Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality worldwide. Non-small cell lung cancer
(NSCLC) accounts for ~85% of all cases of lung cancer (1). Patients diagnosed with NSCLC have low
survival rates and conventional therapies currently available are
rarely beneficial. The optimal chemotherapeutic treatments for
NSCLC are often limited by dose-related toxicity (2). Thus, developing new therapeutic
agents is urgently required for NSCLC patients. Natural products
represent a source of novel antiproliferative agents.
Rhizoma paridis is the root and rhizome of Paris
polyphylla var. yunnanensis, which belongs to the Liliaceae family.
It is widely used in traditional Chinese medicine for its
anti-tumor, antifertility, spermicidal, immunological enhancement
and sedative properties (3–5).
Several studies have demonstrated that extracts from Rhizoma
Paridis possess anti-tumor properties against a variety of
malignant cell lines (6,7). The steroidal Paris saponins are the
active components of Rhizoma paridis. Five Paris saponins have been
identified: Paris saponin I (PSI), also known as polyphyllin D
(8–12), Paris saponin V (PSV), Paris saponin
VI (PSVI), Paris saponin VII (PSVII) and Paris saponin H (PSH). As
the active ingredient, Paris saponin I (PSI) is important in the
treatment of cancer due to its anti-tumor activity, cytotoxic
effects and anti-angiogenic activity (13–16).
It has been demonstrated that hyperthermia can cause
regression of tumors and may be applicable to a wide range of
cancer types (17–20). Hyperthermia is an adjuvant
therapeutic modality to treat cancer by maintaining the temperature
of the tumor region in order to inhibit the regulatory and growth
processes of cancer cells. In addition, hyperthermia causes cancer
cells to become more sensitive to the effects of radiation and
certain anticancer drugs (17–20).
Improvement in clinical outcome involving induced hyperthermia is
suggested to be associated with its ability to inhibit DNA repair,
promote intracellular accumulation of chemical agents, alter
cellular Ca2+ homeostasis, induce cell cycle arrest and
apoptosis, increase membrane permeability and rearrange the
cytoskeleton (21–23). Hyperthermia also has direct
cytotoxic effects, which provide a number of other clinical
advantages, including activation of the immune system against
tumors, improvement of oxygenation and positive effects on drug
delivery (24–26). However, the exact underlying
mechanisms remain to be elucidated.
In our preliminary experiments, it was demonstrated
that PSI significantly inhibited the growth of PC-9 cells in a
dose- and time-dependent manner by causing G2/M arrest, inducing
apoptosis and regulating the B-cell lymphoma 2
(Bcl-2)/Bcl-2-associated X protein (Bax) ratio (27). In the present study, hyperthermia
in tandem with PSI was selected as a combination therapy to
increase the antitumor activity of PSI. In order to further
elucidate the biological effects and mechanisms by which
hyperthermia combined with PSI functions and to improve the effects
of this novel anticancer agent, the role of PSI at different
temperatures in inhibiting NSCLC cell growth was investigated and
cell cycle arrest, apoptosis and the expression of key proteins
were evaluated. This may provide a theoretical and practical basis
for their prospective use in cancer therapy.
Materials and methods
Drugs and reagents
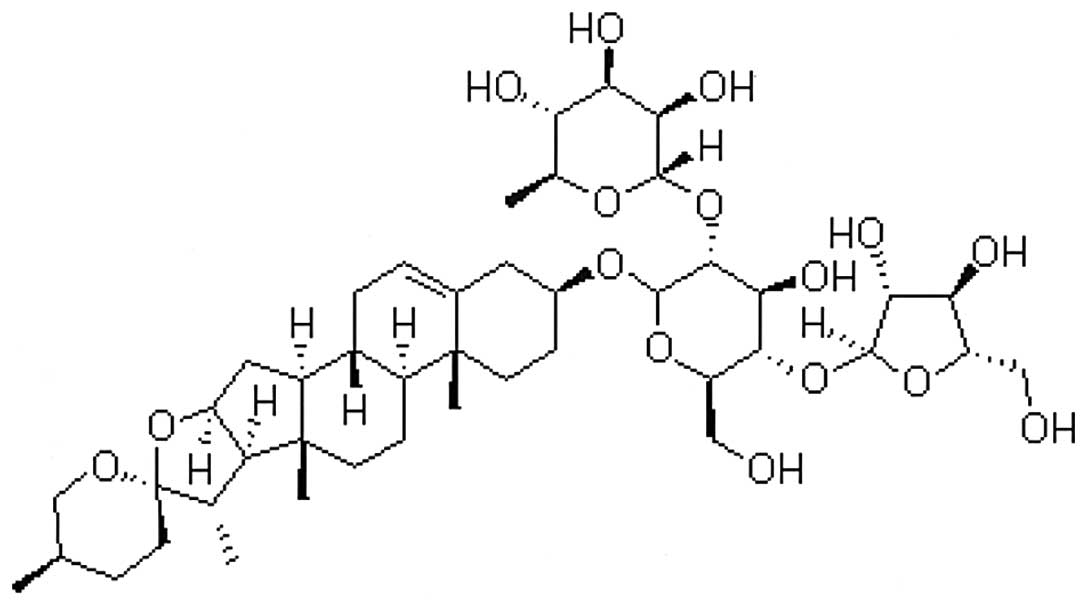
Paris Saponin I
(C44H70O16; Fig. 1) was obtained from the ZheJiang
Institute for Food and Drug Control (Hangzhou, China; cat. no.
