Introduction
Intracellular calcium accumulation is known to cause
oxidative damage in the lens (1,2).
Aberrant Ca2+ homeostasis is known to be involved in
cataract formation (3),
highlighting the importance of understanding the mechanisms by
which Ca2+ is regulated in the lens. Elevated internal
Ca2+ concentrations in the lens can be induced by a
number of processes, including oxidation of external or internal
sulfhydryl groups (4), removal of
external glucose (5), reduced
external Ca2+ levels (6) and aging itself (7).
Calbindin-D28K (CALB1) is a member of the EF-hand
family of Ca2+-binding proteins. It has a molecular
weight of ~28,000 kDa (8) and was
first isolated from chick intestinal mucosa (9). The function of CALB1 has not yet been
fully established. CALB1 has been shown to be a carrier protein,
which may act as a cytoplasmic Ca2+ buffer or facilitate
transcellular Ca2+ transport (10), thereby preventing intracellular
Ca2+ concentrations from reaching toxic levels (10,11).
More recent studies have indicated that CALB1 may act as an
inhibitor of apoptosis (12) and
as a Ca2+ sensor (13).
CALB1 levels in neurons are known to decrease with age and in
neurodegenerative conditions (14). In the spinal cord, motor neurons do
not express CALB1 and have an increased susceptibility to
Ca2+-induced injury (15). CALB1 is expressed widely within the
central nervous system (16),
including in the retina (17) and
in non-neuronal tissue (18),
including the kidney (19–21), bone (22) and pancreas (23). To date, however, to the best of our
knowledge no data describing the expression or function of CALB1 in
the lens has been reported.
In view of the potential role of CALB1 in
Ca2+ regulation, this study aimed to establish the
distribution and level of expression of CALB1 in the lens of
Sprague-Dawley (SD) rats of varying ages. Median sagittal plane
slices from the hematoxylin and eosin (H&E) stained lenses were
used for quantifying lens epithelial cells. Western blot analysis
was used to quantify CALB1 protein level changes with age, and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) was employed to examine mRNA levels.
Materials and methods
Animals
All experiments were conducted with the approval of
the Animal Ethics Committee of the Zhongshan Ophthalmic Center, Sun
Yat-sen University, (Guangzhou, China). Experiments adhered to the
ethical guidelines produced by the Laboratory Animal Care and Use
Committee of the Association for Research in Vision and
Ophthalmology (Zhongshan Opthalmic Center, Sun Yat-sen University,
Guangzhou, China). SD rats were obtained from the Laboratory Animal
Centre of the Zhongshan Ophthalmic Center and were examined at 1,
6, 12 and 18 months of age. Rats were anesthetized and killed by
intraperitoneal injection of 10% chloral hydrate after which the
eyes were immediately enucleated.
Immunohistochemistry
For immunohistochemistry, rat eyes were placed in 4%
paraformaldehyde in 0.1 M phosphate-buffered saline (PBS)
containing 5% glacial acetic acid and 10% acetone (pH 7.4) for 18 h
at 4°C. They were serially cryoprotected in 10, 20 and 30% sucrose
in 0.1 M PBS for 1–2 days at 4°C, respectively. The eyes were then
embedded in optimum cutting temperature compound. Cryostat sections
(5 μm) were placed in an incubator chamber at 50°C for 2 h, and
then stored at room temperature for subsequent use. Frozen tissue
sections were initially rehydrated in 0.01 M PBS (pH 7.2) and then
incubated in 3% hydrogen peroxide solution to block endogenous
peroxidase for 30 min at room temperature. After three 5-min rinses
with 0.01 M PBS, sections were incubated in 5% normal goat serum
for 30 min at 37°C, and then with rabbit-anti-rat polyclonal
antibody against CALB1 (1:3,000; Merck Millipore, Billerica, MA,
USA) overnight at 4°C. Following three 5-min rinses with 0.01 M PBS
containing 0.1% Tween-20 (PBST), polymer helper (Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) was added to the
slides and the slides were incubated for 30 min at 37°C. After
three 5-min rinses with 0.01 M PBST, a polyclonal horseradish
peroxidase (HRP)-conjugated anti-rabbit IgG antibody (Zhongshan
Golden Bridge Biotechnology Co., Ltd) was added to the slides,
which were then incubated for 30 min at 37°C. After three 5-min
rinses with 0.01 M PBST, diaminobenzidine was added. To label cell
nuclei, H&E staining was conducted. Sections were
cover-slipped, dehydrated in a graded ethanol series and cleared
with dimethylbenzene. Control images were obtained using the same
procedure, with the exception that the sections were not exposed to
the CALB1 antibody.
