Introduction
Hepatocellular carcinoma (HCC) is a leading cause of
cancer mortality worldwide, with >600,000 novel cases diagnosed
annually (1). Although there have
been developments in surgical strategies and in clinical care, the
overall outcome for patients with HCC remains poor, as HCC is
commonly detected at a late stage, which precludes potentially
curative treatment (2). Thus,
understanding the molecular mechanisms underlying the pathogenesis
of this type of cancer, and developing sensitive and specific
molecular markers and novel therapies is required.
A disintegrin and metalloproteases (ADAMs) are a
family of zinc-dependent transmembrane proteins (3). The family encodes proteins that
mediate cellular responses to environmental stress by interacting
with various cell surface proteins and regulating diverse cellular
processes. These proteins are involved in a range of human disease
processes, such as cancer metastasis, inflammation and asthma
(4,5). ADAM10, an important member of the
ADAM family, is frequently upregulated in various types of cancer,
including HCC, and is involved in cancer progression and metastasis
(6–12). ADAM10 has been reported to exert an
important role in cell migration, tumor development and metastasis
by proteolytic shedding of cell surface proteins (13,14).
Recently, studies have revealed that RNA interference
(RNAi)-mediated downregulation of endogenous ADAM10 inhibits
adenoid cystic carcinoma cell growth and metastasis (15). In addition, Yang et al
(10) demonstrated that ADAM10 is
crucial in mediating the chemoresistance of HCC cells to
doxorubicin. However, relatively little is known regarding the role
of ADAM10 in HCC cells. Thus, in the present study, the feasibility
of lentiviral vector-delivered small hairpin RNA (shRNA) against
ADAM10 in the treatment of HCC was assessed in vitro and
in vivo, and the molecular pathways involved were
examined.
Materials and methods
Cell culture
HepG2 human HCC cell lines were purchased from the
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China) and were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal bovine serum (FBS; Invitrogen
Life Technologies, Carlsbad, CA, USA) in a humidified incubator in
5% CO2 at 37°C.
Preparation of plasmid-based ADAM10 shRNA
vector and transfection of HepG2 cells
An ADAM10 small interfering RNA (siRNA) sequence
(CAGUGUGCAUUCAAGUCAA) and a scrambled control (NC) sequence that
does not target any gene product and has no significant sequence
similarity to human gene sequences (AATTCTCCGAACGTGTCACGT) were
designed using the siRNA Target Designer software (version 2.0,
Promega Corporation, Madison, WI, USA). Preparation of the RNAi
vector expressing the human ADAM10 and NC shRNA was performed using
the pSuper siRNA expression plasmid with the U6 promoter
(Oligoengine, Seattle, WA, USA) according to previously described
methods (16).
The HepG2 cells were transduced with either the
specific ADAM10 shRNA vector or an empty plasmid using
Lipofectamine™ (Invitrogen Life Technologies) 2000 transfection
reagent. G418 (300 μg/ml) was employed to screen for stably
transfected clones. ADAM10 expression levels were examined using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis using mouse anti human ADAM10
monoclonal antibodies to verify the silencing efficiency of the
target gene following RNAi treatment. Cells with stable
transfection and effective inhibition of the ADAM10 gene were
termed ADAM10-RNAi cells, and cells with stable transfection of the
NC sequence were termed NC-RNAi cells.
RT-qPCR
RT-qPCR analysis of ADAM10 transcripts in HepG2
cells was conducted using TRIzol reagent (Invitrogen Life
Technologies) according to the manufacturer’s instructions. RNA was
reverse-transcribed into cDNA using a Primescript™ RT
reagent kit according to the manufacturer’s instructions (Takara,
Dalian, China). RT-qPCR was conducted using the SYBR Green
fluorescent dye method and a Rotor Gene 3000 real-time PCR
apparatus (Applied Biosystems Life Technologies, Foster City, CA,
USA). ADAM10 gene-specific amplification was confirmed by PCR with
specific primers (5′-CTGCCCAGCATCTGACCCTAA-3′ and
5′-TTGCCATCAGAACTGGCACAC-3′) and subjected to melting curve
analysis. GAPDH served as an internal control for standardization.
The primer sequences for β-actin were as follows: Forward:
5′-GATCATTGCTCCTCCTGAGC-3′ and reverse: 5′-ACTCCTGCTTGCTGATCCAC-3′.
The PCR conditions were as follows: Pre-denaturation at 95°C for 2
min, followed by 40 cycles of denaturation at 95°C for 10 sec and
annealing/extension at 55°C for 20 sec. All RT-qPCR experiments
were conducted in triplicate and were performed at 72 h after
transfection. The data were analyzed using the comparative Ct
method.
Western blot analysis
The cells were lysed by incubation on ice for 30 min
in lysis buffer containing 25 mM Tris-HCl (pH 8.0), 1% Nonidet P40,
0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate (SDS), 125 mM
NaCl and Complete Protease Inhibitor Cocktail (Roche Diagnostics
GmbH, Mannheim, Germany). Equal quantities of protein (15 μg/lane)
from the cell lysates were separated on an 8–15% SDS-polyacrylamide
gel and transferred to nitrocellulose membranes (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The membranes were
incubated for 2 h in phosphate-buffered saline (PBS) with 0.1%
Tween-20 and 5% non-fat milk to block nonspecific binding.