111590). It was dissolved in dimethyl sulfoxide (DMSO) as a 100
μg/μl stock solution and stored at −20°C. This was diluted in
Dulbecco’s modified Eagle’s medium (DMEM) to achieve the final
concentration indicated for each experiment. DMEM and fetal calf
serum were obtained from HyClone Laboratories, Inc. (Logan, UT,
USA). A polyclonal rabbit anti-rat Bax antibody used at 1:2,000
dilution and a monoclonal mouse anti-rat Bcl-2 used at 1:2,000
dilution were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA) (17). A polyclonal
rabbit anti-rat caspase 3 antibody, used at 1:25 dilution was
purchased from Abcam (Cambridge, MA, USA). Cycle Test™ Plus DNA
Reagent kit and Annexin V-fluorescein isothiocyanate and propidium
iodide (PI) Apoptosis Detection kits were purchased from BD
Biosciences (Franklin Lakes, NJ, USA) and Hoechst 33258 was
obtained from Beyotime Institute of Biotechnology. (Jiangsu,
China). All other chemical reagents were purchased from Sigma (St.
Louis, MO, USA). The present study was approved by the ethics
committee of Zhejiang Hospital (Hangzhou, Zhejiang, China).
Cell lines and culture
The NSCLC cell line PC-9, was cultured in Dulbecco’s
modified Eagle’s minimum essential medium (Hyclone Laboratories,
Inc.) supplemented with 10% fetal bovine serum (Hyclone
Laboratories, Inc.), in a humidified incubator (Thermo Fischer
Scientific, Inc., Houston, TX, USA) containing 5% CO2 at
37°C.
MTT assay
The MTT assay was performed to detect cell
proliferation following exposure to PSI with hyperthermia.
Following harvesting by trypsinization, the PC-9 cells (100
μl/well) were seeded in 96-well plates at a density of
1×104 cells/ml. Each group had three wells with a
non-treated group as the control. When the cells had anchored to
the plates, various concentrations of PSI (0.5, 1, 2, 3 and 4
μg/ml) were added and the plates were incubated at different
temperatures (37, 39, 41 and 43°C) for 1 h in a humidified
atmosphere containing 5% CO2. After 24 h, 20 μl of 0.5%
MTT was then added to each well and cultured for another 4 h.
Following this, the supernatant was discarded, MTT formazan
precipitates were dissolved in 150 μl DMSO, agitated mechanically
for 10 min and then the absorbance (A) value was measured at 492 nm
using a multiscanner autoreader (Thermo Fisher Scientific, Inc.)
The following formula was used: Inhibition rate (%) = (1-average A
value of the experimental samples) / average A value of the
control) × 100%.
Cell cycle analysis by flow
cytometry
The experimental groups included the control group,
PSI group, hyperthermia group and PSI + hyperthermia group. Cells
were treated with 1.21 μg/ml PSI and incubated at 43°C, then
harvested at 24, 48 and 72 h, fixed with 70% ethanol and stored
overnight at −20°C. The following day, cells were incubated in 10
μg/ml RNase for 30 min at 37°C and then stained in 50 μg/ml
propidium iodide (PI) for 1 h at 4°C in the dark. Cell cycle
analysis was performed on a fluorescence-activated cell sorting
(FACS) Calibur flow cytometer (Becton-Dickinson, Franklin Lakes,
NJ, USA) and the data were analyzed using BD Cell Quest Pro
software, version 5.1 (Becton-Dickinson, Franklin Lakes, NJ, USA).
The experiments were repeated three times.
Apoptosis analysis by flow cytometry
Cell apoptosis was examined by PI/Annexin V double
staining and Hoechst staining. The experimental groups included the
control group, PSI group, hyperthermia group and PSI + hyperthermia
group. Cells were treated with 1.21 μg/ml PSI and incubated at
43°C, harvested at 24 and 48 h and then stained with PI and Annexin
V. The apoptotic fraction was detected by flow cytometry (Beckman
Coulter, Inc., Miami, FL, USA). Cells were washed in
phosphate-buffered saline (PBS), stained with Hoechst 33528 (5
μg/ml in PBS) for 15 min at room temperature and then observed
under an Olympus BX60 fluorescence microscope (Olympus, Tokyo,
Japan) equipped with 356 nm excitation and 492 nm emission bandpass
filters.
