based on the institutional guidelines. The mice were fed a normal
laboratory diet (22.3% protein, 6.2% fat, 3.0% fiber, 6.5% ash and
47.8% complex carbohydrate) with free access to water, and were
housed with a regular 12-h light/dark cycle according to the Hebei
Medical University Guidelines for the Care and Use of Laboratory
Animals. Following acclimatization for two weeks, B6 mice were fed
a high-fat chow (High-Fat Diet 32; CLEA Japan, Inc., Tokyo, Japan)
for four weeks, then the mice were divided into two groups
(n=7/group); those fed the high-fat chow for four weeks (HF group),
and those fed the high-fat chow with 0.0064% wt/wt ezetimibe (5
mg/kg/day) for four weeks (HF+EZ group). After 16-h fasting, all
mice were sacrificed under anaesthesia by intraperitoneal
administration of pentobarbital (60 mg/kg body weight; Nembutal;
Dainippon Sumimoto Pharma Co., Ltd., Osaka Japan) and medetomidine
(0.3 mg/kg body weight; Domitor; Meiji Seika Kaisha, Ltd., Tokyo,
Japan). The ezetimibe was provided by Merck Sharp & Dohme
(Whitehouse Station, NJ, USA).
Phenotype determination
Alanine aminotransferase (ALT), total serum
cholesterol and triglyceride (TG) were measured as described
previously (14).
Measurement of liver triglyceride
Liver triglyceride content was analyzed using a
Triglyceride Quantification Kit (#ab65336; Abcam, Cambridge, MA,
USA) as previously described (14).
Intraperitoneal glucose tolerance test
(ipGTT)
The mice were given an ipGTT (2 g glucose/kg body
weight) subsequent to overnight fasting. The glucose levels were
measured after fasting prior to glucose administration (0 min), and
at 30, 60, 90 and 120 min post-glucose load.
Histological examination of liver
To study histological changes, liver tissue samples
were formalin-fixed and paraffin-embedded (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and subjected to
hematoxylin-eosin (H&E; Biocare Medical, LLC., Concord, CA,
USA) and Sirius red (Direct Red 80; Sigma-Aldrich, St. Louis, MO,
USA) staining. All the images were acquired and analyzed using a
BZ-8000 Fluorescence Microscope (Keyence Corporation, Osaka,
Japan). H&E-stained sections and Sirius red-stained sections
were graded according to the NASH activity score and the fibrosis
score as previously described (15,16).
The evaluation was performed by two experienced pathologists who
were blinded to the treatments that the mice had received,
according to methods previously described (16).
Hepatic gene expression analysis
Total RNA was extracted from frozen liver samples
using Isogen (Nippon Gene Co., Ltd., Tokyo, Japan), and cDNA was
synthesized using a High Capacity cDNA Reverse Transcription kit
(Applied Biosystems Life Technologies, Carlsbad, CA, USA).
Quantitative polymerase chain reaction (qPCR) was performed using
TaqMan® Gene Expression Master Mix (Applied Biosystems
Life Technologies) with a previously described method (17). The expression of the following
genes was evaluated with probes from Applied Biosystems Life
Technologies as follows: Acc1 (Mm01304282_m1) for
acetyl-coenzyme A (CoA) carboxylase 1; Srebf1
(Mm00550338_m1) for sterol regulatory element binding protein
(SREBP)-1c; Scd1 (Mm00772290) for stearoyl-CoA desaturase 1;
Fasn (Mm00662319_m1) for fatty acid synthase; Srebf2
(Mm01306289_m1) for SREBP-2; Cd36 (Mm00432403) for the fatty
acid translocase (FAT); Dgat2 (Mm00499530) for
diacylglycerol O-acetyltransferase 2; Bax (Mm00432448_m1)
for Bcl-2 associated X protein; Bcl-2 (Mm00477631_m1) for B
cell lymphoma-2; Cpt1a (Mm00550438_m1) for carnitine
palmitoyltransferase 1A; Ppara (Mm00440939_m1) for
peroxisome proliferator-activated receptor α; ApoB
(Mm01545164_m1) for apolipoprotein B; Mttp (Mm00435015_m1)
for microsomal triglyceride transfer protein (MTP); Ccl2
(Mm00441242_m1) for chemokine (C-C motif) ligand 2; Emr1
(Mm00802530_m1) for cell surface glycoprotein F4/80; Tnf
(Mm00443258_m1) for tumor necrosis factor-α; and Tgfb1
(Mm00441724_m1) for transforming growth factor β-1. All experiments
were performed in duplicate and all gene expression levels were
normalized to Hprt1 expression (Mm00446968_m1).
Western blot analysis
Liver samples were collected and proteins were
separated by SDS-PAGE (10% Mini-Protean® TGX™ gel and
Mini-Protean® Tetra Cell Mini Trans-Blot module; Bio-Rad
Laboratories, Hercules, CA, USA), and blotted onto polyvinylidine
fluoride membranes (Bio-Rad Laboratories). The blots were incubated
with polyclonal anti-rabbit SKP2 (L70; #4313; 1:1,000) or β-actin
primary antibodies (#4967, 1:1,000; both from Cell Signaling
Technology, Inc., Danvers, MA, USA) overnight at 4°C using slow
rocking and the horseradish peroxidase (HRP)-conjugated secondary
antibody, anti-rabbit IgG-HRP (1:1,500; Cell Signaling Technology,
Inc.). Thereafter, the membranes were visualized by enhanced
chemiluminescence, and the signals were quantified as previously
described (16).
Statistical analysis
All data are expressed as the means ± standard
deviation. Statistical comparisons were made using the two
independent-samples t-test and Mann-Whitney U test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS for Windows, version
18 (SPSS, Inc., Chicago, IL, USA).