Subsequently, membranes were incubated with a mouse anti-ADAM10
monoclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc., CA,
USA), a mouse anti-Survivin monoclonal antibody (1:2,000; Santa
Cruz, CA, USA), a mouse anti-Bcl-2 monoclonal antibody (1:1,500;
Santa Cruz Biotechnology, Inc.), a mouse anti-phosphorylated Akt
(Ser473; p-Akt) monoclonal antibody (1:500; Cell Signaling
Technology, Inc., Danvers, MA, USA), a mouse anti-Akt monoclonal
antibody (1:2,000; Cell Signaling Technology, Inc.), a mouse
anti-phosphorylated PI3K (Tyr458; p-PI3K) monoclonal
antibody(1:1,000; Cell Signaling Technology, Inc.), a mouse
anti-PI3K monoclonal antibody (1:3,000; Cell Signaling Technology,
Inc.) and a mouse anti-β-actin monoclonal antibody (1:5,000; Cell
Signaling Technology, Inc.) at room temperature for 2 h. Following
washing, anti-mouse secondary horseradish peroxidase-conjugated
(1:10,000; Amersham Biosciences, Uppsala, Sweden) was added for 2
h. Protein bands were visualized with an enhanced
chimioluminescence reagent (ECL, Amersham, GE Healthcare,
Velizy-Villacoublay, France). All assays using ADAM10 knockdown
HepG2 cells were performed at 72 h after of transfection.
Cell proliferation assay
The
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay was used to screen for cell proliferation.
Briefly, the cells were seeded in eight wells of 96-well plates at
a density of 2×103 cells/well. Following culture for 24
h, the cells were treated with ADAM10-RNAi or NC-RNAi. After 48 h
culture, 20 μl MTT (5 mg/ml) was added to each well followed by
incubation at 37°C for 48 h. Subsequently, centrifugation was
performed at 2,000 × g for 10 min. The supernatant was removed and
200 μl dimethylsulfoxide was added to each well followed by
agitation of the plates for 10 min. The absorbance of each well was
measured using a microplate reader (Molecular Devices Corp.,
Sunnyvale, CA, USA) at a wavelength of 490 nm. The experiment was
repeated three times. The mean proliferation of each treatment
group is expressed as a percentage of the mean proliferation of the
cells without any treatment.
Terminal deoxynucleotidyl
transferase-mediated nick end labeling (TUNEL) assay
To measure the effect of RNAi-mediated ADAM10
downregulation on cell apoptosis, a TUNEL assay was conducted.
Briefly, following HepG2 cell treatment with ADAM10 RNAi for 24 h,
cellular DNA fragmentation was measured with the ApoTag Red in
situ Apoptosis Detection kit (Chemicon International, Temecula,
CA, USA) according to the manufacturer’s instructions. To quantify
the apoptotic cells, TUNEL-positive cells were counted using a
confocal microscope (BX2, Olympus Corporation, Tokyo, Japan). In
addition, at the molecular level, the expression levels of Survivin
and Bcl-2 apoptotic proteins were detected by western blotting as
an additional indicator of apoptosis, as described above.
Wound-healing assay
To assess the effect of ADAM10 downregulation by
RNAi on cell migration, a wound-healing assay was performed. A
total of 1×105 HepG2 cells were plated in 12-well plates
in DMEM containing 10% FBS. After 24 h, a scratch was inflicted
through the confluent cell monolayer, and then the cells were
treated with ADAM10- RNAi or NC-RNAi respectively in 3 ml complete
medium. After 48 h treatment, the cells were stained with
hematoxylin and eosin. The cells that invaded the wound line were
observed with an inverted phase-contrast microscope (Leica DMR;
Leica, Wetzlar, Germany). Triplicates were performed in all
experiments.
Transwell invasion assay
Cell invasion was determined using Transwell
chambers (BD Biosciences San Jose, CA, USA) consisting of
polycarbonate membrane filters with a pore size of 8 μm. In brief,
2×105 HepG2 cells in DMEM media with 0.5% FBS were added
to the upper chamber containing 8 mm pore polycarbonate coated with
1 mg/ml Matrigel (BD Biosciences); the lower chamber was filled
with media containing 5% FBS. ADAM10-RNAi or NC-RNAi was added to
the upper chambers, respectively. Cells without any drug treatment
served as a control. After 16 h incubation, the upper membrane
surface was scoured with a cotton-tipped swab. The invading cells
on the lower membrane surface were fixed and stained with 0.5%
crystal violet. Images of random fields (five per membrane) were
captured using an inverted microscope (CKX31; Olympus Corporation)
at magnification ×40 for the calculation of the cell numbers.
Furthermore, the cells were quantified by measuring the absorbance
of dye extracts at 570 nm in 100 ml Sorenson’s solution containing
9 mg tirsodium citrate, 195 ml 0.1 M HCl, 305 ml distilled water
and 500 ml of 90% ethanol. All experiments were performed in
triplicate.
Tumor xenograft assay
All animal experiments were conducted according to
the standards of animal care as outlined in the Guide for the Care
and Use of Experimental Animals of Jilin University (Changchun,
China), following a procedure approved by the Ethics Committees of
the Disease Model Research Center at the First Hospital of Jilin
University. Female BALBc mice, aged 4–5 weeks old were purchased
from the Institute of Laboratory Animal Science of Jilin University
(Changchun, China) and were maintained under specific pathogen-free
conditions, and provided with food and water ad libitum. All
animals were fed a normal pellet diet for one week prior to
experimentation.
