Introduction
Antihistamines have been widely used to relieve a
number of allergic diseases, including allergic rhinitis and
conjunctivitis, chronic urticaria and histamine-induced pruritis.
Terfenadine, a type of second-generation histamine H1-receptor
antagonists which was discovered in the screening of antipsychotic
drugs in the late 1980s, binds preferentially to peripheral rather
than central H1-histamine receptors. Therefore, it exerts its
antihistaminic action without impairing the individual’s
performance and has no effect on psychomotor skills or subjective
feelings (1). However, terfenadine
has been associated with a number of side effects on cardiac
electrical activities. The most noticeable cardiac effect of
terfenadine is the development of ventricular arrhythmias,
including long QT syndrome (LQTS), torsades de pointes (TdP) and
ventricular fibrillation (VF), leading to sudden mortality
(2–4). Therefore, the US Food and Drug
Administration decided to withdraw terfenadine from the market in
the late 1990s for its severe cardiotoxicity. However, terfenadine
has been reclassified as a prescription-only drug in certain
countries, including the UK, Canada and China.
The cardiotoxicity of terfenadine on electrical
activities was closely associated with its blockade of relevant ion
channels in ventricular myocytes. The ventricular arrhythmia
induced by terfenadine is mainly due to the blockade of
K+ channels (5).
Blockade by terfenadine of multiple cardiac K+ currents,
particularly the fast component of delayed rectifier K+
channel current (IKr) in different species, has been
considered to account for the occurrence of TdP and the clinically
observed QT prolongation (6,7).
Previous studies have also found that terfenadine may potentially
inhibit the Na+ current (INa) and L-type
Ca2+ channel current (ICa-L)
though the blockade of the Na+ channel and the
Ca2+ channel, which may produce cardiotoxicity in
patients with a normal heart rhythm (8). Furthermore, terfenadine inhibited
ICa-L, INa and IKr in a
potent and long-lasting manner where the currents were difficult to
restore (8).
Terfenadine may have a potential antiarrhythmic
effect through blocking the above ion channels, particularly
K+ channels. In a previous study, terfenadine was found
to have a beneficial effect in relieving the ischemia-reperfusion
injury by inhibiting the reperfusion arrhythmias in the isolated
rat heart, part of which was attributable to its ability to alter
the cardiac action-potential characteristics (9). A recent study also reported that the
use of terfenadine alone did not produce an effect on the RR and QT
intervals, QRS complex or heart rate in guinea pigs. However, a
combined oral dose of terfenadine and ketoconazole significantly
prolonged the RR and QT intervals and decreased the heart rate in a
time-dependent manner (10). In
another recent study, to detect whether the effect of terfenadine
on K+ channels was separated from the antihistaminic
activity, a series of analogs of terfenadine were prepared with
structural variations, and the results demonstrated that the
ability to inhibit K+ channels was generally in parallel
with the antihistaminic activity (11). All of these studies suggested that
terfenadine may also be used as a potential antiarrhythmic drug to
a certain extent. However, the preventative and therapeutic effects
of terfenadine on ventricular arrhythmias remain controversial.
Furthermore, on the basis of previous studies (12,13),
it was hypothesized that the antiarrhythmic effect of terfenadine
may be similar to that of amiodarone, a widely used class III
antiarrhythmic drug, which also exerts its antiarrhythmic effect
through suppression of associated K+ channels. The
objective of the present study was to address in detail the
protective and therapeutic effects of terfenadine on experimental
ventricular arrhythmia in rats by comparing the antiarrhythmic
activity of terfenadine with that of amiodarone, which may provide
a basis for the discovery and development of novel antiarrhythmic
drugs.
Materials and methods
Animals and reagents
All of the experiments were performed in accordance
with the Guidelines of Animal Experiments from the Committee of
Medical Ethics at the National Health Department of China
(Shanghai, China) and were approved by the Laboratory Center of
Shanghai Tenth People’s Hospital (Shanghai, China). Sprague-Dawley
rats weighing 200–250 g were purchased from the Shanghai Slac
Laboratory Animal Co., Ltd (Shanghai, China), and housed in plastic
cages with well-ventilated stainless steel grid tops at room
temperature with a 12-h light/dark cycle. The temperature of the
animal room was regulated at 23±2°C and the relative humidity was
maintained at 55±15%. All of the animals were provided free access
to drinking water and normal food. Terfenadine, aconitine and
dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich Co. (St.
Louis, MO, USA) and amiodarone was purchased from Sanofi
Pharmaceutical Co., Ltd (Paris, France). Barium chloride
(BaCl2) was purchased from Shanghai Chemical Reagent
Research Institute Co., Ltd (Shanghai, China). The rats were
anaesthetized intraperitoneally with 3% pentobarbital (30 mg/kg;
China National Medicines, Co., Ltd, Shanghai, China). The standard
limb lead II electrocardiogram (ECG) was measured using the BL-420S
data acquisition and analysis system (Chengdu TaiMeng, Sichuan,
China) following subcutaneous penetration of electrodes into four
limbs. ECG intervals were expressed in milliseconds (ms) and the
heart rate was expressed in beats per minute (bpm).
Measurement of ECG parameters following
administration of terfenadine at different concentrations
Previous studies have reported that terfenadine
prolonged the QT interval in a dose-dependent manner (10,14,15).
In the present study, the impact of terfenadine on the QT interval
was also examined. A total of 40 rats were randomly divided into
five groups (n=8, respectively): i) Normal saline group; ii) DMSO
group; iii) terfenadine 6 mg/kg group; iv) terfenadine 12 mg/kg
group; v) terfenadine 18 mg/kg group. All of the drugs were
administered intraperitoneally following anesthetizing the rats,
and the volume of administration was 5 ml/kg. Terfenadine was
dissolved in DMSO and a similar solution lacking any compound was
used as a solvent control (DMSO group). ECG recordings were
conducted for 90 min following drug administration, baseline ECG
data were recorded for several minutes prior to administration of
the compounds and continued for 90 min post-treatment. The ECG
parameters, including heart rate, RR and QT intervals, were
documented. The rate-corrected QT (QTc) interval was also
calculated using Bazett’s formula:
QTc=QT/RR1/2 (16). The QTc intervals in five groups at
different time-points were compared.
Measurements of ECG parameters following
administration of amiodarone
Amiodarone is an effective treatment for atrial and
ventricular arrhythmias; however, its use is limited by a toxic
adverse-effect profile. Amiodarone-induced K+ channel
blockade may result in prolongation of ventricular repolarization,
which finally leads to LQTS, TdP and VF (17,18).
As mentioned above, the impact of amiodarone on QT interval may be
similar to that of terfenadine (12,13).
To compare the effects of these two drugs on electrical activities,
changes in the ECG caused by amiodarone treatment at different
concentrations were also examined. A total of 32 rats were randomly
divided into four groups (n=8/group): The normal saline (5 ml/kg),
amiodarone 18 mg/kg, amiodarone 36 mg/kg and amiodarone 54 mg/kg
groups. All of the drugs were administered intraperitoneally
following anesthetizing the rats. The relevant ECG parameters were
recorded for 90 min and compared at different time intervals.
BaCl2-induced ventricular
arrhythmias in rats
BaCl2 is a highly toxic salt and has
arrhythmogenic effects by impairing ion channels in cardiomyocytes.
Multiple ventricular arrhythmias may be induced following the
administration of BaCl2 in rats, particularly
ventricular premature contraction (VPC) and ventricular tachycardia
(VT) (19,20). In the present study, the protective
and therapeutic effects of terfenadine and amiodarone were
detected. The experimental rats were randomly divided into the
following groups: i) In the control group, 20 rats were randomly
divided into two subgroups with the same treatment of 5 ml/kg
normal saline as terfenadine control and amiodarone control
(n=10/group); ii) in the terfenadine group, 30 rats were divided
into three subgroups according to different doses of terfenadine
(6, 12 and 18 mg/kg; n=10 respectively); iii) in the amiodarone
group, 30 rats were divided into three subgroups according to
different concentrations of amiodarone (18, 36 and 54 mg/kg; n=8
respectively). A total of 40 min following the treatment of
terfenadine or amiodarone, BaCl2 (4 mg/kg) was
administrated via the sublingual vein intravenously within 10 sec.
The onset time of VPC, VT, VF and cardiac arrest (CA) was
recorded.
Aconitine-induced ventricular arrhythmia
in rats
Aconitine is well-known for its acute and high
toxicity in the causation of severe arrhythmia leading to mortality
(21,22). The effect of terfenadine and
amiodarone on the severe arrhythmia induced by aconitine was also
examined. The experimental rats were similarly divided into three
groups, the subgroups and doses of terfenadine and amiodarone were
exactly followed as aforementioned in the evaluation of their
effects on BaCl2-induced ventricular arrhythmia. A total
of 40 min following the administration of terfenadine or
amiodarone, aconitine (0.001%) was administered to rats via the
sublingual vein in rats at a rate of 2 μg/min using an infusion
pump to induce ventricular arrhythmia. The cumulative dosage of
aconitine required to induce VPC, VT, VF and CA was calculated.