Results
Physiological characteristics
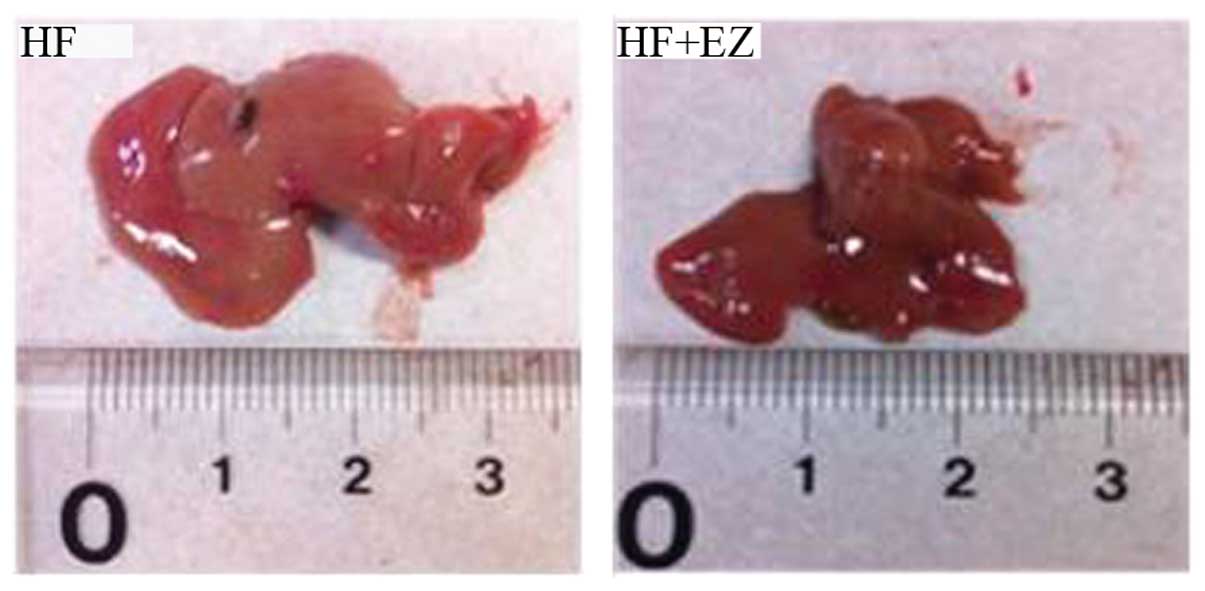
The livers of HF mice were markedly enlarged and
exhibited a paler color as compared with the livers of the HF+EZ
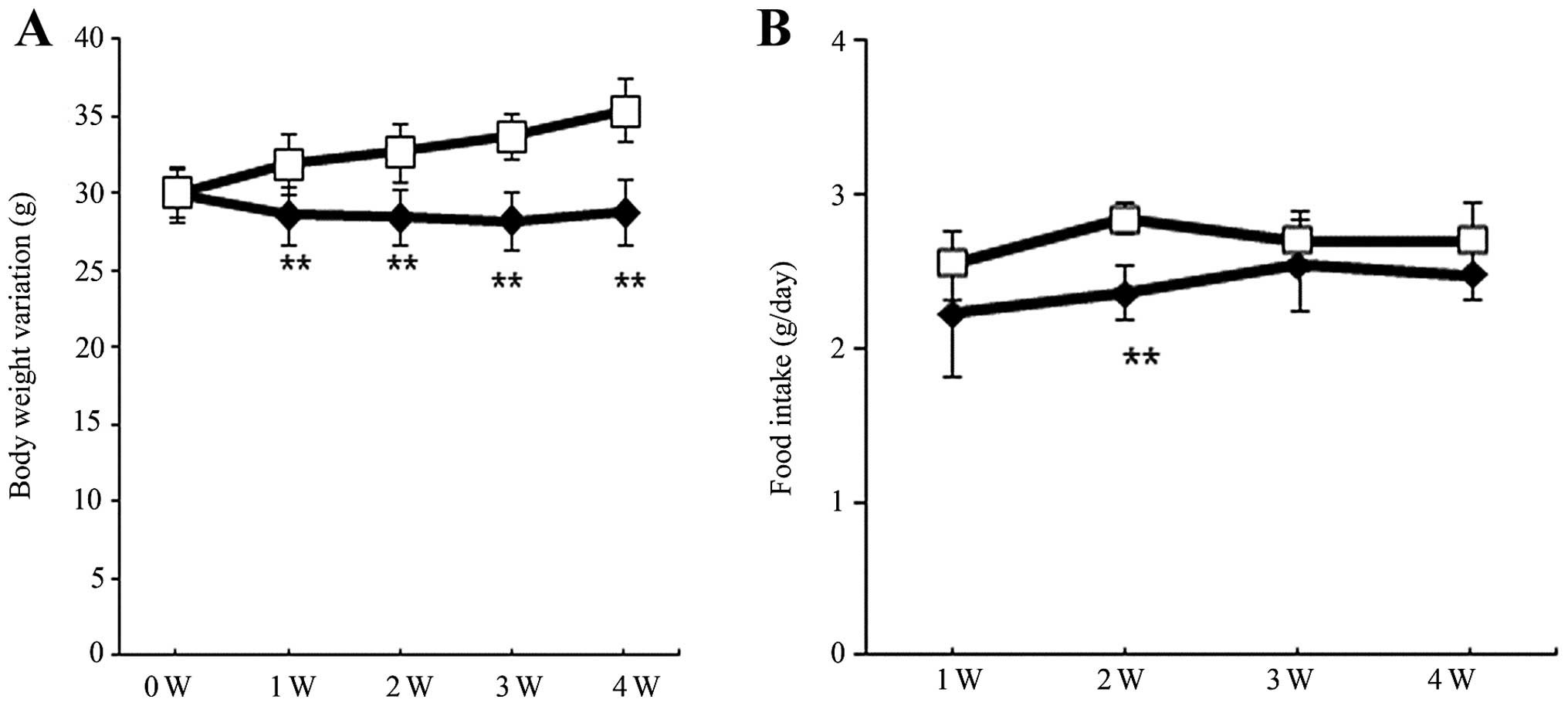
group (Fig. 1, Table I) The food consumption and body
weight of the two groups were monitored throughout the observation
period. Although the baseline body weight was similar between the
two groups (Table I, Fig 2A), the body weight was significantly
lower in the HF+EZ group as compared with the HF group during weeks
1–4 following ezetimibe treatment (P<0.01; Fig. 2A). Food consumption was similar
between HF and HF+EZ groups (Fig.