Exponentially growing HepG2 cells were harvested and
a tumorigenic dose of 2×106 cells was injected
intraperitoneally into the BALB mice. When tumors grew to an
average volume of 75 mm3, the mice were randomly divided
into ADAM10-RNAi, control (untreated) and NC-RNAi groups (n=8 in
each group). The control group received 1% polysorbate resuspended
in deionized water. The other two groups were treated with either
ADAM10-RNAi or NC-RNAi intraperitoneally injected on alternate days
for three weeks. Tumor weight was measured when the mice were
sacrificed by decapitation 21 days after treatment. Tumor volume
was measured prior to the administration of the treatment
injections and on days 7, 14 and 21 of treatment.
Statistical analysis
Statistical analysis between two samples was
performed using Student’s t-test. Statistical comparison of more
than two groups was performed using one-way analysis of variance
followed by a Tukey’s post hoc test. All data are expressed as mean
± standard deviation. SPSS® 19.0 software (SPSS, Inc.,
Chicago, IL, USA) for Windows® was used for statistical
analyses. P<0.05 and P<0.01 were considered to indicate a
statistically significant difference.
Results
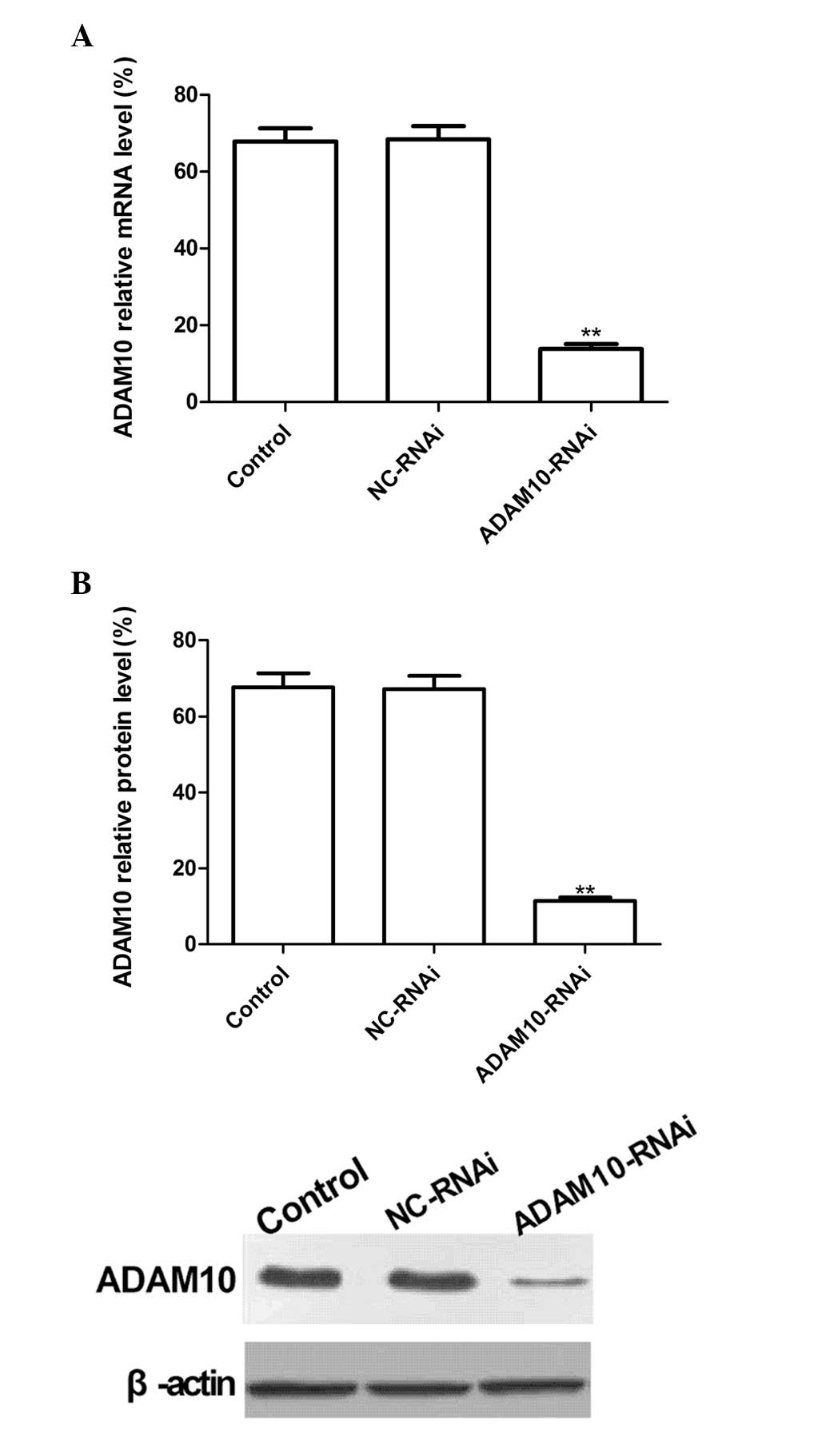
ADAM10-RNAi downregulates ADAM10
expression in HepG2 cells
To evaluate the silencing capacity of ADAM10, HepG2
cells were transfected with ADAM10-RNAi. RT-qPCR and western
blotting were performed to detect the ADAM10 mRNA and protein
expression levels at two days post-transfection. The results
revealed no significant inhibition of ADAM10 expression in the
NC-RNAi group, and no significant differences between the NC-RNAi
and control (PBS) groups (P>0.05). Conversely, ADAM10 mRNA and
protein expression levels in the ADAM10-RNAi group were
significantly reduced following transfection as compared with the
control group (Fig. 1A and B;
P<0.01). These results demonstrate that ADAM10-RNAi
significantly downregulated ADAM10 expression in HepG2 cells
(P<0.01).