Measurement of the duration of VT
The impact of terfenadine and amiodarone on the
duration of VT induced by BaCl2 and aconitine was
further examined. To reduce the incidence of aggravating VF and CA
which may result in cardiac mortality, the concentrations of
BaCl2 and aconitine in each group were altered. A total
of 40 min following treatment with terfenadine or amiodarone,
BaCl2 (2 mg/kg) was administered via the sublingual vein
intravenously within 10 sec. Similarly, aconitine (0.001%; 20
μg/kg) was also injected via the sublingual vein intravenously
within 10 sec in each group. The duration of VT was recorded and
the survival rates of the different groups of animals were
calculated.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis of data was performed by applying
Student’s t-test to determine the significance between the two
groups. Statistical significance of pairwise differences among
three or more groups were determined using one-way analysis of
variance followed by the post-hoc test. P<0.05 was considered to
indicate a statistically significant differnce. Analysis was
performed using SPSS 16.0, (SPSS, Inc., Chicago, IL, USA). The
equivalence test of terfenadine and amiodarone in shortening the
duration of VT was also performed. If the 95% confidence interval
(CI) of the difference between the terfenadine and amiodarone
groups was within the predetermined margin of equivalence (−5 to
5), the two drugs were considered equivalent.
Results
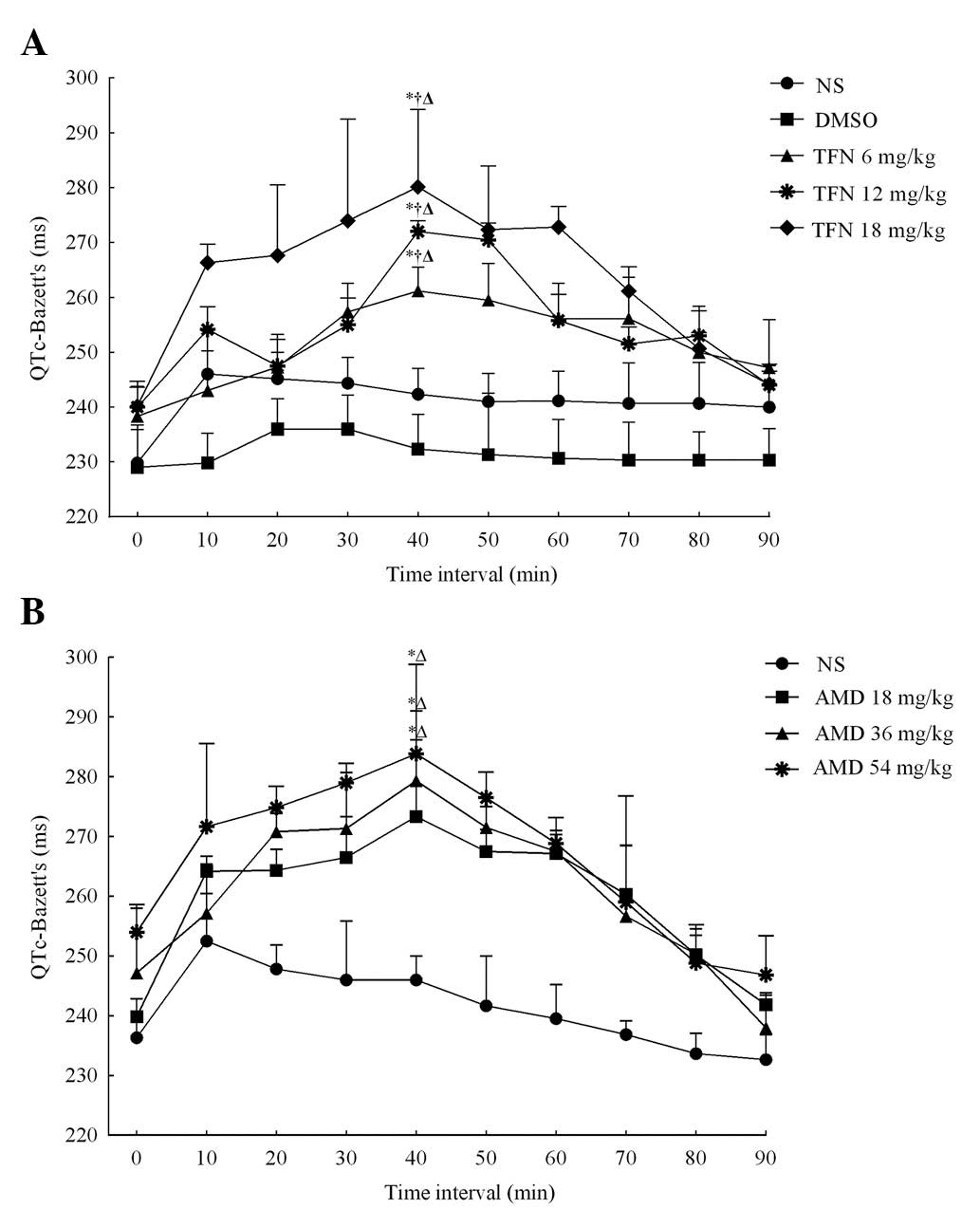
Terfenadine prolongs the QTc interval in
a dose-dependent manner
Table I details the
terfenadine-induced alterations in ECG parameters of rats in five
groups at different time intervals. To eliminate the impact of the
heart rate on the QT interval, the effect of terfenadine on the QTc
interval was detected. A total of 40 min following the
administration of terfenadine at different doses, the QTc intervals
in the 6, 12 and 18 mg/kg terfenadine groups were markedly
increased and reached a peak (261±16, 272±3 and 280±14 ms,
respectively), which demonstrated a significant difference compared
with the QTc intervals immediately following terfenadine
administration (238±10, 240±11 and 240±5 ms respectively, all
P<0.05; Fig. 1A). Following
this, the QTc intervals gradually declined in these groups and
finally restored to baseline in 90 min. The QTc intervals
demonstrated no significant alterations in the normal saline and
DMSO groups following administration and no statistical difference
was detected between these two groups. However, in the presence of
the selected doses of terfenadine, the ECG demonstrated that the
QTc intervals increased in a dose-dependent manner, as demonstrated
in Fig. 1A. Furthermore,
terfenadine may also dose-independently alter RR and QT intervals
as well as the heart rate. All of these results were consistent
with the aforementioned previous studies (14,15),
which suggested terfenadine prolonged the QT interval in a
dose-dependent manner.
 | Table ITerfenadine-induced alterations of RR
(ms), HR (bpm), QT (ms) and QTc-Bazett’s (ms) at different time
intervals. |
Table I
Terfenadine-induced alterations of RR
(ms), HR (bpm), QT (ms) and QTc-Bazett’s (ms) at different time
intervals.