Western blotting
The experimental groups included the control group,
PSI group, hyperthermia group and PSI + hyperthermia group. Cells
were treated with 1.21 μg/ml PSI and incubated at 43°C, then
harvested at 48 h. Samples containing equal quantities of proteins
were electrophoresed on 10% SDS-PAGE gel and transferred onto
polyvinylidene difluoride membranes and then incubated with
specific primary antibodies. The blots reacted with horseradish
peroxidase conjugated secondary antibodies and were detected using
the ECL system (Santa Cruz Biotechnology, Inc.). The density of the
band was quantified by densitometry exposed to X-ray film
(Eastman-Kodak, Rochester, NY, USA) using GAPDH as a control.
Statistical analysis
The experiments were repeated three times. The data
are presented as the mean ± standard deviation. Groups were
compared using one-way analysis of variance. P<0.05 or P<0.01
was considered to indicate a statistically significant
difference.
Results
PSI with hyperthermia inhibits
proliferation of PC-9 cells
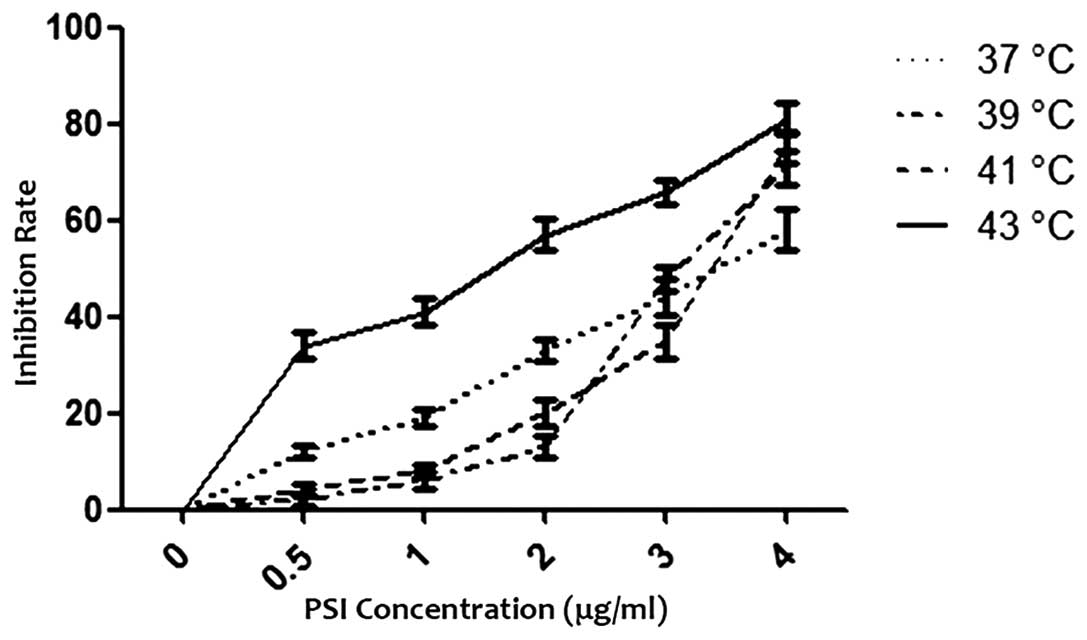
PSI with hyperthermia inhibited the growth of PC-9
cells in a dose-dependent manner with increasing concentrations
between 0.5 and 4 μg/ml at different temperatures for 24 h. The
half maximal inhibitory concentration (IC50) was 2.69 μg/ml (37°C,
control), 3.27 μg/ml (39°C), 3.28 μg/ml (41°C) and 1.21 μg/ml
(43°C), respectively. The effect of PSI with hyperthermia on the
growth of PC-9 cells using an MTT assay is shown in Fig. 2.
PSI with hyperthermia induces G2/M arrest
of PC-9 cells
Flow cytometric analysis revealed the effect of PSI
(IC50=1.21 μg/ml) at 43°C on cell cycle distribution. PSI alone
could induce G2/M arrest in a time-dependent manner. The percentage
of cells at the G2/M phase increased from 21.47 to 29.11% compared
with the control group (P<0.01), however, hyperthermia at 43°C
significantly altered cell cycle distribution of PSI-treated cells
leading to cell cycle arrest at the G2/M phase in a time-dependent
manner. The percentage of cells at the G2/M phase increased from
33.59 to 42.58% compared with the PSI group (P<0.01; Table I).
 | Table IEffect of PSI with hyperthermia on the
G2/M phase of PC-9 cells (%, χ̄±s). |
Table I
Effect of PSI with hyperthermia on the
G2/M phase of PC-9 cells (%, χ̄±s).