2B). The final body and liver weights were significantly lower
in the HF+EZ group than in the HF group (P<0.01; Table I). Liver TG content was
significantly lower in the HF+EZ group, as compared with that of
the HF group (P<0.05; Table
I).
 | Table ICharacteristics of C57BL/6J mice fed
a high-fat diet with or without ezetimibe. |
Table I
Characteristics of C57BL/6J mice fed
a high-fat diet with or without ezetimibe.
| C57BL/6J |
|---|
|
|
|---|
| Variable | HF | HF+EZ |
|---|
| Starting body
weight (g) | 30.0±1.6 | 30.0±1.8 |
| Final body weight
(g) | 35.4±2.0 | 28.8±2.1b |
| Liver weight
(mg) | 1026±63 | 858±47b |
| Liver weight/body
weight | 0.030±0.001 | 0.032±0.003 |
| NASH activity
score | 2.3±0.7 | 1.0±0.5b |
| Fibrosis score | 1.0±0.2 | 0.6±0.1b |
| Serum ALT
(IU/L) | 35.7±8.5 | 30.9±7.2 |
| Serum total
cholesterol (mg/dl) | 119.6±11.3 | 90.0±28.5a |
| Serum triglyceride
(mg/dl) | 14.7±4.6 | 12.0±3.7 |
| Liver TG content
(mg/g liver) | 16.2±1.4 | 13.5±1.5a |
Histological changes in the liver
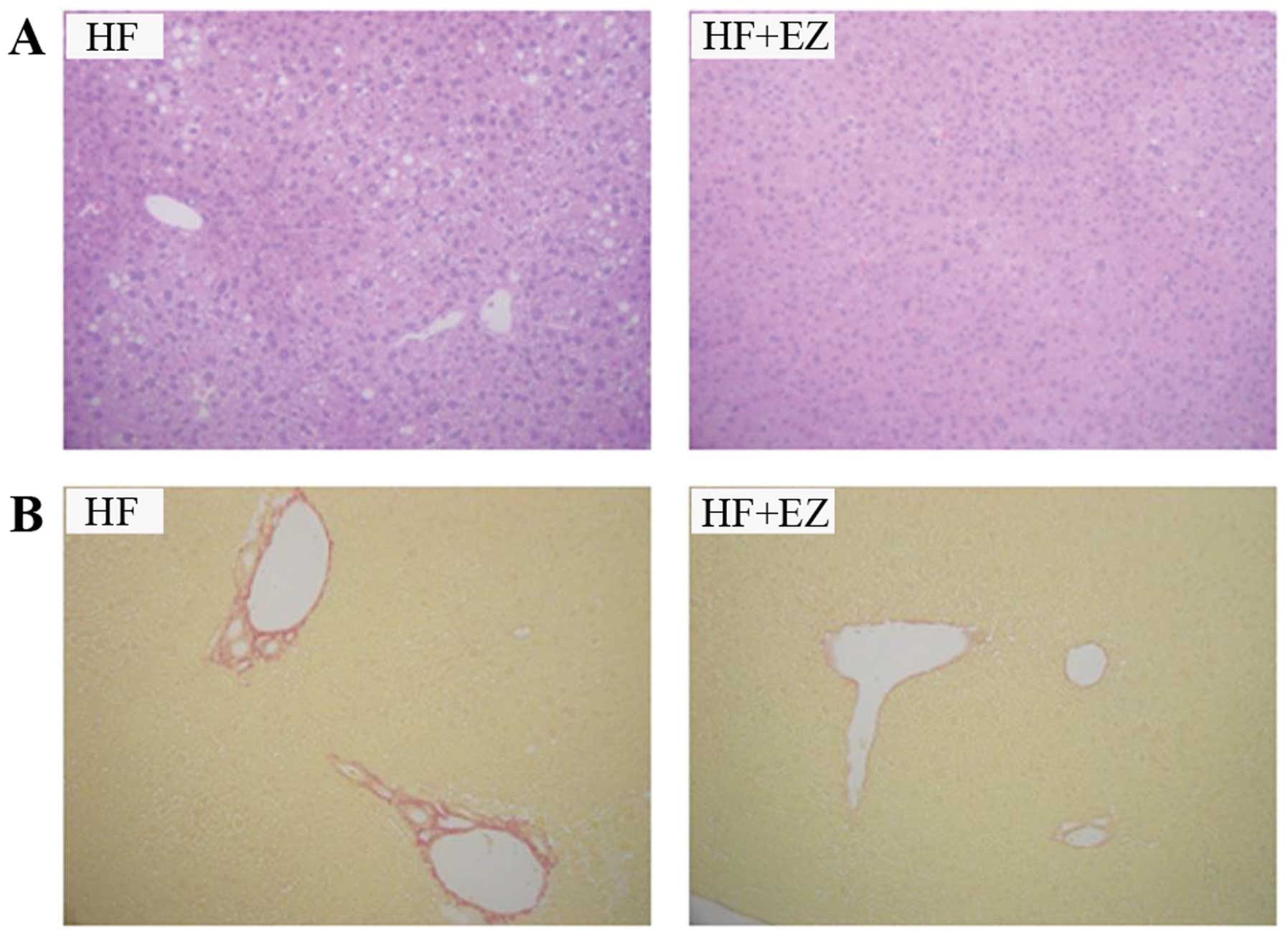
Lipid deposits in the liver were smaller in the
HF+EZ group as compared with those in the HF group (Fig. 3A). Regarding the NASH activity
score, the HF group had a total score of 2.3±0.7, while the HF+EZ
group had a total score of 1.0±0.5, indicating a significant
difference between the two groups (P<0.01; Table I). Sirius red staining of the liver
revealed that the HF+EZ group exhibited a lower degree of liver
fibrosis as compared with the HF group (Fig. 3B). The fibrosis score was
significantly different between the two groups (1.0±0.2 in the HF
group vs. 0.6±0.1 in the HF+EZ group; P<0.01; Table I).