|
Time
interval (min) |
|---|
|
|
|---|
|
Parameters/groups | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
|---|
| RR (ms) |
| NS | 176±8 | 185±6 | 184±7 | 185±6 | 184±5 | 182±11 | 182±5 | 185±7 | 183±9 | 182±12 |
| DMSO | 175±11 | 177±9 | 179±12 | 179±7 | 177±11 | 177±21 | 176±10 | 176±14 | 175±11 | 174±14 |
| TFN 6 mg/kg | 180±17 | 185±14 | 186±4 | 191±1 | 200±17a,b,c | 200±34 | 188±31 | 187±2 | 186±5 | 185±10 |
| TFN 12 mg/kg | 183±11 | 189±7 | 185±7 | 190±12 | 207±23a,b,c | 205±49 | 205±32 | 187±23 | 188±22 | 186±9 |
| TFN 18 mg/kg | 187±12 | 199±6 | 202±31 | 207±23 | 214±28a,b,c | 213±25 | 208±9 | 202±8 | 189±23 | 185±12 |
| HR (bpm) |
| NS | 342±16 | 326±11 | 325±13 | 326±11 | 327±8 | 330±20 | 330±9 | 325±11 | 330±19 | 330±15 |
| DMSO | 345±21 | 341±18 | 337±22 | 336±13 | 339±21 | 342±12 | 343±21 | 343±27 | 344±22 | 345±14 |
| TFN 6 mg/kg | 336±31 | 326±23 | 323±7 | 314±2 | 302±24a,b,c | 308±27 | 328±30 | 322±3 | 323±14 | 327±9 |
| TFN 12 mg/kg | 328±22 | 319±12 | 325±11 | 316±20 | 294±14a,b,c | 308±19 | 208±49 | 325±42 | 328±17 | 328±6 |
| TFN 18 mg/kg | 322±20 | 303±11 | 303±40 | 293±33 | 284±26a,b,c | 285±20 | 288±11 | 298±13 | 320±18 | 326±23 |
| QT (ms) |
| NS | 96.4±3.3 | 106±4 | 105±3 | 105±3 | 104±2 | 103±4 | 103±2 | 103±4 | 103±4 | 102±9 |
| DMSO | 95.6±4.1 | 96.5±3.2 | 99.7±9.9 | 99.7±1 | 97.9±5.3 | 97.2±9.6 | 96.6±3.4 | 96.4±1.7 | 96.7±2.6 | 96.1±4.9 |
| TFN 6 mg/kg | 101±3 | 104±4 | 106±2 | 113±2 | 117±7a,b,c | 116±10 | 110±10 | 111±3 | 105±8 | 104±13 |
| TFN 12 mg/kg | 103±3 | 110±2 | 106±3 | 111±5 | 123±8a,b,c | 122±16 | 115±15 | 109±6 | 105±6 | 104±21 |
| TFN 18 mg/kg | 104±4 | 106±3 | 120±10 | 125±8 | 129±8a,b,c | 126±8 | 125±3 | 117±2 | 106±15 | 103±11 |
| QTc-Bazett’s
(ms) |
| NS | 230±11 | 246±10 | 245±5 | 244±9 | 242±15 | 241±5 | 241±15 | 241±7 | 241±8 | 240±8 |
| DMSO | 229±8 | 230±5 | 237±15 | 236±6 | 232±6 | 231±13 | 231±7 | 230±7 | 230±5 | 230±6 |
| TFN 6 mg/kg | 238±10 | 243±7 | 247±6 | 257±15 | 261±16a,b,c | 260±7 | 254±6 | 254±7 | 250±8 | 247±9 |
| TFN 12 mg/kg | 240±11 | 254±4 | 248±5 | 255±9 | 272±3a,b,c | 271±3 | 256±5 | 252±13 | 253±5 | 244±3 |
| TFN 18 mg/kg | 240±5 | 266±4 | 268±14 | 274±19 | 280±14a,b,c | 274±12 | 273±4 | 261±4 | 251±3 | 244±4 |
Amiodarone exerts similar effects on the
QTc interval to that of terfenadine
By contrast, the effects of amiodarone on the QTc
intervals of rats were further assessed and the data revealed that
amiodarone had a similar effect on the ECG parameters to that of
terfenadine. The results are outlined in Table II. Intraperitoneal injection of
amiodarone also resulted in dose-dependent QTc interval
prolongation, as is demonstrated in Fig. 1B. A total of 40 min following
administration, the QTc intervals in the different groups all
reached a peak. The QTc interval was 240±3 ms, 247±10 ms and 254±5
ms immediately following amidarone administration and increased to
272±17, 279±14 and 284±14 ms following 40 min in 18, 36 and 54
mg/kg amiodarone groups, respectively (all P<0.05), which
demonstrated a significant difference when compared with the normal
saline group 40 min following amidarone administration (246±4 ms)
in these groups (all P<0.05). The data demonstrated that the
mechanism underlying the prolongation of QTc intervals induced by
terfenadine and amiodarone was similar, since terfenadine as well
as amiodarone are blockers of the K+ channel encoded by
the human ether-à-go-go-related gene (hERG) (23,24).
 | Table IIAmiodarone-induced alterations of RR
(ms), HR (bpm), QT (ms) and QTc-Bazett’s (ms) at different time
intervals. |
Table II
Amiodarone-induced alterations of RR
(ms), HR (bpm), QT (ms) and QTc-Bazett’s (ms) at different time
intervals.
|
Time
interval (min) |
|---|
|
|
|---|
|
Parameters/Groups | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
|---|
| RR (ms) |
| NS | 179±24 | 191±14 | 187±13 | 185±19 | 185±22 | 185±19 | 182±12 | 180±19 | 177±14 | 177±21 |
| AMD 18 mg/kg | 184±20 | 201±12 | 201±16 | 202±18 | 203±15a,b | 201±26 | 201±13 | 200±13 | 191±9 | 183±11 |
| AMD 36 mg/kg | 185±5 | 191±28 | 205±25 | 206±18 | 218±16a,b | 203±27 | 202±18 | 193±12 | 191±5 | 179±5 |
| AMD 54 mg/kg | 192±26 | 208±20 | 214±18 | 216±6 | 222±7a,b | 214±6 | 202±24 | 200±17 | 191±9 | 186±6 |
| HR (bpm) |
| NS | 341±28 | 316±22 | 322±23 | 332±27 | 333±11 | 328±36 | 332±22 | 337±36 | 343±12 | 342±18 |
| AMD 18 mg/kg | 329±37 | 305±11 | 301±25 | 299±18 | 297±23a,b | 303±11 | 308±15 | 302±20 | 314±31 | 328±24 |
| AMD 36 mg/kg | 325±8 | 320±20 | 296±17 | 294±15 | 276±21a,b | 300±25 | 306±32 | 311±18 | 317±15 | 339±17 |
| AMD 54 mg/kg | 317±45 | 290±28 | 283±24 | 278±7 | 271±9a,b | 281±8 | 310±35 | 302±12 | 316±5 | 328±13 |
| QT (ms) |
| NS | 99.6±6.1 | 110±10 | 107±4 | 106±12 | 105±8 | 104±10 | 102±3 | 100±5 | 100±9 | 99.8±5.7 |
| AMD 18 mg/kg | 103±7 | 118±9 | 118±5 | 120±6 | 122±6a,b | 120±9 | 119±12 | 116±9 | 112±5 | 109±8 |
| AMD 36 mg/kg | 106±14 | 112±8 | 123±7 | 123±6 | 130±10a,b | 122±8 | 120±12 | 113±13 | 111±7 | 102±15 |
| AMD 54 mg/kg | 111±8 | 124±7 | 127±16 | 123±2 | 134±17a,b | 128±2 | 120±12 | 116±19 | 111±5 | 106±12 |
| QTc-Bazett’s
(ms) |
| NS | 236±3 | 253±14 | 248±4 | 246±11 | 246±4 | 242±9 | 240±6 | 237±2 | 234±3 | 233±4 |
| AMD 18 mg/kg | 240±3 | 264±3 | 264±4 | 267±16 | 272±17a,b | 268±4 | 267±3 | 260±9 | 250±3 | 242±2 |
| AMD 36 mg/kg | 247±10 | 257±3 | 271±3 | 271±2 | 279±14a,b | 272±4 | 268±4 | 257±12 | 250±4 | 238±6 |
| AMD 54 mg/kg | 254±5 | 272±4 | 275±11 | 279±3 | 284±14a,b | 277±4 | 269±4 | 259±18 | 249±6 | 247±7 |
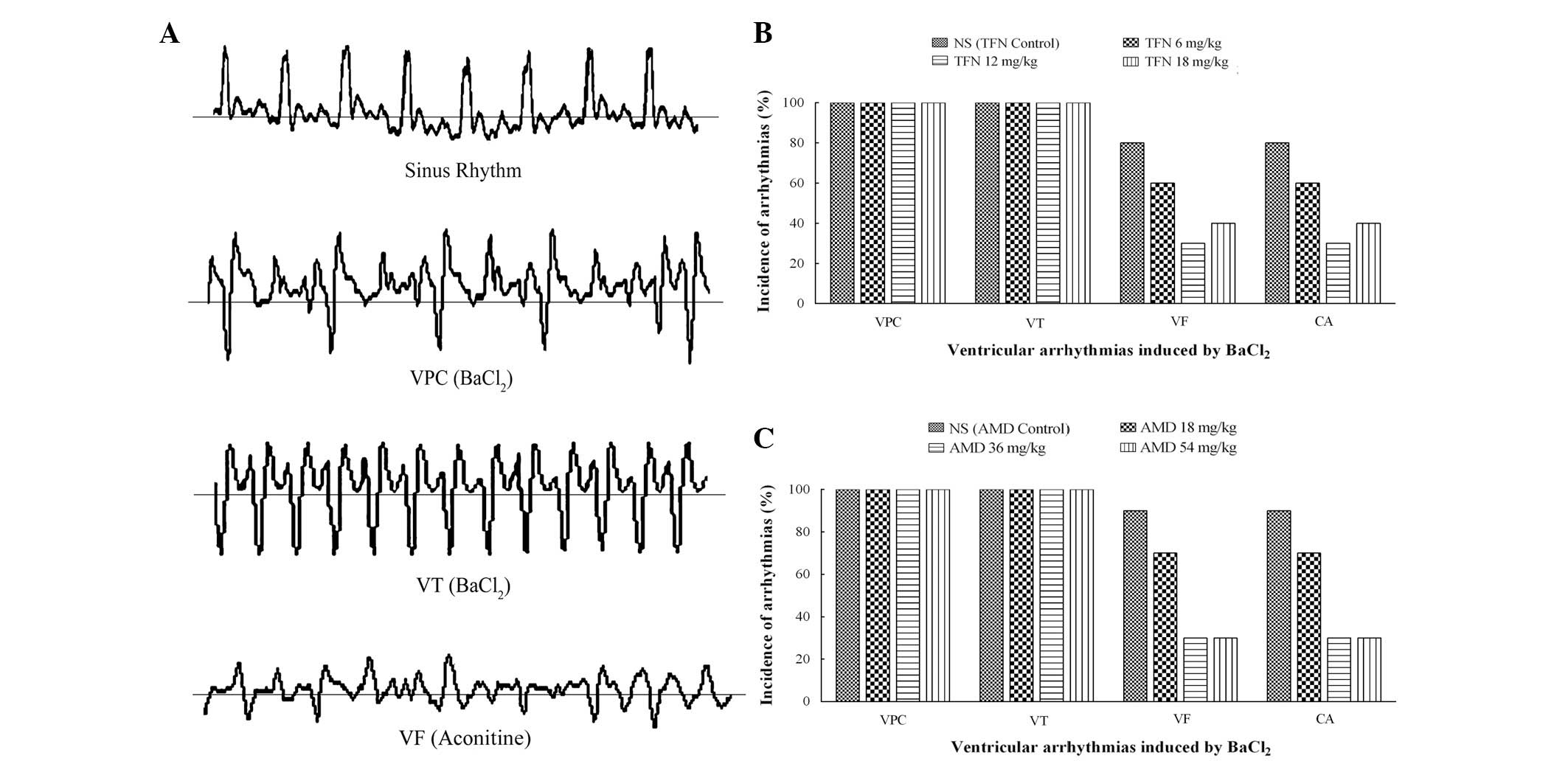
Effects of terfenadine and amiodarone on
BaCl2-induced ventricular arrhythmia in rats
Intravenous injection of BaCl2 into rats
produced disturbances of the cardiac rhythm, including VPC, VT, VF
or CA (Fig. 2A). Generally, VPC
occurred first and was followed by aggravating VT and VF.