| Group | 24 h | 48 h | 72 h |
|---|
| Control | 8.17±1.88 | 11.34±2.46 | 10.22±1.63 |
| PSIa | 21.47±2.75 | 26.71±2.58 | 29.11±2.92 |
| Hyperthermia | 11.33±2.17 | 10.67±1.65 | 14.52±2.18 |
| PSI +
Hyperthermiab | 33.59±2.24 | 38.18±3.73 | 42.58±3.14 |
PSI with hyperthermia induces apoptosis
in PC-9 cells
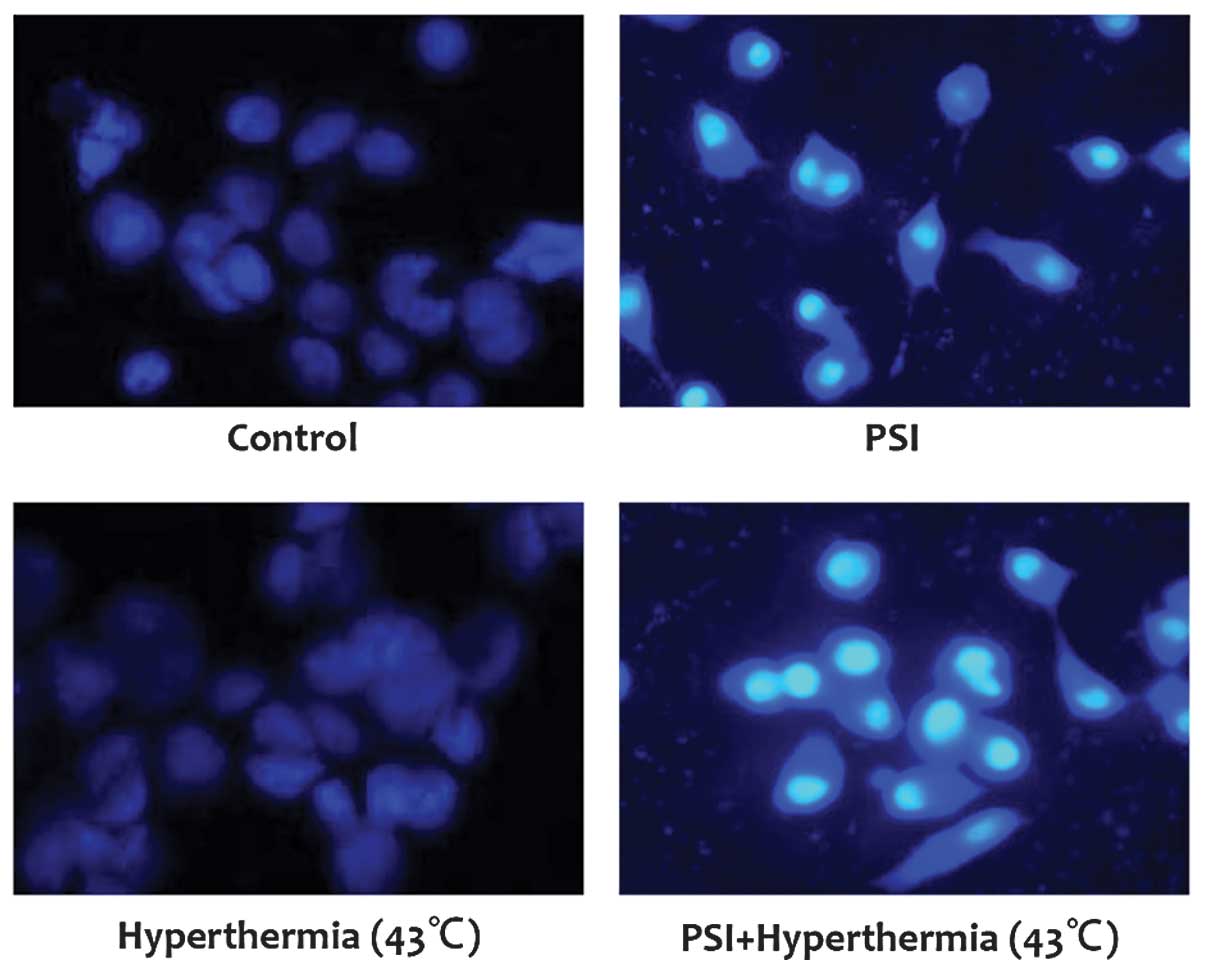
The ability of PSI combined with hyperthermia to
induce apoptosis in PC-9 cells was assessed using the Annexin-V/PI
double staining and Hoechst staining assay. PSI (IC50=1.21 μg/ml)
induced significant levels of apoptosis in PC-9 cells and PSI
(IC50=1.21 μg/ml) with hyperthermia at 43°C increased the apoptotic
ratio at 24 and 48 h (P<0.01; Table II). From the Hoechst staining
assay, it was demonstrated that the cells in the control and
hyperthermia at 43°C groups were morphologically normal and the
nuclei were regularly-shaped with even staining. However, typical
morphological alterations associated with apoptosis were
identified, including nuclear shrinkage, DNA condensation and
chromatin fragmentation in the PSI (IC50=1.21 μg/ml) and PSI
(IC50=1.21 μg/ml) with hyperthermia at 43°C group (Fig. 3). This indicates that hyperthermia
at 43°C can further increase the apoptosis induced by PSI.
 | Table IIEffect of PSI with hyperthermia on the
apoptosis of PC-9 cells (%, χ̄±s). |
Table II
Effect of PSI with hyperthermia on the
apoptosis of PC-9 cells (%, χ̄±s).