Glucose tolerance
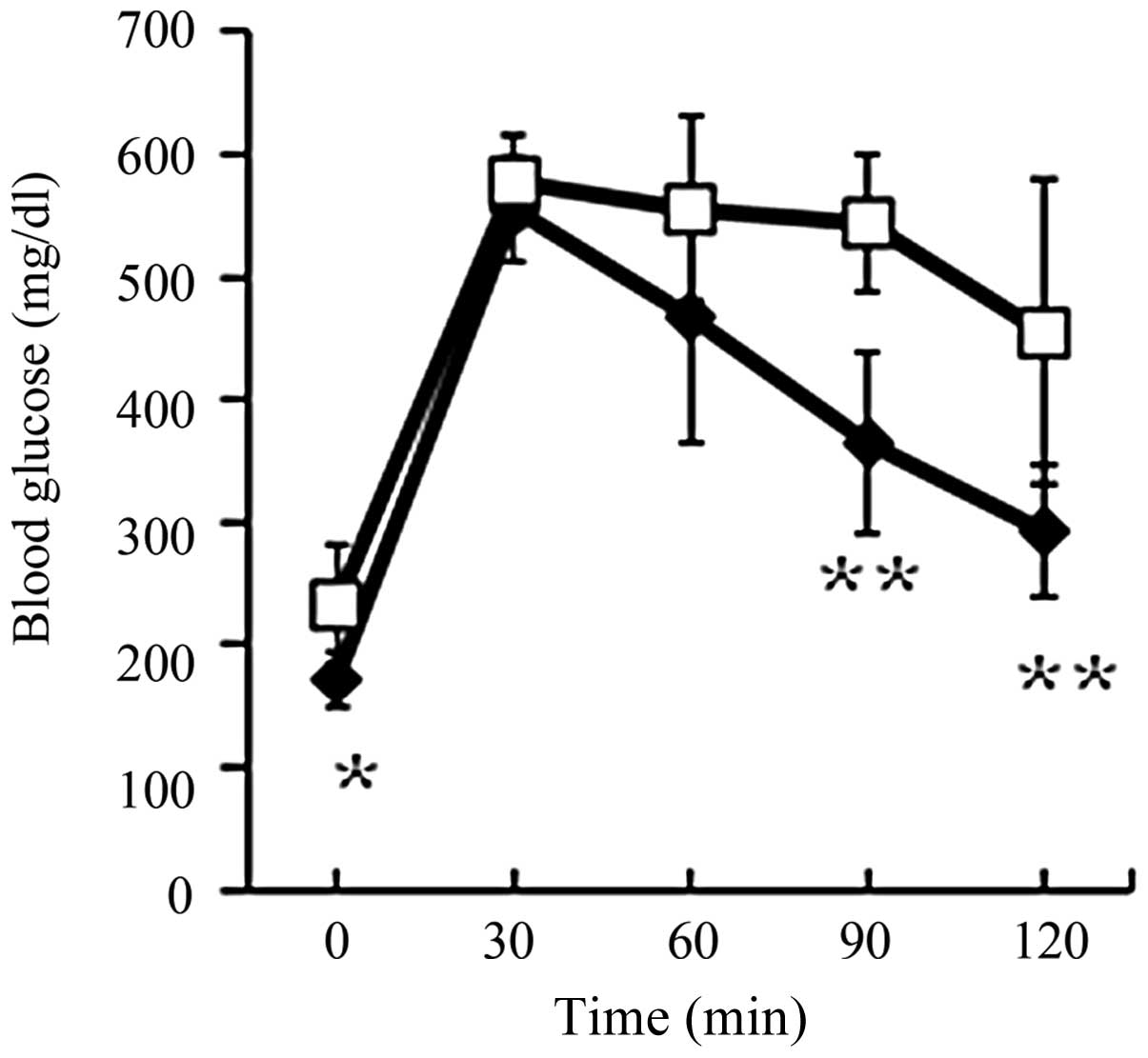
The fasting glucose level in the HF+EZ group was
significantly lower than that of the HF group (P<0.05; Fig. 4). In addition, the blood glucose
level at 90 and 120 min during the ipGTT were significantly lower
in the HF+EZ group as compared with the HF group (P<0.01;
Fig. 4).
Serum biochemical markers
Serum total cholesterol levels in the HF+EZ group
were significantly lower than that of the HF group (P<0.05;
Table I). Serum ALT and TG levels
in the HF+EZ group were not significantly lower than those in the
HF group (Table I).