Cell counts
Lenses were dissected from eyeballs following
immersion fixation for 18 h in a solution of 4% paraformaldehyde in
PBS (pH 7.0–7.4). Lenses were embedded with paraffin following
dehydration with varying concentrations of ethanol (70 -> 75
-> 80 -> 85 -> 90 -> 95 -> 100% × 2)and cleared with
dimethylbenzene. Paraffin sections (5 μm) were made and stained
with H&E. Epithelial cell nuclei were counted in median
sagittal plane slices, taken from the H&E stained lenses of
rats aged 1, 6, 12 or 18 months, under ×400 magnification. A total
of 12 lenses from different animals was analyzed at each age. For
each specimen, two slides from the aequator lentis and lens
subcapsular epithelium were analyzed.
RT-qPCR
After enucleation, the lenses were isolated and
stored in an RNAsafer stabilizer reagent (Omega Scientific,
Tarzana, CA, USA) prior to use. Total RNA was isolated with TRIzol
reagent (Takara Bio, Inc., Shiga, Japan), according to the
manufacturer’s instructions. Contaminated DNA was removed using the
TURBO DNA-freeTM kit (Applied Biosystems, Inc., Foster
City, CA, USA) and cDNA was synthesized from 2 μg total RNA using
the PrimeScriptTM RT Reagent kit (Takara Bio, Inc.) in
20 μl reaction mixture. The cDNA was then used as a template for
PCR amplification. PCR was conducted using the TProfessional
thermocycler (Biometra GmbH, Göttingen, Germany). All primers were
designed online using Integrated DNA Technologies SciTools software
(Integrated DNA Technologies, Inc., Coralville, IA, USA). Primer
sequences are listed in Table I
and were designed according to the cDNA sequences of rat
Calb1 listed in the GenBankTM database
(http://www.ncbi.nlm.nih.gov/genbank/). GAPDH was used
as the internal control for each reaction. All primers were tested
for their specificity by conventional PCR prior to being used for
the RT-qPCR quantitative studies. The following PCR scheme was
used: 5 min at 94°C, 32 cycles of 60 sec at 94°C; 45 sec at 60°C;
60 sec at 72°C, 10 min at 72°C, and then 4°C thereafter. Following
the PCR reaction, the expression levels of the genes were observed
using agarose gel electrophoresis. The change of Calb1
expression was recorded as the fold change of the densitometric
ratio between Calb1 and GAPDH for each lens.
 | Table IReverse transcription-quantitative
polymerase chain reaction primers. |
Table I
Reverse transcription-quantitative
polymerase chain reaction primers.
| Rat gene | Forward primer | Reverse primer |
|---|
| Calb1 (417
bp) |
5′-ACACTGACCACAGTGGCTTC-3′ |
5′-GTTCGGTACAGCTTCCCTCC-3′ |
| GAPDH (321
bp) |
5′-GGACCAGGTTGTCTCCTGTG-3′ |
5′-GGCCCCTCCTGTTGTTATGG-3′ |
Western blot analysis
Individual rat lenses were homogenized in ice-cold
cell lysis buffer (20 mM Tris-HCl buffer, pH 7.5, 150 mM NaCl, 1%
Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate,
1 mM EDTA, 1 mM Na3VO4 and 1 μg/ml leupeptin)
according to the manufacturer’s instructions (Beyotime Institute of
Biotechnology, Haimen, China). Homogenates were centrifuged at
12,000 × g for 5 min at 4°C, and the clear supernatants were stored
at −80°C until use. Protein concentrations were determined using a
bicinchoninic acid kit (Comwin Biotech, Co., Ltd., Beijing, China).
Samples (20 μg protein/well) were loaded and electrophoresed on 12%
sodium dodecyl sulfate-polyacrylamide gels for 60 min at 100 V.
Proteins were then transferred to polyvinylidene fluoride (PVDF)
transfer membranes (Bio-Rad, Hercules, CA, USA) for 80 min at 200
mA. Following transfer, the PVDF membranes were blocked with
blocking solution containing Tris-buffered saline with 0.1%
Tween-20 (TBST) and 5% non-fat milk for 60 min at room temperature.