| Group | 24 h | 48 h |
|---|
| Control | 2.43±0.67 | 5.47±1.91 |
| PSIa | 18.27±2.45 | 29.17±2.55 |
| Hyperthermia | 8.16±0.97 | 13.05±0.31 |
| PSI +
Hyperthermiab | 28.82±2.46 | 39.63±2.18 |
Effects of PSI with hyperthermia on the
levels of Bcl-2, Bax and caspase-3 in PC-9 cells
In order to examine the potential signaling pathways
by which PSI induces apoptosis and cell cycle arrest, western
blotting was used to evaluate the expression of the Bcl-2 family
and caspase-3 protein. The level of Bcl-2 protein decreased, while
the level of Bax and caspase-3 protein increased following
treatment with PSI (IC50=1.21 μg/ml) for 48 h, which is a
significant increase to PSI (IC50=1.21 μg/ml) with hyperthermia at
43°C (Fig. 4A–C).
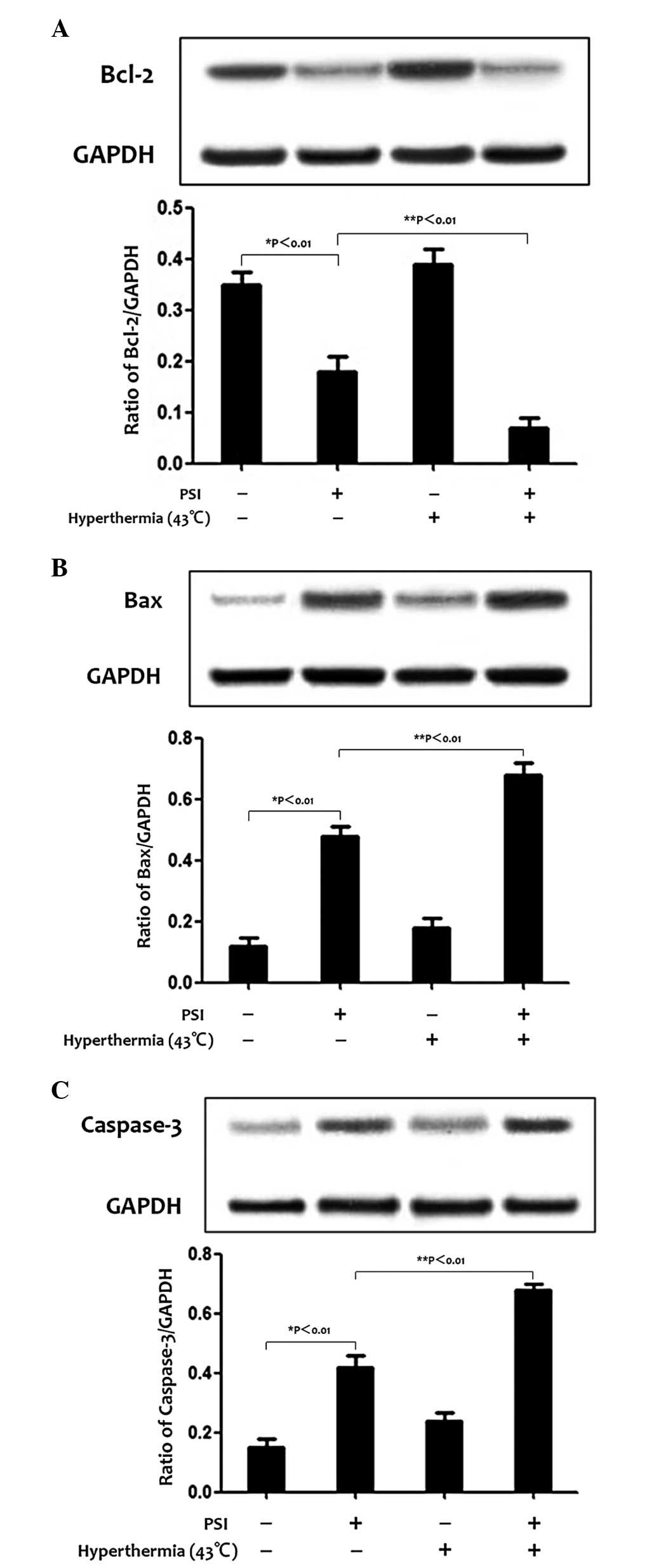 | Figure 4Effect of PSI with hyperthermia on the
levels of Bcl-2, Bax and caspase-3 protein expression in the
control group and treatment groups. Protein levels were detected by
western blotting and the graphs show the percentage of protein
levels in the treatment and control groups. Values are presented as
the means of triplicate analysis. Error bars show the standard
deviations. *P<0.01 and **P<0.01,
significant differences between the treatment group compared with
the control group and between treatment groups, respectively. (A)
PSI decreased the expression of Bcl-2 in PC-9 cells, however, this
decrease was more marked in the PSI with hyperthermia at 43°C
treatment group. (B and C) PSI increased the expression of Bax and
caspase-3, however, this effect was more marked in the PSI with
hyperthermia at 43°C treatment group. PSI, paris saponin 1; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein. |
Discussion
Rhizoma Paridis and its components have been
extensively used in China as antibacterial, antifungal,
antimicrobial, anti-inflammatory and hemostatic regulatory
medications (28). PSI has been
approved for cancer therapy due to its potential involvement in the
suppression of tumor growth (13–16).