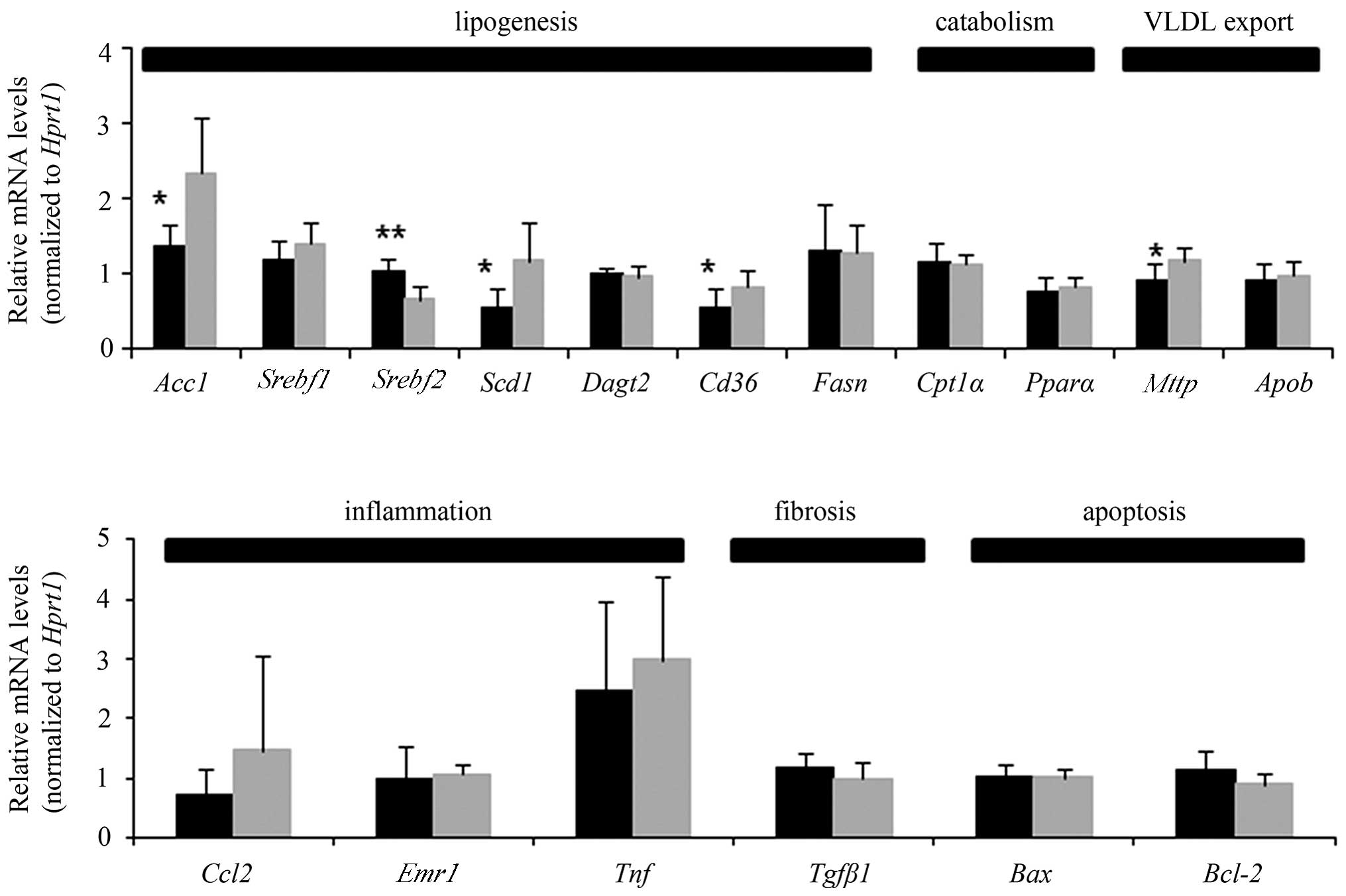
Hepatic gene expression for lipid
metabolism, inflammation, fibrosis and apoptosis
The mRNA levels of specific hepatic
lipogenesis-related genes were evaluated, including the following:
Acc1, Srebf1, Srebf2, Scd1,
Dagt2, Cd36 and Fasn. Hepatic expression
levels of Acc1, Scd1 and Cd36 were
significantly lower in the HF+EZ group, compared with the HF group
(P<0.05; Fig. 5), while those
of Srebf2 were significantly higher (P<0.01). Notably,
the hepatic expression level of Mttp was also significantly
lower in the HF+EZ group, as compared with the HF group
(P<0.05). Expression levels of genes associated with lipid
catabolism, fibrosis and apoptosis were not significantly different
between the two groups. Regarding the expression of genes involved
in inflammation, Ccl2 and Tnf were slightly lower in
the HF+EZ group than in the HF group, however this was not
significant, and there was no difference in the expression of
Emr1 between the two groups (Fig. 5).
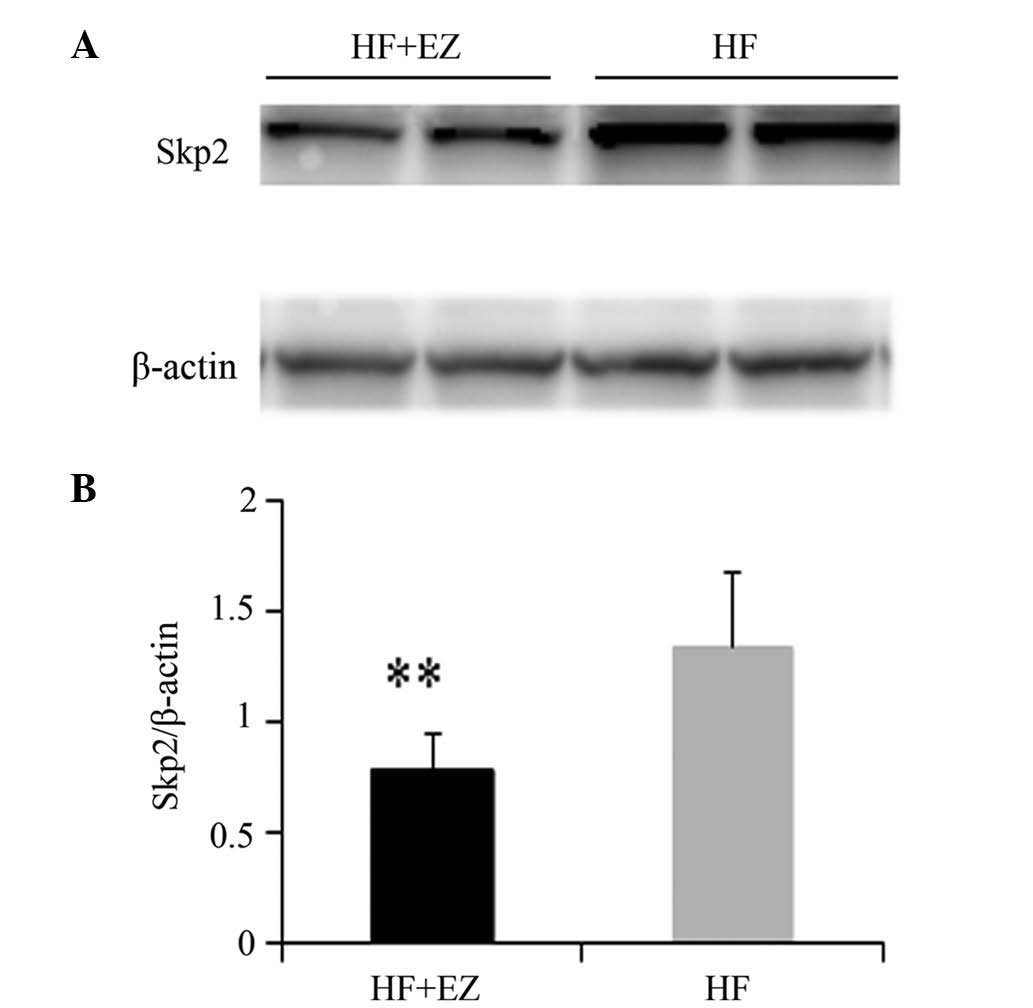
Western blot analysis of SKP2
The protein expression level of SKP2 in the HF+EZ
group was significantly lower than that in the HF group (P<0.01;
Fig. 6).