Eventually, several animals died as a result of VF or CA. VPC and
VT appeared in all of the animals following BaCl2
treatment. The incidence of VF or CA varied in the different groups
and was possibly reduced by terfenadine and amiodarone. As revealed
in Fig. 2B, only six, three and
four animals exhibited VF in the 6, 12 and 18 mg/kg terfenadine
groups as opposed to animals in the terfenadine control group. VF
was triggered in seven, three and three animals in the 18, 36 and
54 mg/kg amiodarone groups, respectively. Compared with nine
animals in the amiodarone control group, the incidence of VF was
significantly reduced by amiodarone (Fig. 2C). Likewise, the CA incidence
declined with varying degrees following treatment with terfenadine
and amiodarone at different doses.
Intravenous administration of 18, 36 and 54 mg/kg
amiodarone significantly delayed the onset time of VPC, VT, VF and
CA (all P<0.05 vs. amiodarone control group). In a similar
manner, 12 and 18 mg/kg terfenadine also markedly delayed the onset
time of VPC, VT, VF and CA (all P<0.05 vs. the terfenadine
control group; Table III and
Fig. 3). However, it should be
noted that 6 mg/kg terfenadine was not able to delay the onset time
of VPC (P=0.07 vs. the terfenadine control group), but was able to
delay the onset time of VT, VF and CA (all P<0.05 vs. the
terfenadine control group).
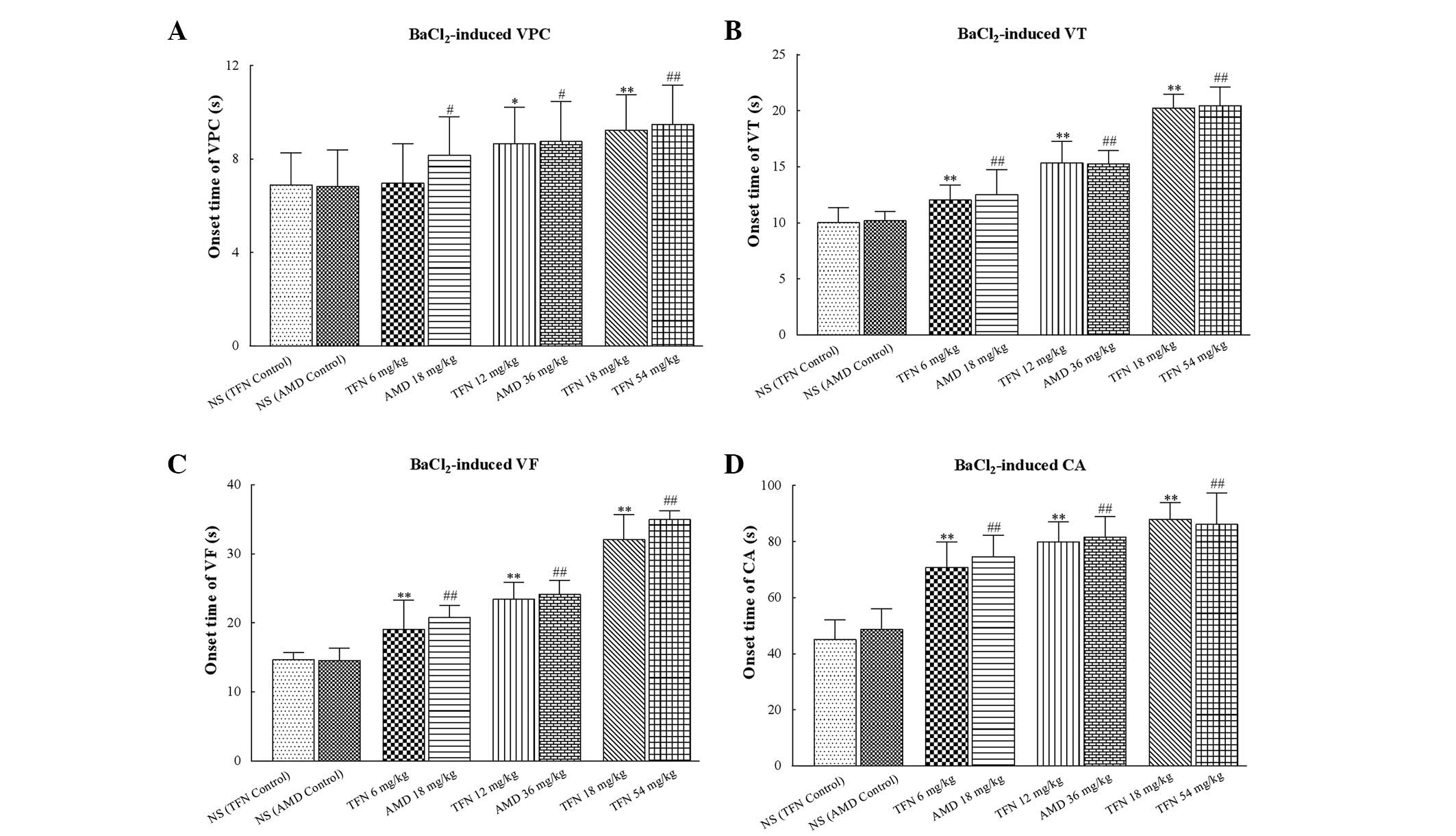 | Figure 3Onset time of
BaCl2-induced ventricular arrhythmias in each group. (A,
B, C and D) The onset time of VPC, VT, VF and CA induced by
BaCl2, respectively. Low dose (6 mg/kg) terfenadine may
not delay the onset time of VPC but delayed the onset time of VT,
VF and CA. AMD of different concentrations significantly delayed
the onset time of VPC, VT, VF and CA. In addition, 12 and 18 mg/kg
terfenadine also markedly delayed the onset time of VPC, VT, VF and
CA. In each group (n=10), values are expressed as the mean ± SD
(columns, mean; error bars, ± SD) *P<0.05 and
**P<0.01 vs the NS (TFN control) group;
#P<0.05 and ##P<0.01 vs. the NS (AMD
control) group. VPC, ventricular premature contraction; VT,
ventricular tachycardia; VF, ventricular fibrillation; CA, cardiac
arrest; NS, normal saline; TFN, terfenadine; AMD, amiodarone; SD,
standard deviation. |
 | Table IIIOnset time of
BaCl2-induced ventricular arrhythmias in different
groups. |
Table III
Onset time of
BaCl2-induced ventricular arrhythmias in different
groups.
| Groups | Onset time of VPC
(s) | Onset time of VT
(s) | Onset time of VF
(s) | Onset time of CA
(s) |
|---|
| Terfenadine |
| NS (TFN
control) | 6.89±1.37 | 10.02±1.35 | 14.68±1.05 | 45.07±7.03 |
| TFN 6 mg/kg | 6.96±1.70 | 12.05±1.33b | 19.10±4.17b | 70.82±8.98b |
| TFN 12 mg/kg | 8.66±1.56a | 15.35±1.94b | 23.44±2.47b | 79.93±7.15b |
| TFN 18 mg/kg | 9.24±1.51b | 20.23±1.25b | 32.07±3.62b | 87.90±5.91b |
| Amiodarone |
| NS (AMD
control) | 6.83±1.55 | 10.19±0.84 | 14.58±1.77 | 48.67±7.42 |
| AMD 18 mg/kg | 8.17±1.63 | 12.50±2.24d | 20.82±1.76d | 74.62±7.57d |
| AMD 36 mg/kg | 8.75±1.71c | 15.27±1.20d | 24.12±2.01d | 81.57±7.41d |
| AMD 54mg/kg | 9.48±1.69d | 20.46±1.63d | 34.97±1.31d | 86.13±11.24d |
Effects of terfenadine and amiodarone on
aconitine-induced ventricular arrhythmia in rats
Ventricular premature beats were followed by VT and
VF appearing in all treated rats following administration of
aconitine (Fig. 2A). Treatment of
the rats with terfenadine or amiodarone prior to aconitine caused a
significant increase in the cumulative dosage of aconitine required
to induce VPC, VT, VF and CA compared with the terfenadine control
and amiodarone control groups, respectively (P<0.05 or
P<0.01; Table IV and Fig. 4).