Membranes were incubated with rabbit-anti-rat polyclonal antibody
against CALB1 (1:3,000: Merck Millipore), overnight at 4°C.
Membranes with GAPDH protein were incubated with rabbit-anti-rat
monoclonal antibody against GAPDH (1:1,000, Abcam, Cambridge, UK).
Blots were then washed three times (5 min each) with TBST. All
membranes were then incubated with secondary antibodies
(HRP-labeled goat-anti-rabbit polyclonal IgG, 1:500; Abcam) for 1 h
at room temperature whilst being shaken. Membranes were rinsed
between incubations. Finally, blots were developed by incubating in
enhanced chemiluminescence reagent (Comwin Biotech, Co., Ltd) and
subsequently exposed to BiomaxTM Light film (Eastman
Kodak, Rochester, NY, USA) for 20–30 min. Signal specificity was
confirmed by blotting in the absence of primary antibody, and bands
were normalized to GAPDH-immunoreactive bands visualized in the
same membrane after stripping. Density measurements for each band
were performed with ImageJ version 1.41o software (National
Institutes of Health, Bethesda, MA, USA). Background samples from
an area near each lane were subtracted from each band to obtain the
mean band density. Densitometric ratios between CALB1 and GAPDH
were calculated to determine the relative levels of CALB1.
Statistical analysis
All data are presented as the mean ± standard error.
Data were evaluated by one-way analysis of variance, and the least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Distribution of CALB1 in the rat
lens
An antibody specific for CALB1 was used to
investigate age-related changes in CALB1 levels in the SD rat lens.
Immunohistochemical staining showed that CALB1 is widely