Discussion
NAFLD is a growing public health concern in
developed and developing countries due to the increasing prevalence
of obesity, diabetes and the metabolic syndrome induced by the
excessive consumption of high-fat and cholesterol-containing diets
and the lack of exercise in the general population.
In the present study, the effects of ezetimibe on
NAFLD were investigated using a high-fat induced mouse model. The
results demonstrated that ezetimibe significantly reduced the NASH
activity and fibrosis score in mice fed the high-fat diet compared
with the untreated mice, indicating favorable effects of ezetimibe
on liver steatosis and fibrosis. In addition, ezetimibe improved
serum cholesterol, hepatic fat accumulation and insulin resistance
in the livers of mice fed a high-fat diet. Furthermore, ezetimibe
significantly reduced mRNA levels of Acc1, Scd1 and
Cd36 in the liver, which are all associated with hepatic
lipogenesis and free fatty acid transportation. The level of SKP2
protein upregulation, which induces uncontrolled cell proliferation
and tumor progression, was also reduced by ezetimibe.
Ezetimibe administration reduced the body and liver
weights. The current study considered the possibility that the
effect of ezetimibe may be mediated by food intake, as reduced food
intake would significantly affect body weight, and therefore
influence hepatic steatosis. However, the reduced body and liver
weights were not associated with reduced food consumption in the
present study, and weight-specific food intake was indicated to be
similar between the two groups. This suggests that ezetimibe
directly protected against obesity and hepatic steatosis,
independent of food intake (18).
A previous study in C57BL/6 mice demonstrated that
fat overconsumption is key in the etiology of hepatic steatosis
(19). Another study suggested
that lipid accumulation in the liver of mice and rats can be
induced by a high-fat diet (20).
Long-term fat overconsumption may increase the risk of insulin
resistance and obesity, which enhance susceptibility to NAFLD. In
rodent models and humans, hepatic steatosis is consistently
associated with the development of hepatic insulin resistance
(2). In the current study,
ezetimibe ameliorated insulin resistance in mice fed the high-fat
diet, and significantly altered fasting glucose in addition to
glucose levels at 90 and 120 min of the ipGTT, which strongly
indicated that ezetimibe improved insulin sensitivity. Ezetimibe
administration may inhibit high fat-induced insulin resistance by
reducing intestinal fat absorption and weight gain, rather than via
downregulation of Srebf1 as suggested previously (6,21).
CD36 functions in the development of hepatic
steatosis in rodents, and is the most well-characterized free fatty
acid transporter. Thus far, the significance of CD36 in human liver
diseases remains unclear. Hepatic expression of CD36 is abnormally
increased in non-alcoholic fatty liver disease, and a previous
study indicated that hepatic CD36 upregulation was significantly
associated with insulin resistance, hyperinsulinemia and increased
steatosis in patients with NAFLD (22). Hepatic CD36 expression is normally
low, but its expression is increased in rodents with fatty liver
(23). Additionally, another study
demonstrated that CD36 mRNA levels increased concomitantly with
hepatic TG content in a number of animal models of fatty liver
(24,25). Notably, experimental amelioration
of steatosis in mice was accompanied by hepatic CD36 downregulation
(26,27). Modulation of CD36 expression in
hepatocytes may prove useful for the prevention or treatment of
liver fat accumulation in patients with NAFLD. In the present
study, ezetimibe significantly reduced CD36 gene expression in the
liver. Hence, ezetimibe may ameliorate hepatic insulin resistance
in addition to dyslipidemia and hepatic steatosis, partly via a
pathway involving CD36 in high-fat diet-induced B6 mouse models of
NAFLD.
SREBP-1c is a key transcriptional activator of
lipogenesis, and is responsible for regulating genes involved in
lipogenesis, including Acc1, Fas and Scd1
(5). In the present study,
ezetimibe treatment reduced the mRNA expression levels of
Acc1 and Scd1, which were correlated with hepatic
lipogenesis, and upregulated the gene expression of Srebf2,
as previously reported (6).
Inhibition of cholesterol absorption may occur by
ezetimibe-activated hepatic expression of SREBP-2, which is
established as a key regulator of cholesterol synthesis and uptake
(28). The hepatic mRNA expression
level of Mttp was increased in the HF group compared with
the level in the HF+EZ group in the present study, suggesting a
compensatory mechanism to release excess lipid as VLDL, as
previously suggested (29).
Overexpression of SKP2, a positive regulator of
G1-to-S phase transition, has been observed in numerous cases of
human cancer, including hepatocellular carcinoma (HCC) (30,31).