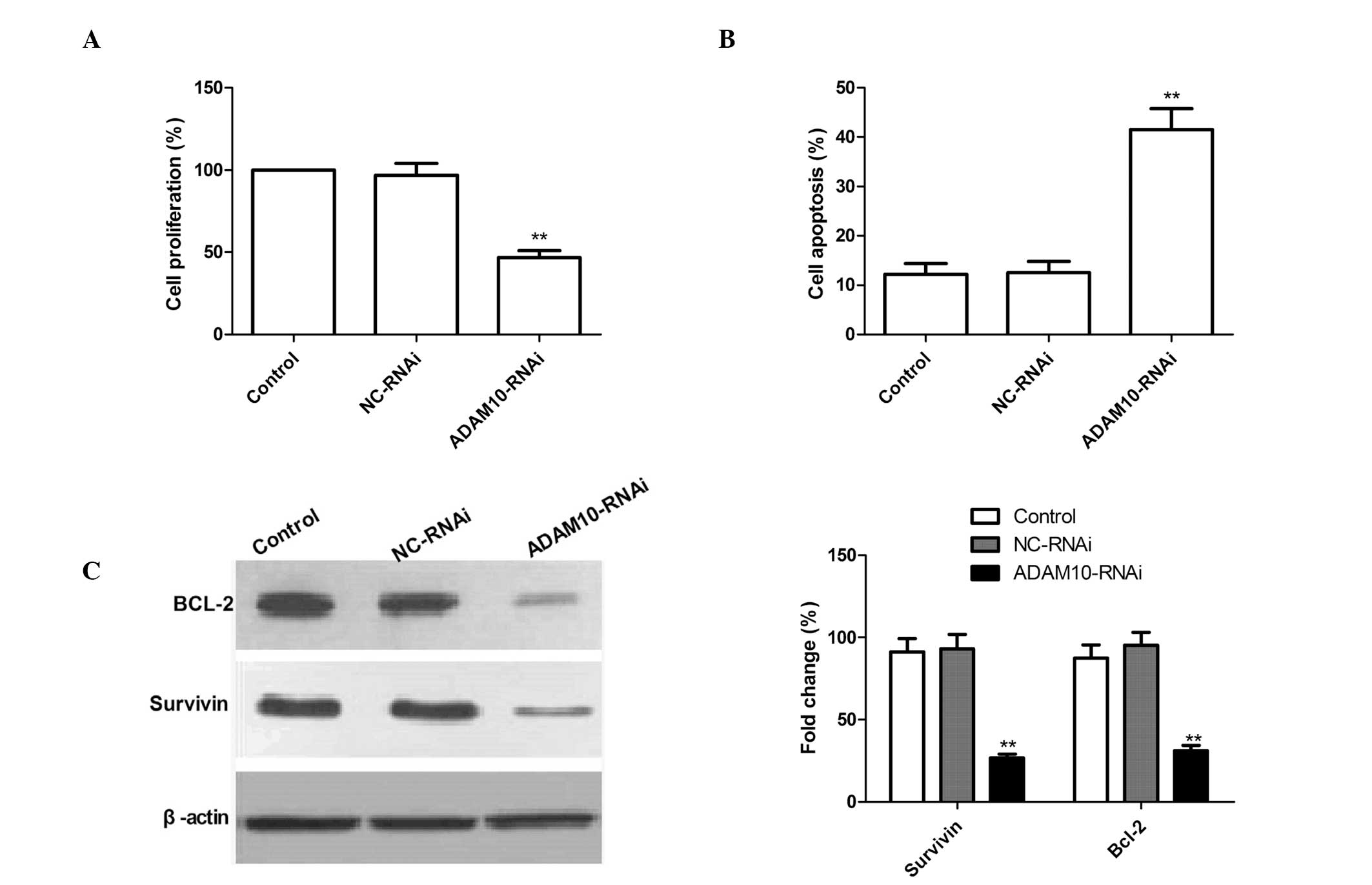
Silencing ADAM10 reduces cell
proliferation and induces cell apoptosis in HepG2 cells
To examine whether the knockdown of ADAM10
expression exerts any effect on cell proliferation, an MTT assay
was performed. As compared with the control and NC-RNAi cells,
ADAM10-RNAi-transfected cells exhibited significantly reduced cell
proliferation, which suggests the involvement of ADAM10 in HepG2
cell growth (P<0.02; Fig.
2A).
In addition, the affect of ADAM10 gene silencing on
HepG2 cell apoptotic ability was investigated by a TUNEL assay.
ADAM10 silencing resulted in a significant increase in the number
of apoptotic cells as compared with the control and NC-RNAi groups
(Fig. 2B; P<0.01). No
significant differences in the induction of HCC apoptosis were
identified between the control and NC-RNAi groups.
To investigate the possible mechanism of the
pro-apoptotic effect of silencing ADAM10 in HepG2 cells, Survivin
and Bcl-2 expression patterns were determined by western blotting.