The inhibitory effects of PSI were associated with increased levels
of pro-apoptotic Bax, cytochrome c, active caspase-9 and active
caspase-3. PSI also decreased anti-apoptotic Bcl-2 expression
levels and phosphorylated extracellular signal-regulated kinase 1/2
in treated cells (16,29–31).
Hyperthermia can be a highly effective cancer
treatment, particularly when combined with chemotherapy,
radiotherapy or immunotherapy (32–34).
Hyperthermia can inhibit DNA repair, promote intracellular
accumulation of chemical agents, alter cellular Ca2+
homeostasis, induce cell cycle arrest and apoptosis, increase
membrane permeability and rearrange the cytoskeleton (20–25).
Thus, hyperthermia in combination with PSI was selected to increase
the antitumor activity of PSI.
In our previous study, PSI exhibited anti-tumor
effects in PC-9 cells (27).
However, in the present study, four temperatures were selected
combined with PSI on PC-9 cells in order to decide which is the
optimum temperature for PSI to inhibit the growth of NSCLC cells
and elucidate the potential mechanisms.
Current literature reported that hyperthermia
affects various cellular targets, including DNA, proteins,
membranes and the cytoskeleton of carcinoma cells following
exposure to temperatures between 41.5 and 45.5°C (35). There are a number of studies
demonstrating that hyperthermia at 43°C could improve the
inhibitory effect of antitumor agents, including curcumin and
triptonide in Hep-2 cells (36,37).
In the present study it was demonstrated that PSI with hyperthermia
inhibited the growth of PC-9 cells in a dose-dependent manner with
increasing concentrations between 0.5 and 4 μg/ml at different
temperatures, and the IC50 of PSI was 2.69 μg/ml (37°C, control),
3.27 μg/ml (39°C), 3.28 μg/ml (41°C) and 1.21 μg/ml (43°C),
respectively. It was revealed that hyperthermia at 43°C was the
most effective temperature to enable PSI to inhibit the PC-9 cells.
Therefore, hyperthermia at 43°C with PSI (IC50=1.21 μg/ml) was used
to further elucidate the potential underlying mechanisms.
Cell cycle regulation is important for cell
proliferation. In the present study, it was found that PSI altered
the cell cycle distribution of PC-9 cells, leading to cell cycle
arrest at the G2/M phase, but hyperthermia at 43°C altered the cell
cycle distribution of PSI-treated cells further, leading to cell
cycle arrest at the G2/M phase in a time-dependent manner. The
percentage of cells at the G2/M phase increased from 33.59 to
42.58% compared with the PSI group. PSI can also increase apoptosis
in PC-9 cells. PSI can induce significant apoptosis in PC-9 cells
and in combination with hyperthermia at 43°C can increase the
apoptotic ratio further. Caspases are crucial mediators of
apoptosis. Among them, caspase-3 is a frequently activated death
protease, catalyzing the specific cleavage of numerous key cellular
proteins (38). The Bcl-2 family,
which comprise of anti-apoptotic (Bcl-2 and Bcl-xl) and
pro-apoptotic members (Bax and Bak), was the main controller and
mediator of cell apoptosis (39,40).
The high Bcl-2/Bax ratio is considered a crucial factor of cell
resistance to apoptosis (41,42).
In the present study, the results indicated that the level of Bcl-2
protein decreased, while the level of Bax and caspase-3 protein
increased following treatment with PSI and increased further in PSI
with hyperthermia at 43°C. Thus, hyperthermia at 43°C increases the
number of cells arresting at the G2/M phase and promotes apoptosis
induced by PSI through the association between Bcl-2 and Bax and
caspase-3, eventually leading to inhibition of cell
proliferation.
In conclusion, PSI is a potent antitumor agent and
hyperthermia at 43°C can significantly enhance the inhibitory
effect of PSI on PC-9 cells. This occurs through inducing G2/M
arrest and apoptosis via a decrease of Bcl-2 expression and
increase in the protein expression of Bax and caspase-3. This
modality may have significant therapeutic potential in clinical
settings.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (nos. 81303274 and
81202947).
References
|
1
|
Sher T, Dy GK and Adjei AA: Small cell
lung cancer. Mayo Clin Proc. 83:355–367. 2008. View Article : Google Scholar
|
|
2
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group. Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar
|
|
3
|
Wang SL, Zhao YL, Li X, Li Y and Li H:
Study on amyloid and colloidal Paris polyphylla var. yunnanensis.
Acta Botanica Yunnanica. 18:345–348. 1996.
|
|
4
|
Chen CX, Nagasawa J and Zhou H: Two minor
steroidal saponins from the aerial parts of Paris polyphylla var.
yunnanensis. Acta Botanica Yunnanica. 17:215–220. 2001.
|
|
5
|
Matsuda H, Pongpiriyadacha Y, Morikawa T,
Kishi A, Kataoka S and Yoshikawa M: Protective effects of steroid
saponins from Paris polyphylla var. yunnanensis on ethanol- or
indomethacin-induced gastric mucosal lesions in rats: structural
requirement for activity and mode of action. Bioorg Med Chem Lett.