Previous reports have demonstrated in various types of cancer, that
SKP2 mRNA or protein expression is increased compared with normal
tissues (32–35). Uncontrolled SKP2 upregulation may
favor cell transformation in vitro and tumor progression
(36,37). It has been reported that a mouse
knockout for Skp2 results in a reduction of cell
proliferation and mouse body size (38). Another study indicated that
silencing of Skp2 by siRNA in HuH7 and HepG2 cells led to
growth restraint, enhanced apoptosis, and a rise in protein levels
of cell cycle inhibitors, with consequent reduction of their
ubiquitination (30). Other
studies have indicated that Skp2 serves as an oncogene in
HCC and thus is upregulated by increased transcriptional activity
(39,40). Therefore, the Skp2 gene may
be a therapeutic target for NAFLD-related HCC. In the present
study, the protein expression of SKP2 was reduced by ezetimibe
administration, providing a clue that ezetimibe may prevent the
progression of NAFLD-related HCC through downregulation of SKP2
protein expression. However, further research is required to
confirm this contention.
In conclusion, ezetimibe administration in an
HF-induced model of NAFLD resulted in lower serum cholesterol
levels, and amelioration of glucose tolerance, histological lesions
and hepatic expression of lipogenesis-related genes. In addition,
B6 mice that received ezetimibe treatment presented lower
Cd36 gene expression in the liver, suggesting ezetimibe may
ameliorate hepatic insulin resistance in addition to dyslipidemia
and hepatic steatosis, in part via a pathway involving Cd36.
Furthermore, the protein level of SKP2, a therapeutic target for
NAFLD-related HCC, was reduced by ezetimibe administration. These
data suggest that ezetimibe may have the potential to be used as an
effective drug for NAFLD and NAFLD-related HCC.
Acknowledgements
The authors would like to thank Ms. Chunhuan Ma for
her technical assistance.
Abbreviations:
|
MTP
|
microsomal triglyceride transfer
protein
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
HCC
|
hepatocellular carcinoma
|
|
EZ
|
ezetimibe
|
|
HF
|
high fat diet
|
|
TG
|
triglyceride
|
|
Chol
|
cholesterol
|
|
B6 mouse
|
C57BL/6J mouse
|
|
ALT
|
alanine aminotransferase
|
References
|
1
|
Wieckowska A, McCullough AJ and Feldstein
AE: Noninvasive diagnosis and monitoring of nonalcoholic
steatohepatitis: present and future. Hepatology. 46:582–589. 2007.
View Article : Google Scholar
|
|
2
|
Deushi M, Nomura M, Kawakami A, et al:
Ezetimibe improves liver steatosis and insulin resistance in obese
rat model of metabolic syndrome. FEBS Lett. 581:5664–5670. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Altmann SW, Davis HR Jr, Zhu LJ, et al:
Niemann-Pick C1 Like 1 protein is critical for intestinal
cholesterol absorption. Science. 303:1201–1204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng S, Hoos L, Cook J, et al: Ezetimibe
improves high fat and cholesterol diet-induced non-alcoholic fatty
liver disease in mice. Eur J Pharmacol. 584:118–124. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matono T, Koda M, Tokunaga S, et al:
Therapeutic effects of ezetimibe for non-alcoholic steatohepatitis
in fatty liver shionogi-ob/ob mice. Hepatol Res. 41:1240–1248.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muraoka T, Aoki K, Iwasaki T, et al:
Ezetimibe decreases SREBP-1c expression in liver and reverses
hepatic insulin resistance in mice fed a high-fat diet. Metabolism.
60:617–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oza N, Takahashi H, Eguchi Y, et al:
Efficacy of ezetimibe for reducing serum low-density lipoprotein
cholesterol levels resistant to lifestyle intervention in patients
with non-alcoholic fatty liver disease. Hepatol Res. 44:812–817.
2014. View Article : Google Scholar
|
|
8
|
Ushio M, Nishio Y, Sekine O, et al:
Ezetimibe prevents hepatic steatosis induced by a high-fat but not
a high-fructose diet. Am J Physiol Endocrinol Metab. 305:E293–E304.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishino Y, Tanaka Y, Ikeda T, et al:
Ezetimibe increases hepatic iron levels in mice fed a high-fat
diet. J Pharmacol Exp Ther. 345:483–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
George V, Tremblay A, Després JP, Leblanc
C and Bouchard C: Effect of dietary fat content on total and
regional adiposity in men and women. Int J Obes. 14:1085–1094.
1990.PubMed/NCBI
|
|
11
|
Tucker LA and Kano MJ: Dietary fat and
body fat: a multivariate study of 205 adult females. Am J Clin
Nutr. 56:616–622. 1992.PubMed/NCBI
|
|
12
|
Takahashi Y, Soejima Y and Fukusato T:
Animal models of nonalcoholic fatty liver disease/nonalcoholic
steatohepatitis. World J Gastroenterol. 18:2300–2308. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buettner R, Schölmerich J and Bollheimer
LC: High-fat diets: modeling the metabolic disorders of human
obesity in rodents. Obesity (Silver Spring). 15:798–808. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Sugimoto K, Fujisawa T, et al:
Novel effect of ezetimibe to inhibit the development of
non-alcoholic fatty liver disease in Fatty Liver Shionogi mouse.
Hepatol Res. 44:102–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kleiner DE, Brunt EM, Van Natta M, et al:
Nonalcoholic Steatohepatitis Clinical Research Network: Design and
validation of a histological scoring system for nonalcoholic fatty
liver disease. Hepatology. 41:1313–1321. 2005. View Article : Google Scholar
|
|
16
|
Shindo N, Fujisawa T, Sugimoto K, et al:
Involvement of microsomal triglyceride transfer protein in
nonalcoholic steatohepatitis in novel spontaneous mouse model. J
Hepatol. 52:903–912. 2010. View Article : Google Scholar
|
|
17
|
Oze-Fukai A, Fujisawa T, Sugimoto K, et
al: A novel mouse model for type 2 diabetes and non-alcoholic fatty
liver disease: spontaneous amelioration of diabetes by augmented
beta cell mass. Endocr J. 56:227–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung HY, Kim YH, Kim IB, et al: The Korean
mistletoe (Viscum album coloratum) extract has an
antiobesity effect and protects against hepatic steatosis in mice
with high-fat diet-induced obesity. Evid Based Complement Alternat
Med. 2013:1682072013.PubMed/NCBI
|
|
19
|
Jia L, Betters JL and Yu L: Niemann-pick
C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol
transport. Annu Rev Physiol. 73:239–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oosterveer MH, van Dijk TH, Tietge UJ, et
al: High fat feeding induces hepatic fatty acid elongation in mice.