The results are shown in Fig. 2C.
Silencing ADAM10 significantly reduced the expression levels of the
Survivin and Bcl-2 apoptosis-inhibiting genes compared with control
or NC-RNAi treatment. These data support the hypothesis that ADAM10
expression is required for promoting cell proliferation and
inhibiting apoptosis’.
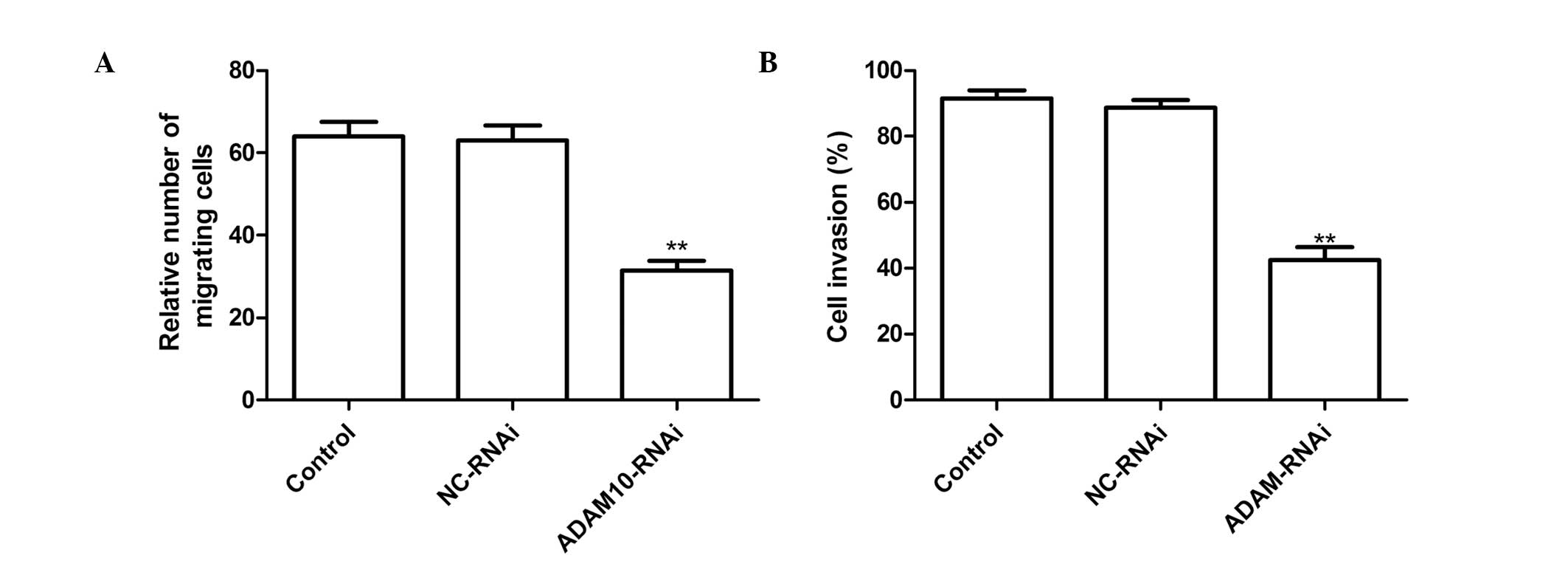
Silencing ADAM10 reduces cell migration
and invasion in HepG2 cells
To ascertain the inhibitory effect of silencing
ADAM10 on HepG2 cell migration, a wound-healing assay was
performed. It was found that hepG2 cells transfected with
ADAM10-RNAi migrated significantly less than those in the untreated
cells or cells transfected with NC-RNAi (Fig. 3A P<0.01).
The ability of ADAM10 silencing to reduce the
invasiveness of HepG2 cells was further investigated by a Transwell
system assay. Invasion was found to be significantly reduced
following ADAM10-RNAi treatment as compared with the control and
NC-RNAi treatments (P<0.01; Fig.
3B).