distributed in the SD rats lens, including in lens epithelial cells
and lens fiber cells in the cortex lentis and nucleus lentis. At
all ages, CALB1 is predominantly expressed in the lens epithelial
cells, although the density of CALB1 labeling appeared lower in
lenses obtained from older rats (Fig.
1).
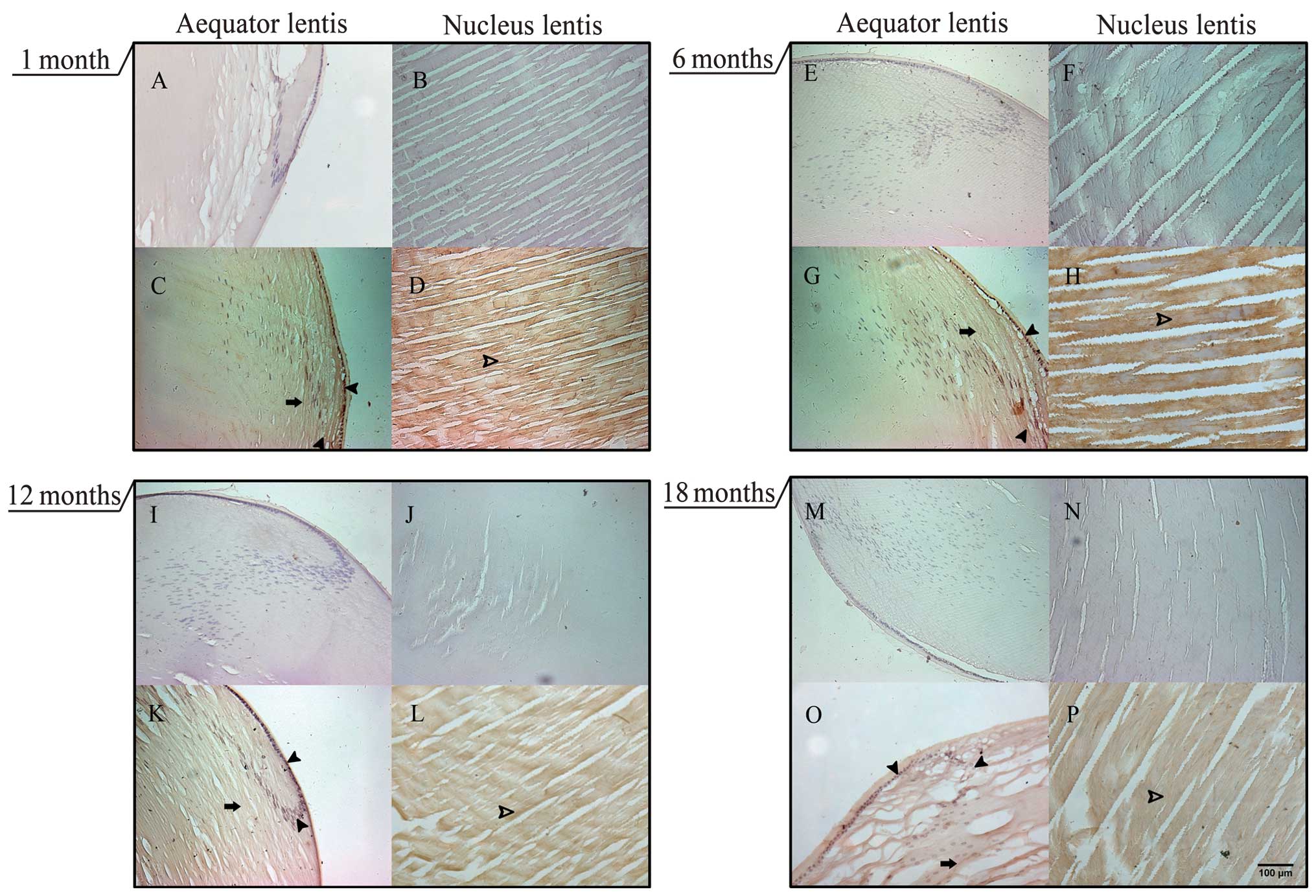 | Figure 1Localization of CALB1 in the aging SD
rat lens. Immunohistochemistry was performed to show CALB1 protein
in sections of lenses obtained from SD rats at the ages indicated.
A–D, 1 month; E–H, 6 months; I–L, 12 months; and M–P, 18 months, by
diaminobenzine staining. At each age, the upper pair of images (A,
B, E, F, I, J, M and N) were obtained from sections prepared
without exposure to the CALB1 primary antibody (control images).
Left and right columns show cross-sections through the aequator
lentis or nucleus lentis, respectively. Arrows, CALB1-positive
resions in the lens cortex; filled arrowheads, CALB1-positive cells
in the lens epithelial cells; open arrowheads, CALB1-positive lens
fiber cells in the lenticular nucleus. CALB1, Calbindin-D28K; SD,
Sprague-Dawley. The scale bar indicates 100 μm. |
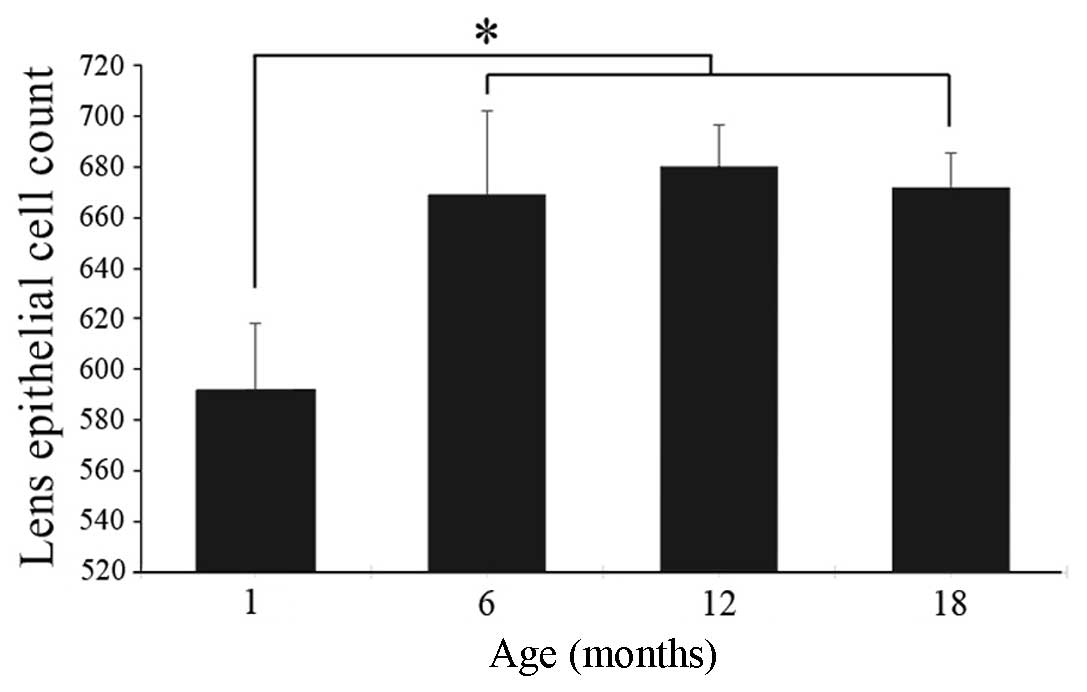
Density of lens epithelial cells
In order to determine whether the age-related
decrease in CALB1 labeling reflected a decrease in the number of
lens epithelial cells, the number of epithelial cells in lenses
obtained from rats aged 1, 6, 12 or 18 months was counted. Cell
numbers were significantly lower in lenses obtained from
1-month-old rats than from older rats. No significant difference
was identified in lens density between the 6-, 12- or 18-month-old
rats (Fig. 2).