13:1101–1106. 2003. View Article : Google Scholar
|
|
6
|
Jin WD, Chen XP and Cai HJ: In vitro
cytotoxicity of Paridis extract on HepG2 cells. J Huazhong Univ Sci
Tech Med Sci. 35:103–106. 2006.
|
|
7
|
Sun J, Liu BR, Hu WJ, Yu LX and Qian XP:
In vitro anticancer activity of aqueous extracts and ethanol
extracts of fifteen traditional Chinese medicines on human
digestive tumor cell lines. Phytother Res. 21:1102–1104. 2007.
View Article : Google Scholar
|
|
8
|
Lee MS, Yuet-Wa JC, Kong SK, Yu B,
Eng-Choon VO, Nai-Ching HW, Chung-Wai TM and Fung KP: Effects of
polyphyllin D, a steroidal saponin in Paris polyphylla, in growth
inhibition of human breast cancer cells and in xenograft. Cancer
Biol Ther. 4:1248–1254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung JY, Ong RC, Suen YK, Ooi V, Wong
HN, Mak TC, Fung KP, Yu B and Kong SK: Polyphyllin D is a potent
apoptosis inducer in drug-resistant HepG2 cells. Cancer Lett.
217:203–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng S, Yu B, Hui Y, Yu H and Han X:
Synthesis of three diosgenyl saponins: dioscin, polyphyllin D, and
balanitin 7. Carbohydr Res. 317:53–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Yu B, Hui Y, Li M, Han X and Fung
KP: An improved synthesis of the saponin, polyphyllin D. Carbohydr
Res. 331:1–7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siu FM, Ma DL, Cheung YW, Lok CN, Yan K,
Yang Z, Yang M, Xu S, Ko BC, He QY and Che CM: Proteomic and
transcriptomic study on the action of a cytotoxic saponin
(Polyphyllin D): induction of endoplasmic reticulum stress and
mitochondria-mediated apoptotic pathways. Proteomics. 8:3105–3117.
2008. View Article : Google Scholar
|
|
13
|
Chan JY, Koon JC, Liu X, Detmar M, Yu B,
Kong SK and Fung KP: Polyphyllin D, a steroidal saponin from Paris
polyphylla, inhibits endothelial cell functions in vitro and
angiogenesis in zebra fish embryos in vivo. J Ethnopharmacol.
137:64–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rose CYO, Jenny YNC, Henry NCW, Thomas
CWM, Kong SK, Yu B and Fung KP: Saponin Polyphyllin D induced
apoptosis in human hepatocellular carcinoma and multidrug resistant
liver cancer cells. Proc Amer Assoc Cancer Res. 45:29792004.
|
|
15
|
Ma DD, Lu HX, Xu LS and Xiao W:
Polyphyllin D exerts potent anti-tumour effects on Lewis cancer
cells under hypoxic conditions. J Int Med Res. 37:631–640. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao X and Bai P: The antitumoral effect
of Paris Saponin I associated with the induction of apoptosis
through the mitochondrial pathway. Mol Cancer Ther. 8:1179–1188.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Zee J, González González D, van
Rhoon GC, van Dijk JD, van Putten WL and Hart AA: Comparison of
radiotherapy alone with radiotherapy plus hyperthermia in locally
advanced pelvic tumours: a prospective, randomised, multicentre
trial. Dutch Deep Hyperthermia Group. Lancet. 355:1119–1125.
2000.
|
|
18
|
Jones EL, Samulski TV, Dewhirst MW,
Alvarez-Secord A, Berchuck A, Clarke-Pearson D, Havrilesky LJ,
Soper J and Prosnitz LR: A pilot Phase II trial of concurrent
radiotherapy, chemotherapy, and hyperthermia for locally advanced
cervical carcinoma. Cancer. 98:277–282. 2003. View Article : Google Scholar
|
|
19
|
Sakurai H, Kitamoto Y, Saitoh J, Nonaka T,
Ishikawa H, Kiyohara H, Shioya M, Fukushima M, Akimoto T, Hasegawa
M and Nakano T: Attenuation of chronic thermotolerance by KNK437, a
benzylidene lactam compound, enhances thermal radiosensitization in
mild temperature hyperthermia combined with low dose-rate
irradiation. Int J Radiat Biol. 81:711–718. 2005. View Article : Google Scholar
|
|
20
|
Han SI, Duong HQ, Choi JE, Lee TB, Kim CH,
Lee SY, Jeon HM, Shin SH, Lim SC and Kang HS: Hyperthermia switches
glucose depletion-induced necrosis to apoptosis in A549 lung
adenocarcinoma cells. Int J Oncol. 32:851–860. 2008.PubMed/NCBI
|
|
21
|
Coss RA and Linnemans WA: The effects of
hyperthermia on the cytoskeleton: a review. Int J Hyperthermia.