PLoS One. 4:e60662009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia L, Ma Y, Rong S, et al: Niemann-Pick
C1-Like 1 deletion in mice prevents high-fat diet-induced fatty
liver by reducing lipogenesis. J Lipid Res. 51:3135–3144. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miquilena-Colina ME, Lima-Cabello E,
Sánchez-Campos S, et al: Hepatic fatty acid translocase CD36
upregulation is associated with insulin resistance,
hyperinsulinaemia and increased steatosis in non-alcoholic
steatohepatitis and chronic hepatitis C. Gut. 60:1394–1402. 2011.
View Article : Google Scholar
|
|
23
|
Inoue M, Ohtake T, Motomura W, et al:
Increased expression of PPARgamma in high fat diet-induced liver
steatosis in mice. Biochem Biophys Res Commun. 336:215–222. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buqué X, Martínez MJ, Cano A, et al: A
subset of dysregulated metabolic and survival genes is associated
with severity of hepatic steatosis in obese Zucker rats. J Lipid
Res. 51:500–513. 2010.PubMed/NCBI
|
|
25
|
Degrace P, Moindrot B, Mohamed I, et al:
Upregulation of liver VLDL receptor and FAT/CD36 expression in
LDLR−/− apoB100/100 mice fed trans-10, cis-12 conjugated linoleic
acid. J Lipid Res. 47:2647–2655. 2006.PubMed/NCBI
|
|
26
|
Liu LF, Purushotham A, Wendel AA and
Belury MA: Combined effects of rosiglitazone and conjugated
linoleic acid on adiposity, insulin sensitivity, and hepatic
steatosis in high-fat-fed mice. Am J Physiol Gastrointest Liver
Physiol. 292:G1671–G1682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
López-Parra M, Titos E, Horrillo R, et al:
Regulatory effects of arachidonate 5-lipoxygenase on hepatic
microsomal TG transfer protein activity and VLDL-triglyceride and
apoB secretion in obese mice. J Lipid Res. 49:2513–2523.
2008.PubMed/NCBI
|
|
28
|
Nozaki Y, Fujita K, Yoneda M, et al:
Long-term combination therapy of ezetimibe and acarbose for
non-alcoholic fatty liver disease. J Hepatol. 51:548–556. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higuchi N, Kato M, Tanaka M, et al:
Effects of insulin resistance and hepatic lipid accumulation on
hepatic mRNA expression levels of apoB, MTP and L-FABP in
non-alcoholic fatty liver disease. Exp Ther Med. 2:1077–1081.
2011.PubMed/NCBI
|
|
30
|
Calvisi DF, Ladu S, Pinna F, et al: SKP2
and CKS1 promote degradation of cell cycle regulators and are
associated with hepatocellular carcinoma prognosis.
Gastroenterology. 137:1816–1826. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao D, Inuzuka H, Tseng A, Chin RY, Toker
A and Wei W: Phosphorylation by Akt1 promotes cytoplasmic
localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction.
Nat Cell Biol. 11:397–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang G, Ayala G, De Marzo A, et al:
Elevated Skp2 protein expression in human prostate cancer:
association with loss of the cyclin-dependent kinase inhibitor p27
and PTEN and with reduced recurrence-free survival. Clin Cancer
Res. 8:3419–3426. 2002.
|
|
33
|
Li SH, Li CF, Sung MT, et al: Skp2 is an
independent prognosticator of gallbladder carcinoma among
p27(Kip1)-interacting cell cycle regulators: an immunohistochemical
study of 62 cases by tissue microarray. Mod Pathol. 20:497–507.
2007. View Article : Google Scholar
|
|
34
|
Masuda TA, Inoue H, Sonoda H, et al:
Clinical and biological significance of S-phase kinase-associated
protein 2 (Skp2) gene expression in gastric carcinoma: modulation
of malignant phenotype by Skp2 overexpression, possibly via p27
proteolysis. Cancer Res. 62:3819–3825. 2002.
|
|
35
|
Shigemasa K, Gu L, O’Brien TJ and Ohama K:
Skp2 overexpression is a prognostic factor in patients with ovarian
adenocarcinoma. Clin Cancer Res. 9:1756–1763. 2003.PubMed/NCBI
|
|
36
|
Calvisi DF, Pinna F, Ladu S, et al: The
degradation of cell cycle regulators by SKP2/CKS1 ubiquitin ligase
is genetically controlled in rodent liver cancer and contributes to
determine the susceptibility to the disease. Int J Cancer.
126:1275–1281. 2010.PubMed/NCBI
|
|
37
|
Gstaiger M, Jordan R, Lim M, et al: Skp2
is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci
USA. 98:5043–5048. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu L: Skp2 knockout reduces cell
proliferation and mouse body size: and prevents cancer? Cell Res.
20:605–607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koga H, Harada M, Ohtsubo M, et al:
Troglitazone induces p27Kip1-associated cell-cycle arrest through
down-regulating Skp2 in human hepatoma cells. Hepatology.
37:1086–1096. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Signoretti S, Di Marcotullio L, Richardson
A, et al: Oncogenic role of the ubiquitin ligase subunit Skp2 in
human breast cancer. J Clin Invest. 110:633–641. 2002. View Article : Google Scholar : PubMed/NCBI
|