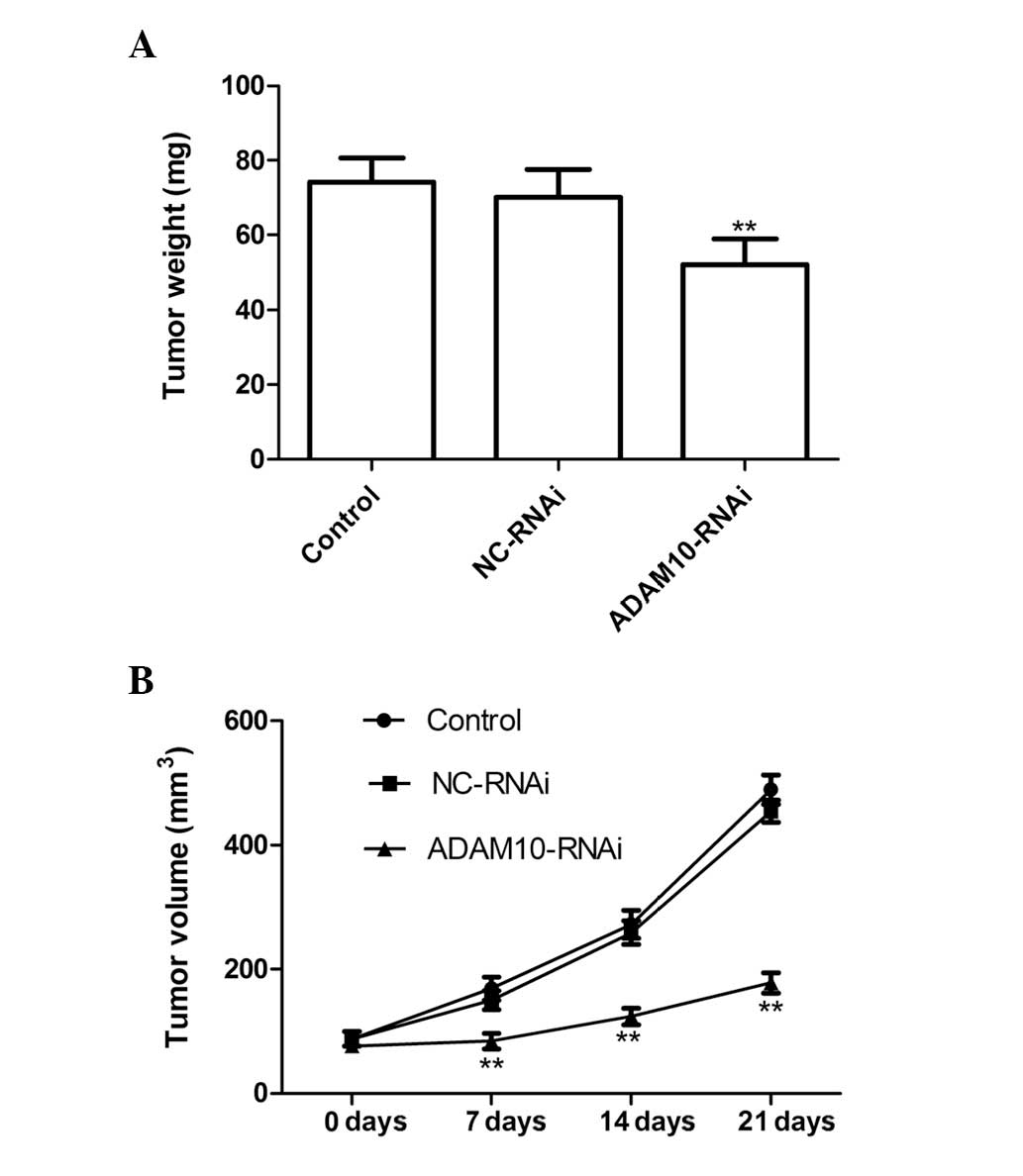
Silencing ADAM10 suppresses tumor growth
in vivo
The in vivo therapeutic efficacy of ADAM10
downregulation was assessed in female BALB mice bearing HepG2
tumors. The tumor weight in the ADAM10-RNAi group was significantly
lower than that in the control and NC-RNAi groups (P<0.01;
Fig. 4A). In addition, tumor
volume following treatment with ADAM10-RNAi was found to be
significantly lower and increased at a markedly lower rate, as
compared with the tumor volumes following control or NC-RNAi
treatment (P<0.01; Fig. 4B).
These results indicate that downregulation of ADAM10 expression in
HCC cells markedly suppressed tumor growth in mice.
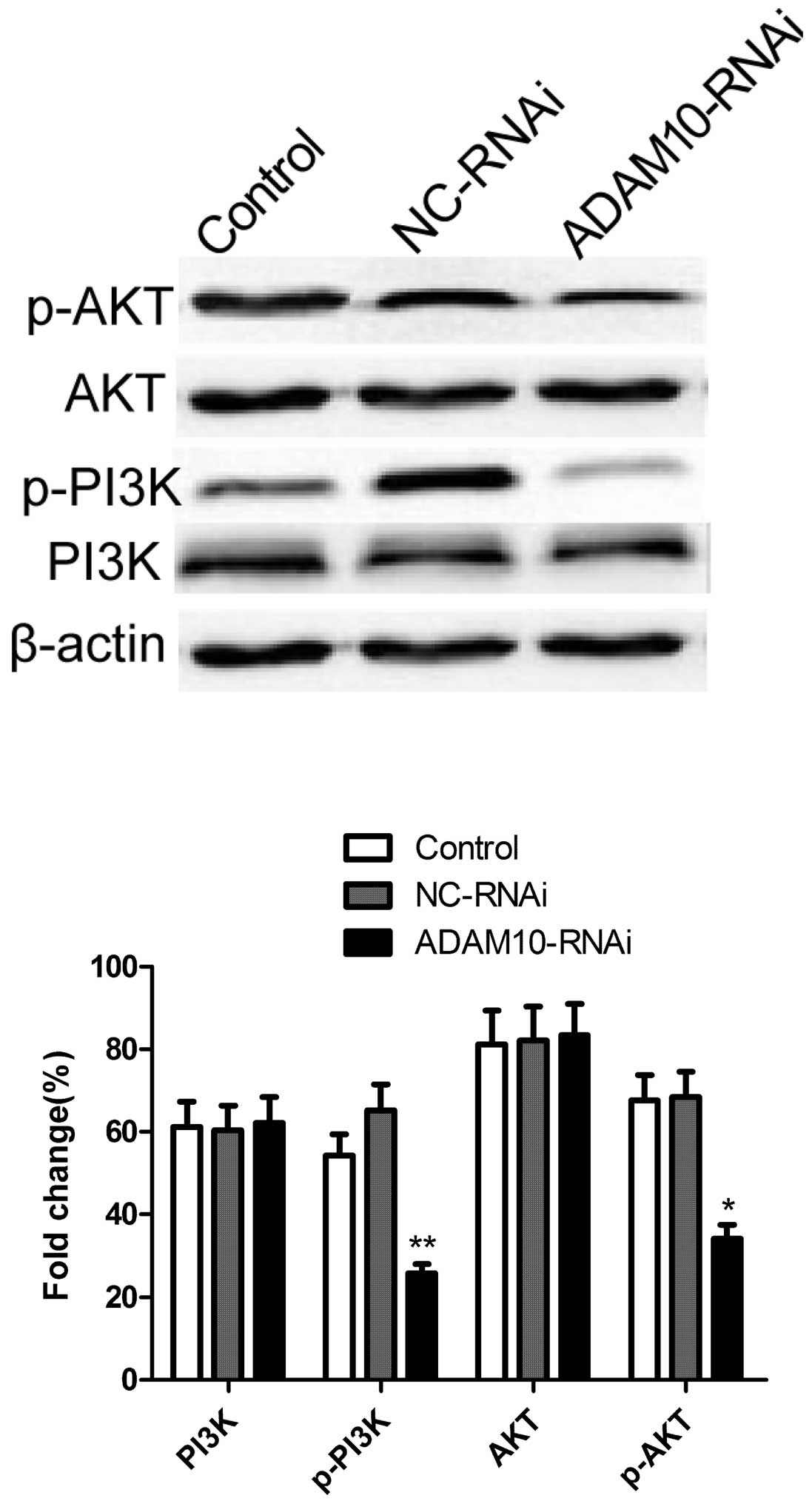
Silencing ADAM10 inhibits the
phosphorylation of Akt and PI3K in HepG2 cells
The antiapoptotic enzyme Akt was shown to be an
important factor in apoptosis resistance in HCCs (17). Therefore, the effect of ADAM10
silencing on the phosphorylation of Akt and PI3K proteins was
investigated by western blotting. As shown in Fig. 5, as compared with the control and
NC-RNAi groups, silencing ADAM10 resulted in a significant
suppression of Akt and PI3K phosphorylation. These results indicate
that silencing ADAM10 induced apoptosis, to a certain extent, by
suppression of the PI3K/Akt signaling pathway.
Discussion
ADAMs are involved in multiple cellular processes,
such as cell proliferation, differentiation, migration and invasion
(4,5). Accumulating evidence suggests that
ADAMs are also important in cell survival (18,19).
Certain members of the ADAM family, including ADAM9, ADAM10 and
ADAM17, are closely involved in tumorigenesis, and the development
and metastasis of tumors (13,20,21).
In addition, Yang et al (10) demonstrated that ADAM10 induces a
prosurvival response in HCC cells upon doxorubicin treatment. Bai
et al (22) found that
expression of microRNA-122, as a negative regulator of ADAM10,
sensitizes HCC cells to sorafenib, a multikinase inhibitor
clinically effective against HCC. Yuan et al (23) observed that ADAM10 silencing
resulted in inhibition of proliferation and migration as well as
HepG2 human hepatoma cell invasion (P<0.05), a finding that is
in concordance with the results of the present study. However, Yuan
et al (23) did not
investigate responses in an in vivo model. The results from