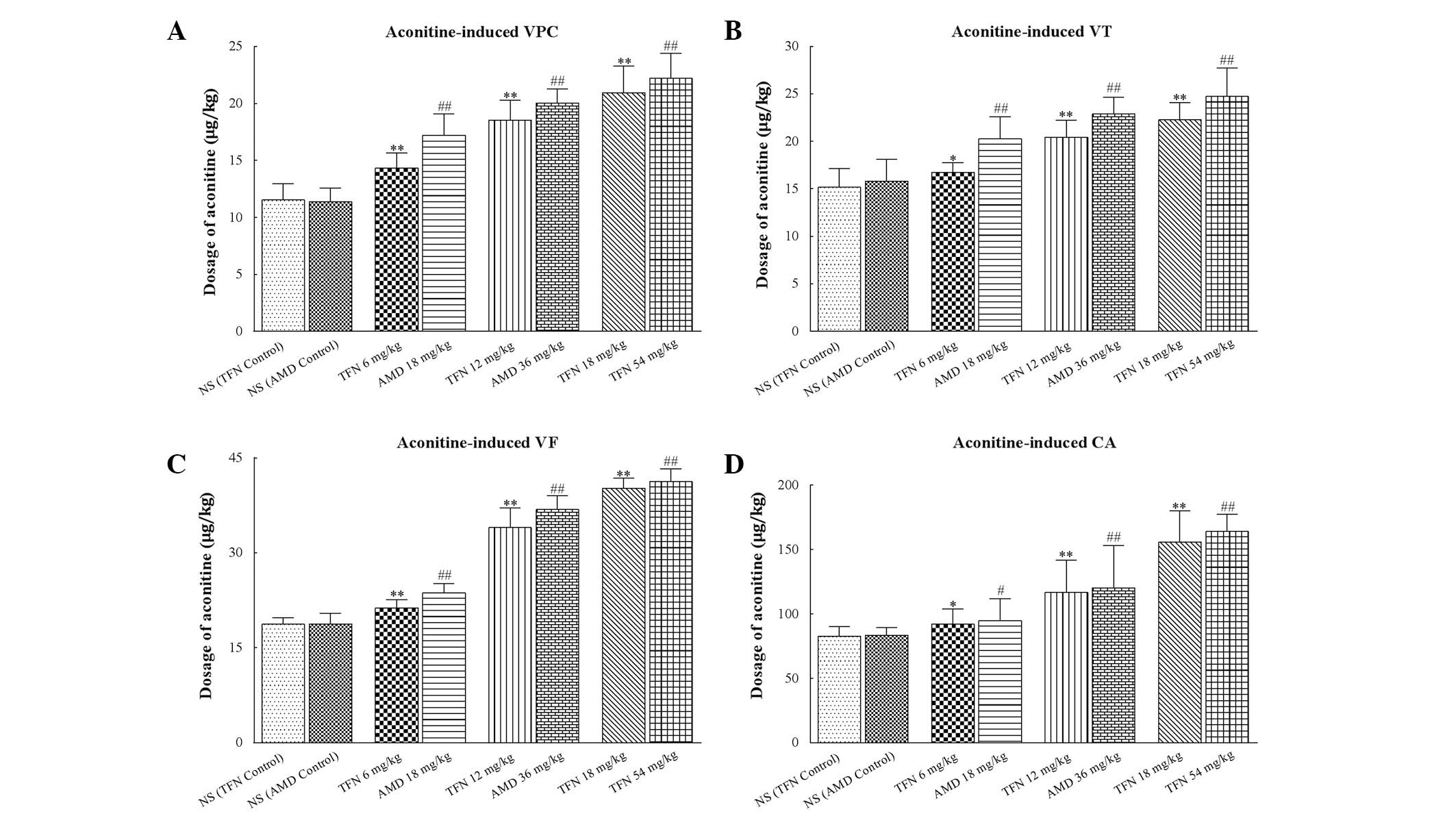 | Figure 4Dosage of aconitine required to
induce ventricular arrhythmias in each group. (A–D) The cumulative
dosage of aconitine required to induce VPC, VT, VF and CA,
respectively. Treatment of rats with terfenadine and amiodarone
prior to aconitine both caused significantly increase in the
cumulative dosage of aconitine required to induce VPC, VT, VF and
CA. In each group (n=10) values are presented as the mean ± SD
(columns, mean; error bars, ± SD). #P<0.05 and
##P<0.01 versus NS (AMD control) group. VPC,
ventricular premature contraction; VT, ventricular tachycardia; VF,
ventricular fibrillation; CA, cardiac arrest; NS, normal saline;
TFN, terfenadine; AMD, amiodarone. *P<0.05 and
**P<0.01 versus NS (TFN control) group. |
 | Table IVDosage of aconitine required to
induce ventricular arrhythmias in different groups. |
Table IV
Dosage of aconitine required to
induce ventricular arrhythmias in different groups.
| Dosage of aconitine
(μg/kg) |
|---|
|
|
|---|
| Groups | VPC | VT | VF | CA |
|---|
| Terfenadine |
| NS (TFN
control) | 11.52±1.43 | 15.17±1.99 | 18.70±1.04 | 82.65±7.73 |
| TFN 6 mg/kg | 14.34±1.30b | 16.75±0.98a | 21.32±1.29b | 92.08±11.59a |
| TFN 12 mg/kg | 18.54±1.73b | 20.45±1.76b | 33.97±3.11b |
116.61±25.29b |
| TFN 18 mg/kg | 20.95±1.35b | 22.28±1.83b | 40.19±1.61b |
155.83±24.14b |
| Amiodarone |
| NS (AMD
control) | 11.36±1.19 | 15.83±2.26 | 18.75±1.68 | 83.41±5.98 |
| AMD 18 mg/kg | 17.17±1.90d | 20.27±2.32d | 23.68±1.47d | 94.81±16.88c |
| AMD 36 mg/kg | 20.04±1.22d | 22.90±1.74d | 36.85±2.18d |
120.29±33.05d |
| AMD 54 mg/kg | 22.20±2.20d | 24.74±2.98d | 41.27±2.01d |
164.21±13.41d |
Effect of terfenadine and amiodarone on
the duration of VT in rats
As demonstrated in Table V and Fig. 5, treatment of rats with 12 and 18
mg/kg terfenadine prior to BaCl2 or aconitine
significantly shortened the duration of VT (P<0.05 or
P<0.01). Similarly, the duration of
BaCl2/aconitine-induced VT in 18, 36 and 54 mg/kg
amiodarone groups was markedly reduced compared with that of the
amiodarone control group. However, 6 mg/kg terfenadine exerted no
essential impact on the duration of VT induced by BaCl2
and aconitine (P=0.06 and P=0.09 vs. the terfenadine control group,
respectively). Furthermore, according to the results of the
equivalence test of 12 mg/kg terfenadine and 36 mg/kg amiodarone,
18 mg/kg terfenadine and 54 mg/kg amiodarone in reducing the
duration of VT caused by BaCl2, the 95% CI of the
difference (−3.6-2.4) and (−0.2–2.9) were both within the
predetermined margin of equivalence. Similarly, regarding the
aspect of suppressing aconitine-induced VT, the 95% CI (−4.5-4.2)
and (−4.3-3.9) also lay within the predetermined margin of
equivalence. These results indicated that the potential
antiarrhythmic effect of terfenadine was not inferior to that of
amiodarone.