12:173–196. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wachsberger PR and Iliakis G: Hyperthermia
does not affect rejoining of DNA double-strand breaks in a
cell-free assay. Int J Radiat Biol. 76:313–326. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luchetti F, Mannello F, Canonico B,
Battistelli M, Burattini S, Falcieri E and Papa S: Integrin and
cytoskeleton behaviour in human neuroblastoma cells during
hyperthermia-related apoptosis. Apoptosis. 9:635–648. 2004.
View Article : Google Scholar
|
|
24
|
Song CW, Shakil A, Osborn JL and Iwata K:
Tumour oxygenation is increased by hyperthermia at mild
temperatures. Int J Hyperthermia. 12:367–373. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong G, Anyarambhatla G, Petros WP, Braun
RD, Colvin OM, Needham D and Dewhirst MW: Efficacy of liposomes and
hyperthermia in a human tumor xenograft model: importance of
triggered drug release. Cancer Res. 60:6950–6957. 2000.PubMed/NCBI
|
|
26
|
Milani V, Noessner E, Ghose S, Kuppner M,
Ahrens B, Scharner A, Gastpar R and Issels RD: Heat shock protein
70: role in antigen presentation and immune stimulation. Int J
Hyperthermia. 18:563–575. 2002.PubMed/NCBI
|
|
27
|
Jiang H, Su D and Ma SL: The effect of
chonglou saponin I on proliferation and apoptosis in lung
adenocarcinoma cell line PC9. J Chin Oncol. 18:166–169. 2012.
|
|
28
|
Huang Y, Cui LJ, Liu WN and Wang Q:
Quantitative analysis of steroidal saponins in Chinese material
medica Rhizoma Paridis by HPLC-ELSD. Zhongguo Zhong Yao Za Zhi.
31:1230–1233. 2006.(In Chinese).
|
|
29
|
Gu LH, Feng JG, Qian LJ and Ma SL:
Research on proliferation inhibitory effect of Paris Saponin on
high metastatic human ovarian cell line HO-8910PM in vitro. Chin
Arch Trad Chin Med. 30:2212–2215. 2012.
|
|
30
|
Hua YH, Ma SL, Fu ZF, Mou HZ and Jiang H:
Effect of polyphyllin I on radiosensitivity in nasopharyngeal
carcinoma cell line CNE-2 in vitro. Chin Arch Trad Chin Med.
29:1387–1390. 2011.
|
|
31
|
Xiao M, Dai X, He X, Zhou R, Zhang B, Hu
G, Huang Z and Fan X: Paris Saponin I induces G2/M cell cycle
arrest and apoptosis in human gastric carcinoma SGC7901 cells. J
Huazhong Univ Sci Technol. 31:768–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wust P, Hildebrandt B, Sreenivasa G, Rau
B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in
combined treatment of cancer. Lancet Oncol. 3:487–497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mizuuchi H, Yoshiga K, Sakurai K, Tsumura
M and Takada K: Antitumor effect of carboplatin combined with
hyperthermia on Ehrlich-ascites tumor in vivo. Anticancer Res.
16:381–387. 1996.PubMed/NCBI
|
|
34
|
Saga T, Sakahara H, Nakamoto Y, Sato N,
Ishimori T, Mamede M, Kobayashi H, Masunaga S, Sasai K, Kuroki M
and Konishi J: Enhancement of the therapeutic outcome of
radio-immunotherapy by combination with wholebody mild
hyperthermia. Eur J Cancer. 37:1429–1434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Speit G and Schütz P: Hyperthermia-induced
genotoxic effects in human A549 cells. Mutat Res. 747–748:1–5.
2013.PubMed/NCBI
|
|
36
|
Li SW and Liu YM: Curcumin combined with
hyperthermia on Hep-2 cells apoptosis and cell cycle. Clin J Lab
Diagn. 13:1616–1617. 2009.(In Chinese).
|
|
37
|
Feng QJ, Sun LQ, Teng B and Xu YP:
Triptolide combined with hyperthermia on Hep-2 cell proliferation.
J Jilin Univ. 35:862–865. 2009.
|
|
38
|
Porter AG and Jänicke RU: Emerging roles
of caspase 3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shroff EH, Snyder C and Chandel NS: Bcl-2
family members regulate anoxia-induced cell death. Antioxid Redox
Signal. 9:1405–1409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reed JC, Miyashita T, Takayama S, Wang HG,
Sato T, Krajewski S, Aimé-Sempé C, Bodrug S, Kitada S and Hanada
MB: BCL-2 family proteins: regulators of cell death involved in the
pathogenesis of cancer and resistance to therapy. J Cell Biochem.
60:23–32. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sedlak TW, Oltvai ZN, Yang E, Wang K,
Boise LH, Thompson CB and Korsmeyer SJ: Multiple Bcl-2 family
members demonstrate selective dimerizations with Bax. Proc Natl
Acad Sci USA. 92:7834–7838. 1995. View Article : Google Scholar : PubMed/NCBI
|