the present study revealed that downregulation of ADAM10 expression
using the RNA silencing approach in HepG2 tumor cells significantly
suppressed cell proliferation, cell migration and cell invasion
in vitro, and tumor growth in vivo. These results,
combined with the findings from previous studies, suggest that
ADAM10 may be a promising target for HCC anticancer therapy.
Although numerous studies have demonstrated that
ADAM10 is involved in tumor cell proliferation and cell invasion,
the underlying molecular mechanisms remain unclear. Extensive
investigation has revealed that ADAM10 cleaves amyloid precursor
protein (24,25), a protein associated with the growth
of several types of cell (26,27).
ADAM10 has also been reported to activate Notch signaling by
suppressing the ectodomain shedding of Δ-1, which subsequently
results in a marked inhibitory effect on tumor cell proliferation
(28). Endres et al
(13) observed that ADAM10 cleaves
collagen type IV in the basement membrane, a process associated
with tumor metastasis and tumor cell proliferation. Murai et
al (14) revealed that the
cleavage of CD44 catalyzed by ADAM10 contributed to the migration
and invasion of glioblastoma tumor cells. Yang et al
(10) demonstrated that ADAM10
exerts an important role in modulating the chemosensitivity of HCC
cells, which involved the activation of the PI3K/Akt signaling
pathway. In the present study, the results revealed that
downregulation of ADAM10 expression using RNA silencing suppressed
the phosphorylation of Akt and PI3K, which indicates that ADAM10
silencing inhibits tumor cell growth, to a certain extent, by
suppressing the activation of the PI3K/Akt signaling pathway. These
studies imply that different mechanisms may be involved in the
antiproliferative effects of ADAM10 against tumor cells.
In conclusion, in the present study, the results
demonstrated that downregulation of ADAM10 expression using RNA
silencing significantly suppressed the proliferation, cell
migration and cell invasion in vitro, and tumor growth in
vivo. Thus, considering the significance of cell invasion in
metastatic progression, ADAM10 is a potential therapeutic target in
the treatment of HCC.
Acknowledgements
This study was supported by the Jilin Provincial
Science and Technology Research and Innovation Team Fund (grant no.