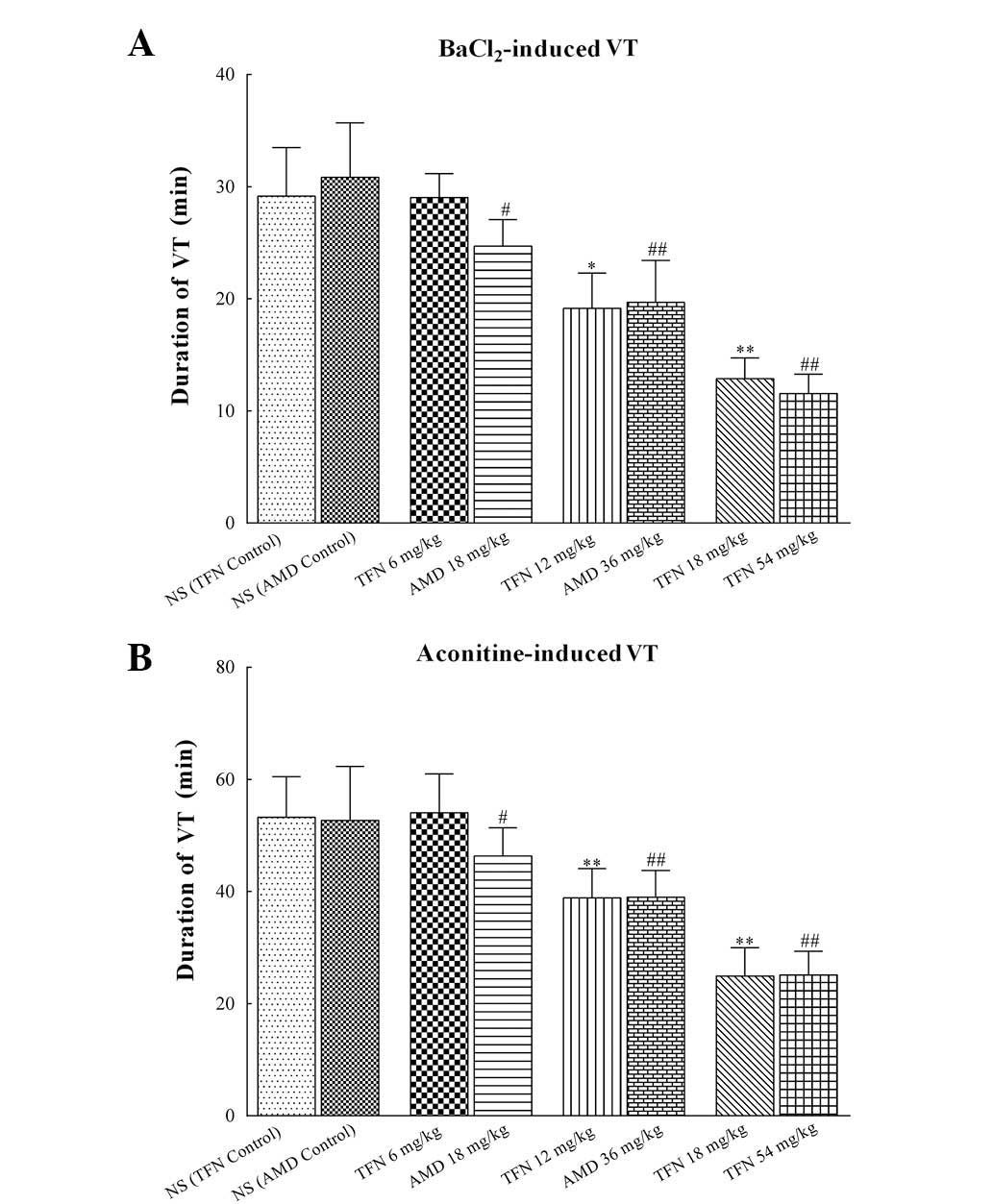 | Figure 5Duration of
BaCl2/aconitine-induced VT in each group. (A) Duration
of BaCl2-induced VT in different groups. (B) Duration of
aconitine-induced VT in different groups. Administration of 12 and
18 mg/kg terfenadine significantly shortened the duration of VT.
Similarly, the duration of BaCl2/aconitine-induced VT in
18, 36 and 54 mg/kg amiodarone groups were markedly shortened. In
each group (n=10) values are expressed as the mean ±SD (columns,
mean; error bars, ± SD). *P<0.05 and
**P<0.01 vs. the NS (TFN control) group;
#P<0.05 and ##P<0.01 vs. the NS (AMD
control) group. VT, ventricular tachycardia; BaCl2,
barium chloride; NS, normal saline; TFN, terfenadine; AMD,
amiodarone; SD, standard deviation. |
 | Table VDuration of
BaCl2/aconitine-induced VT in different groups. |
Table V
Duration of
BaCl2/aconitine-induced VT in different groups.
| Groups | Duration of
BaCl2-induced VT (min) | Duration of
aconitine-induced VT (min) |
|---|
| Terfenadine |
| NS (TFN
control) | 29.17±4.32 | 53.27±7.20 |
| TFN 6 mg/kg | 29.03±2.13 | 54.03±6.94 |
| TFN 12 mg/kg | 19.15±3.15a | 38.89±5.22b |
| TFN 18 mg/kg | 12.88±1.84b | 24.93±5.04b |
| Amiodarone |
| NS (AMD
control) | 30.83±4.88 | 52.71±9.62 |
| AMD 18 mg/kg | 24.69±2.37c | 46.33±5.06c |
| AMD 36 mg/kg | 19.69±3.75d | 39.02±4.76d |
| AMD 54 mg/kg | 11.55±1.73d | 25.12±4.23d |
Administration of BaCl2 caused
life-threatening VT in several animals in all of the groups. The
incidence of mortality reached 50% (5/10 animals) in the
terfenadine control and amiodarone control groups, 40% (4/10
animals) in the 18 mg/kg terfenadine and 18 mg/kg amiodarone
groups, 30% (3/10 animals) in the 6 mg/kg terfenadine group, 20%
(2/10 animals) in the 36 and 54 mg/kg amiodarone groups, and 10%
(1/10 animals) in the 12 mg/kg terfenadine group (Fig. 6A). Aconitine also caused
life-threatening VT in each group. The incidence of mortality
reached 60% (6/10 animals) in the terfenadine control group, 50%
(5/10 animals) in the amiodarone control group, 40% (4/10 animals)
in the 6 and 18 mg/kg terfenadine groups, 30% (3/10 animals) in the
18 and 36 mg/kg amiodarone groups, and 20% (2/10 animals) in the 54
mg/kg amiodarone group (Fig.
6B).
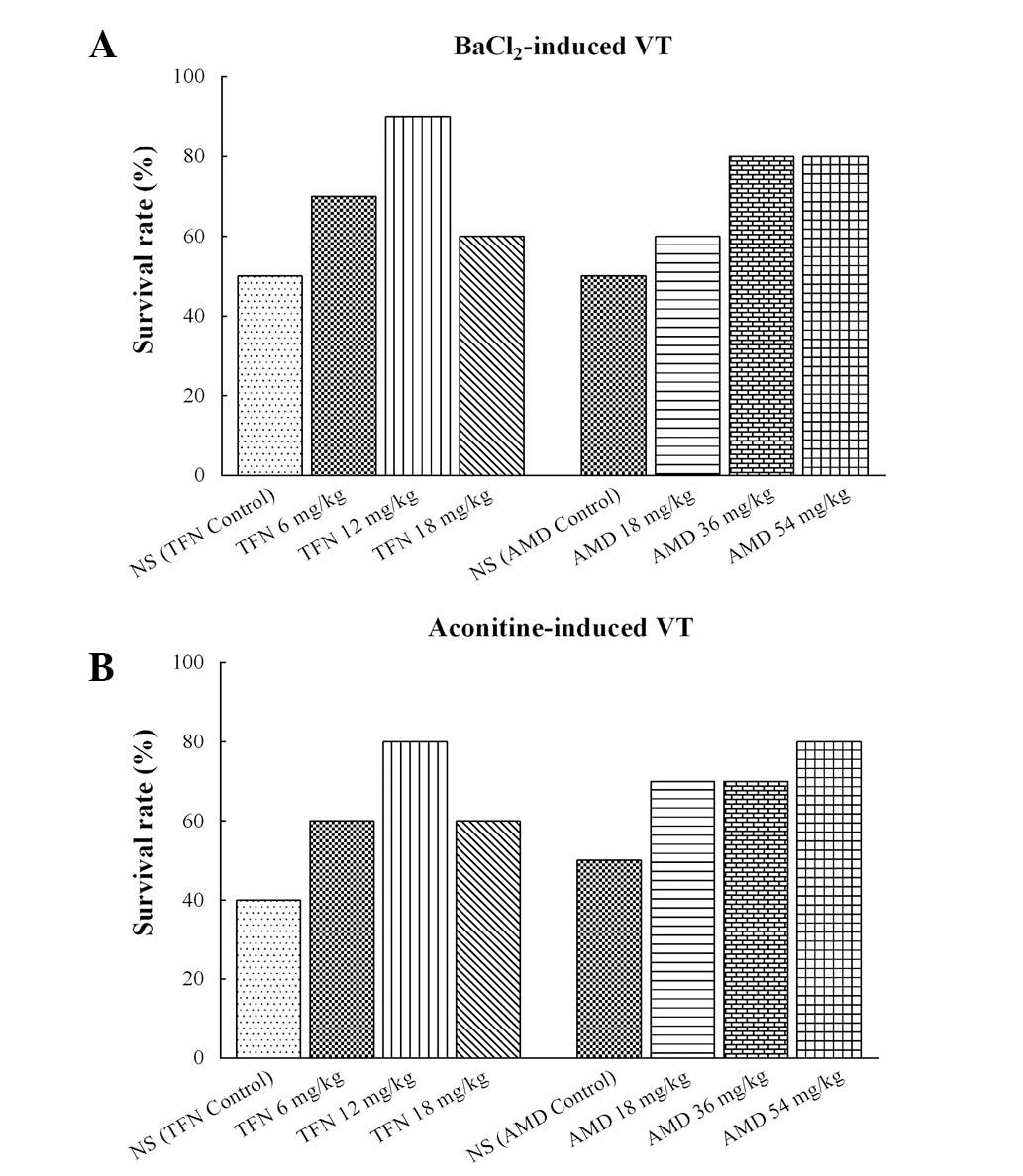 | Figure 6Survival rates in different groups
following triggering of VT with BaCl2 or aconitine in
rats. (A) Survival rates of terfenadine- and amiodarone-treated
rats with BaCl2-induced ventricular arrhythmia. The
survival rate in the normal control group was 50%, in the 18 mg/kg
terfenadine and 18 mg/kg amiodarone groups it was 60%, in the 6
mg/kg terfenadine group it was 70%, in the 36 and 54 mg/kg
amiodarone groups it was 80%, and in the 12 mg/kg terfenadine it
was 90%. (B) Survival rates of terfenadine and amiodarone on
aconitine-induced ventricular arrhythmia. The survival rate in the
terfenadine control group was 40%, in the amiodarone control group
it was 50%, in the 6 and 18 mg/kg terfenadine groups it was 60%, in
the 18 and 36 mg/kg amiodarone groups it was 70%, and in the 12
mg/kg terfenadine and 54 mg/kg amiodarone groups it was 80%. Each
group, n=10. VT, ventricular tachycardia; NS, normal saline; TFN,
terfenadine; AMD, amiodarone. |
Discussion
In the present study,
BaCl2/aconitine-induced animal models of ventricular
arrhythmia were established to investigate and compare the
protective effects of terfenadine and amiodarone at different
concentrations on ventricular arrhythmia. The results demonstrated
that both terfenadine and amiodarone significantly prolonged the
QTc intervals and altered the other ECG parameters in a
dose-dependent manner. In the meantime, these two drugs
demonstrated similar preventative and therapeutic effects on
various arrhythmias triggered by BaCl2 or aconitine.
It is well established that infusion of
BaCl2 leads to delayed afterdepolarization and triggered
activity through increases in Na+ and Ca2+
inflow to the myocardium, promoting the genesis of arrhythmia
(25,26). Most importantly,
electrophysiological studies have found that Ba2+ was
the potential blocker of various K+ channels and
permeation of Ba2+ through the K+ channel may
impede the presence of K+ in the external solution,
which may result in the increase of autorhythmicity of the
myocardium to produce ventricular arrhythmia (27–29).
The present study demonstrated that terfenadine and amiodarone at
different concentrations not only delayed the onset time of VPC,
VT, VF and CA in BaCl2-induced ventricular arrhythmia in
rats, but terfenadine and amiodarone at different doses also
shortened the duration of VT. Additionally, the survival rates in
all of the terfenadine and amiodarone groups were higher than those
of the control groups. The above results may be mainly explained by
K+ channel blockade of terfenadine and amiodarone, and
partially attributed to the Na+ and Ca2+
channel blockade of these two drugs (30,31).
Aconitine, a specific Na+ channel blocker
able to prolong the open state of the channel, may induce
intracellular Na+ accumulation and intracellular
Ca2+ overload, which may eventually result in
polymorphic ventricular arrhythmia (32). The present study indicated that
there was an obvious protective effect of the different
concentrations of terfenadine and amiodarone on aconitine-induced
arrhythmia. Terfenadine and amiodarone of different doses
significantly increased the threshold dose of aconitine required to
induce ventricular arrhythmias, including VPC, VT, VF and CA.
Furthermore, the duration of ventricular arrhythmias and the
mortality were significantly reduced compared with those in the
control groups. The protective effect may be due to decrease of
INa and ICa-L currents induced by
these two drugs in ventricular cardiomyocytes (4,33,34).
However, low-dose terfenadine (6 mg/kg) exerted no
significant impact on VPC and the duration of ventricular
tachycardia induced by BaCl2. Additionally, although the
incidence of mortality was significantly reduced following
administration of terfenadine and amiodarone, a high dose of
terfenadine (18 mg/kg) was more lethal than a moderate dose of
terfenadine (12 mg/kg) in the BaCl2-induced as well as
in the aconitine-induced VT. Furthermore, terfenadine and
amiodarone as K+ channel agonists have been widely
investigated, whereas little information is available on the
antiarrhythmic effects of terfenadine and amiodarone as
Na+ and Ca2+ channel blockers. More complex
mechanisms may be involved in the protective role of terfenadine on
the arrhythmia model, and elucidation of the effects requires
further studies.
In humans, terfenadine is well absorbed and
metabolized in liver microsomes to form hydroxyterfenadine.
Hydroxyterfenadine then undergoes subsequent oxidation to the
corresponding carboxylic acid, which is considered to be the
biologically active antihistamine (35). This carboxylic acid has been proved
no effect on K+ channels in ventricular myocytes, even
at high plasma levels (36).
However, terfenadine itself produced cardiotoxicity in isolated
rabbit hearts and human atrial myocytes (4). CYP3A4 has been demonstrated to be the
principal cytochrome P450 enzyme involved in the metabolism of
terfenadine, and the adverse clinical drug interactions of
terfenadine have been associated with the inhibition of its
CYP3A4-mediated metabolism (36).
Drugs including ketoconazole and erythromycin may inhibit the
activity of CYP3A4, which leads to an increase in terfenadine
plasma levels and finally produces toxicity in the heart. The
present data indicated that terfenadine dose-dependently prolonged
the QTc interval, and this effect was similar to that of
amiodarone. Furthermore, similar side effects occurred following
overdose of antiarrhythmic drugs, including quinidine and sotalol.
Therefore, the QTc interval prolongation caused by terfenadine may
be similar to the adverse effects of several antiarrhythmic
drugs.
Amiodarone is one of the few remaining treatment
options for ventricular arrhythmia and for reducing the incidence
of atrial fibrillation, particularly in heart failure patients with
severely impaired left ventricular function, where class I
antiarrhythmic drugs or dronedarone are considered as
contra-indicated (37–39). The present study demonstrated that
terfenadine and amiodarone may not only similarly delay the onset
time of ventricular arrhythmia induced by BaCl2, but
also increase the cumulative dosage of aconitine required to induce
VPC, VT, VF and CA. Furthermore, the two drugs were equally
efficient in shortening the duration of ventricular arrhythmia.
Aside from cardiotoxicity, the accumulation of amiodarone may also
lead to thyroid dysfunction and pulmonary fibrosis (40,41).
Since these adverse drug reactions have limited the application of
amiodarone, the development of new antiarrhythmic drugs is urgently
required. The present study provided preliminary evidence that
terfenadine had potential antiarrhythmic effects, which greatly
facilitates the discovery and development of novel antiarrhythmic
drugs.
Several limitations of the present study should be
noted. The investigation was performed only in ventricular
arrhythmia animal models, the antiarrhythmic activity of
terfenadine should be confirmed in cardiomyocytes through in
vitro experiments. The preventative and therapeutic effects of
terfenadine were only detected in ventricular antiarrhythmia
through comparing with the K+ channel blocker
amiodarone. Since terfenadine may potentially inhibit
Na+ channels and L-type Ca2+ channels,
similar comparisons with Na+ channels and
Ca2+ channel blockers should be conducted to determine
the antiarrhythmic effect of terfenadine. The present investigation
was performed following anesthetizing of the animals, which may
have had an effect on ECG parameters. Whether terfenadine may be
suitable as a novel antiarrhythmic drug requires additional
studies.
In conclusion, it was identified that terfenadine
and amiodarone similarly prolonged the QTc interval in rats. In
BaCl2/aconitine induced ventricular arrhythmia models,
terfenadine and amiodarone not only similarly delayed the onset
time of arrhythmia induced by barium chloride (all P<0.05), but
also increased the cumulative dosage of aconitine required to
induce various types of arrhythmia. Furthermore, the two drugs
equivalently caused a significant decrease in the duration of VT.
The potential antiarrhythmic effect of terfenadine was shown to not
be inferior to that of amiodarone in experimental ventricular
arrhythmia rat models.
Acknowledgements
The present study was supported by a grant of the
National Natural Science Foundation of China (no. 81200198) to
Professor Yawei Xu. This study was conducted at the Central
Laboratory of the Shanghai Tenth People’s Hospital of Tongji
University. The authors are grateful to Dr Han Yan, Dr Rong Guo and
Dr Ke Wang from Tongji University School of Medicine (Shanghai,
China) for their help.
References
|
1
|
Ghoneim M, Issa R and Tawfik A: Assay of
terfenadine in pharmaceutical formulation and human plasma by
adsorptive stripping voltammetry. J Pharm Biomed Anal. 26:593–598.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monahan BP, Ferguson CL, Killeavy ES, et
al: Torsades de pointes occurring in association with terfenadine
use. JAMA. 264:2788–2790. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith SJ: Cardiovascular toxicity of
antihistamines. Otolaryngol Head Neck Surg. 111:348–354.