JL2013011).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Kern MA, Breuhahn K and Schirmacher P:
Molecular pathogenesis of human hepatocellular carcinoma. Adv
Cancer Res. 86:67–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu X, Lu D, Scully M and Kakkar V: ADAM
proteins - therapeutic potential in cancer. Curr Cancer Drug
Targets. 8:720–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seals DF and Courtneidge SA: The ADAMs
family of metalloproteases: multidomain proteins with multiple
functions. Genes Dev. 17:7–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klein T and Bischoff R: Active
metalloproteases of the A Disintegrin and Metalloprotease (ADAM)
family: biological function and structure. J Proteome Res.
10:17–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kohga K, Takehara T, Tatsumi T, et al:
Anticancer chemotherapy inhibits MHC class I-related chain a
ectodomain shedding by downregulating ADAM10 expression in
hepatocellular carcinoma. Cancer Res. 69:8050–8057. 2009.
View Article : Google Scholar
|
|
7
|
Lee SB, Schramme A, Doberstein K, et al:
ADAM10 is upregulated in melanoma metastasis compared with primary
melanoma. J Invest Dermatol. 130:763–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaida MM, Haag N, Günther F, et al:
Expression of A disintegrin and metalloprotease 10 in pancreatic
carcinoma. Int J Mol Med. 26:281–288. 2010.PubMed/NCBI
|
|
9
|
Wang YY, Ye ZY, Li L, et al: ADAM 10 is
associated with gastric cancer progression and prognosis of
patients. J Surg Oncol. 103:116–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang CL, Jiang FQ, Xu F and Jiang GX:
ADAM10 overexpression confers resistance to doxorubicin-induced
apoptosis in hepatocellular carcinoma. Tumour Biol. 33:1535–1541.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko SY, Lin SC, Wong YK, et al: Increase of
disintergin metalloprotease 10 (ADAM10) expression in oral squamous
cell carcinoma. Cancer Lett. 245:33–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gavert N, Conacci-Sorrell M, Gast D, et
al: L1, a novel target of beta-catenin signaling, transforms cells
and is expressed at the invasive front of colon cancers. J Cell
Biol. 168:633–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Endres K and Fahrenholz F: Upregulation of
the alpha-secretase ADAM10 - risk or reason for hope? FEBS J.
277:1585–1596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murai T, Miyazaki Y, Nishinakamura H, et
al: Engagement of CD44 promotes Rac activation and CD44 cleavage
during tumor cell migration. J Biol Chem. 279:4541–4550. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Q, Liu X, Chen W and Zhang Z:
Inhibiting adenoid cystic carcinoma cells growth and metastasis by
blocking the expression of ADAM 10 using RNA interference. J Transl
Med. 8:1362010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brummelkamp TR, Bernards R and Agami R: A
system for stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leng J, Han C, Demetris AJ, Michalopoulos
GK and Wu T: Cyclooxygenase-2 promotes hepatocellular carcinoma
cell growth through Akt activation: Evidence for Akt inhibition in
celecoxib-induced apoptosis. Hepatology. 38:756–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kyula JN, Van Schaeybroeck S, Doherty J,
et al: Chemotherapy-induced activation of ADAM-17: a novel
mechanism of drug resistance in colorectal cancer. Clin Cancer Res.
16:3378–3389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rocks N, Estrella C, Paulissen G, et al:
The metalloproteinase ADAM-12 regulates bronchial epithelial cell
proliferation and apoptosis. Cell Prolif. 41:988–1001. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu K, Liao M, Liu B and Deng Z: ADAM-17
over-expression in gallbladder carcinoma correlates with poor
prognosis of patients. Med Oncol. 28:475–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zubel A, Flechtenmacher C, Edler L and
Alonso A: Expression of ADAM9 in CIN3 lesions and squamous cell
carcinomas of the cervix. Gynecol Oncol. 114:332–336. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai S, Nasser MW, Wang B, et al:
MicroRNA-122 inhibits tumorigenic properties of hepatocellular
carcinoma cells and sensitizes these cells to sorafenib. J Biol
Chem. 284:32015–32027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan S, Lei S and Wu S: ADAM10 is
overexpressed in human hepatocellular carcinoma and contributes to
the proliferation, invasion and migration of HepG2 cells. Oncol
Rep. 30:1715–1722. 2013.
|
|
24
|
Allinson TM, Parkin ET, Turner AJ and
Hooper NM: ADAMs family members as amyloid precursor protein
alpha-secretases. J Neurosci Res. 74:342–352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jorissen E, Prox J, Bernreuther C, et al:
The disintegrin/metalloproteinase ADAM10 is essential for the
establishment of the brain cortex. J Neurosci. 30:4833–4844. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan X, Liu Y, Jiang J, et al: miR-20a
promotes proliferation and invasion by targeting APP in human
ovarian cancer cells. Acta Biochim Biophys Sin (Shanghai).
42:318–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Venkataramani V, Rossner C, Iffland L, et
al: Histone deacetylase inhibitor valproic acid inhibits cancer
cell proliferation via down-regulation of the alzheimer amyloid
precursor protein. J Biol Chem. 285:10678–10689. 2010. View Article : Google Scholar
|
|
28
|
Zhao H, Zhu J, Cui K, et al:
Bioluminescence imaging reveals inhibition of tumor cell
proliferation by Alzheimer’s amyloid beta protein. Cancer Cell Int.
9:152009.PubMed/NCBI
|