1994.PubMed/NCBI
|
|
4
|
Lu HR, Hermans AN and Gallacher DJ: Does
terfenadine-induced ventricular tachycardia/fibrillation directly
relate to its QT prolongation and Torsades de Pointes? Br J
Pharmacol. 166:1490–1502. 2012. View Article : Google Scholar
|
|
5
|
Kamiya K, Niwa R, Morishima M, Honjo H and
Sanguinetti MC: Molecular determinants of hERG channel block by
terfenadine and cisapride. J Pharmacol Sci. 108:301–307. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Testai L, Breschi MC, Martinotti E and
Calderone V: QT prolongation in guinea pigs for preliminary
screening of torsadogenicity of drugs and drug-candidates. II. J
Appl Toxicol. 27:270–275. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iribarren C, Round AD, Peng JA, et al:
Validation of a population-based method to assess drug-induced
alterations in the QT interval: a self-controlled crossover study.
Pharmacoepidemiol Drug Saf. 22:1222–1232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li XW, Niu SC, Zhang XP, et al:
Differences of promethazine and terfenadine on ion channels in
guinea pig ventricular myocytes. Chin Med J (Engl). 119:944–947.
2006.PubMed/NCBI
|
|
9
|
Woosley RL, Chen Y, Freiman JP and Gillis
RA: Mechanism of the cardiotoxic actions of terfenadine. JAMA.
269:1532–1536. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajput SK, Singh JN and Sharma SS:
Evaluation of terfenadine and ketoconazole-induced QT prolongation
in conscious telemetered guinea pigs. Pharmacol Rep. 62:683–688.
2010. View Article : Google Scholar
|
|
11
|
Aslanian R, Piwinski JJ, Zhu X, et al:
Structural determinants for histamine H(1) affinity, hERG affinity
and QTc prolongation in a series of terfenadine analogs. Bioorg Med
Chem Lett. 19:5043–5047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mehta A, Chung Y, Sequiera GL, et al:
Pharmacoelectrophysiology of viral-free induced pluripotent stem
cell-derived human cardiomyocytes. Toxicol Sci. 131:458–69. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stork D, Timin EN, Berjukow S, et al:
State dependent dissociation of HERG channel inhibitors. Br J
Pharmacol. 151:1368–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davies AJ, Harindra V, McEwan A and Ghose
RR: Cardiotoxic effect with convulsions in terfenadine overdose.
BMJ. 298:3251989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hess P, Rey M, Wanner D, Steiner B and
Clozel M: Measurements of blood pressure and electrocardiogram in
conscious freely moving guineapigs: a model for screening QT
interval prolongation effects. Lab Anim. 41:470–80. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang W, Wang Y, Cao YG, et al:
Antiarrhythmic effects and ionic mechanisms of allicin on
myocardial injury of diabetic rats induced by streptozotocin.
Naunyn Schmiedebergs Arch Pharmacol. 386:697–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilhelms M, Rombach C, Scholz EP, Dössel O
and Seemann G: Impact of amiodarone and cisapride on simulated
human ventricular electrophysiology and electrocardiograms.
Europace. 14:v90–v96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taniguchi T, Uesugi M, Arai T, et al:
Chronic probucol treatment decreases the slow component of the
delayed-rectifier potassium current in CHO cells transfected with
KCNQ1 and KCNE1: a novel mechanism of QT prolongation. J Cardiovasc
Pharmacol. 59:377–386. 2012. View Article : Google Scholar
|
|
19
|
Khisatmutdinova RIu, Baschenko NZh,
Zarudii FS, et al: Some aspects of the antiarrhythmic effect of
glialin. Eksp Klin Farmakol. 69:26–28. 2006.PubMed/NCBI
|
|
20
|
Huang W, Wang Y, Cao YG, et al:
Antiarrhythmic effects and ionic mechanisms of allicin on
myocardial injury of diabetic rats induced by streptozotocin.
Naunyn Schmiedebergs Arch Pharmacol. 386:697–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wada K, Nihira M, Hayakawa H, et al:
Effects of long-term administrations of aconitine on
electrocardiogram and tissue concentrations of aconitine and its
metabolites in mice. Forensic Sci Int. 148:21–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao Y, Li P, Ma LX, et al: Effects of
acute administration of ethanol on experimental arrhythmia. Chin J
Physiol. 55:307–313. 2012.PubMed/NCBI
|
|
23
|
Testai L, Cecchetti V, Sabatini S, et al:
Effects of K openers on the QT prolongation induced by
HERG-blocking drugs in guinea-pigs. J Pharm Pharmacol. 62:924–930.
2010.PubMed/NCBI
|
|
24
|
Zhang YH, Cheng H, Alexeenko VA, Dempsey
CE and Hancox JC: Characterization of recombinant hERG K(+) channel
inhibition by the active metabolite of amiodarone
desethyl-amiodarone. J Electrocardiol. Sep–Oct;43(5): 440–8. 2010.
View Article : Google Scholar
|
|
25
|
Coast GM: Intracellular Na+,
K+ and Cl− activities in Acheta domesticus Malpighian
tubules and the response to a diuretic kinin neuropeptide. J Exp
Biol. 215:2774–2785. 2012.PubMed/NCBI
|
|
26
|
Discala F, Belachgar F, Planelles G, Hulin
P and Anagnostopoulos T: Barium- or quinine-induced depolarization
activates K+, Na+ and cationic conductances
in frog proximal tubular cells. J Physiol. 448:525–537. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kehl SJ, Fedida D and Wang Z: External
Ba(2+) block of Kv4.2 channels is enhanced in the
closed-inactivated state. Am J Physiol Cell Physiol. 304:C370–C381.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rowley CN and Roux B: A computational
study of barium blockades in the KcsA potassium channel based on
multi-ion potential of mean force calculations and free energy
perturbation. J Gen Physiol. 142(4): 451–463. 2013. View Article : Google Scholar
|
|
29
|
Kubo Y, Baldwin TJ, Jan YN and Jan LY:
Primary structure and functional expression of a mouse inward
rectifier potassium channel. Nature. 362:127–33. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ming Z and Nordin C: Terfenadine blocks
time-dependent Ca2+, Na+, and K+
channels in guinea pig ventricular myocytes. J Cardiovasc
Pharmacol. 26:761–769. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lalevée N, Nargeot J, Barrére-Lemaire S,
Gautier P and Richard S: Effects of amiodarone and dronedarone on
voltage-dependent sodium current in human cardiomyocytes. J
Cardiovasc Electrophysiol. 14:885–90. 2003.PubMed/NCBI
|
|
32
|
Zhou SS, Yang J, Li YQ, et al: Effect of
Cl− channel blockers on aconitine-induced arrhythmias in rat heart.
Exp Physiol. 90:865–872. 2005.
|
|
33
|
Moreno JD and Clancy CE: Pathophysiology
of the cardiac late Na current and its potential as a drug target.
J Mol Cell Cardiol. 52:608–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goegelein H, Gautier P, Roccon A, O’Connor
S and Ruetten H: Effects of the novel amiodarone-like compound
SAR114646A on cardiac ion channels and ventricular arrhythmias in
rats. Naunyn Schmiedebergs Arch Pharmacol. 384:231–244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garteiz DA, Hook RH, Walker BJ and
Okerholm RA: Pharmacokinetics and biotransformation studies of
terfenadine in man. Arzneimittelforschung. 32:1185–1190.
1982.PubMed/NCBI
|
|
36
|
Jones BC, Hyland R, Ackland M, Tyman CA
and Smith DA: Interaction of terfenadine and its primary
metabolites with cytochrome P450 2D6. Drug Metab Dispos. 26:875–82.
1998.PubMed/NCBI
|
|
37
|
Eckardt L and Breithardt G: Is there a
role for amiodarone in the era of the implantable
cardioverter-defibrillator? Heart Rhythm. 3:484–487. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Echt DS, Liebson PR, Mitchell LB, et al:
Mortality and morbidity in patients receiving encainide,
flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial N
Engl J Med. 324:781–788. 1991.PubMed/NCBI
|
|
39
|
Naccarelli GV, Wolbrette DL, Samii S, et
al: A review of the appropriate and inappropriate use of
dronedarone: lessons learned from controlled studies and regulatory
submission. J Cardiovasc Pharmacol Ther. 15:24S–30S.
2010.PubMed/NCBI
|
|
40
|
Maseeh-uz-Zaman, Fatima N and Sajjad Z:
Amiodarone therapy: don’t forget thyroid. J Pak Med Assoc.
62:268–272. 2012.PubMed/NCBI
|
|
41
|
Garg J, Agrawal N, Marballi A, et al:
Amiodarone induced pulmonary toxicity: An unusual response to
steroids. Am J Case Rep. 13:62–65. 2012. View Article : Google Scholar : PubMed/NCBI
|