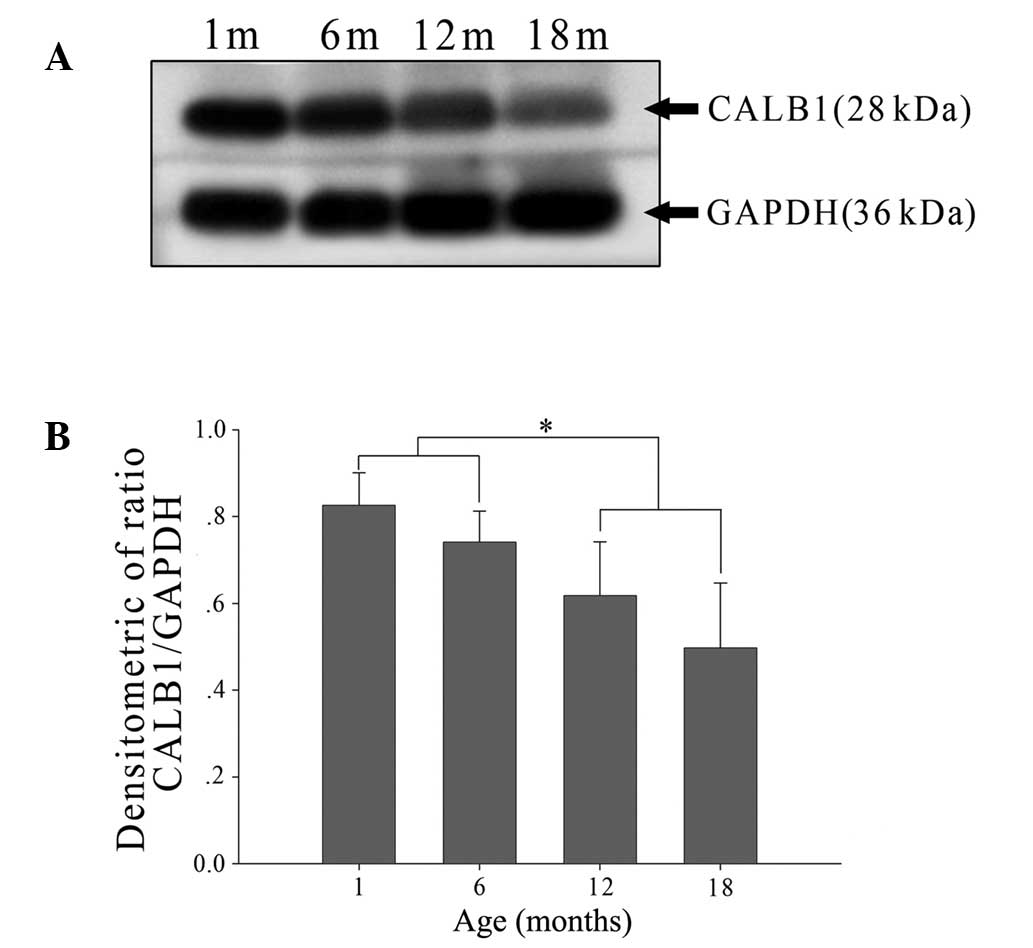
Age-related changes in CALB1 protein and
Calb1 mRNA expression
Western blot analysis was used to define the time
course over which CALB1 levels vary with age. At all ages examined,
CALB1 levels were detectable using the procedures described in the
Materials and methods section (Fig.
3A). When CALB1 levels were analyzed relative to GAPDH levels,
no difference was found between CALB1 levels in lenses from rats
aged 1 or 6 months (Fig. 3B). By
contrast, CALB1 levels were significantly reduced in lenses
obtained from rats aged 12 and 18 months compared with those from
the younger age groups (Fig. 3B).
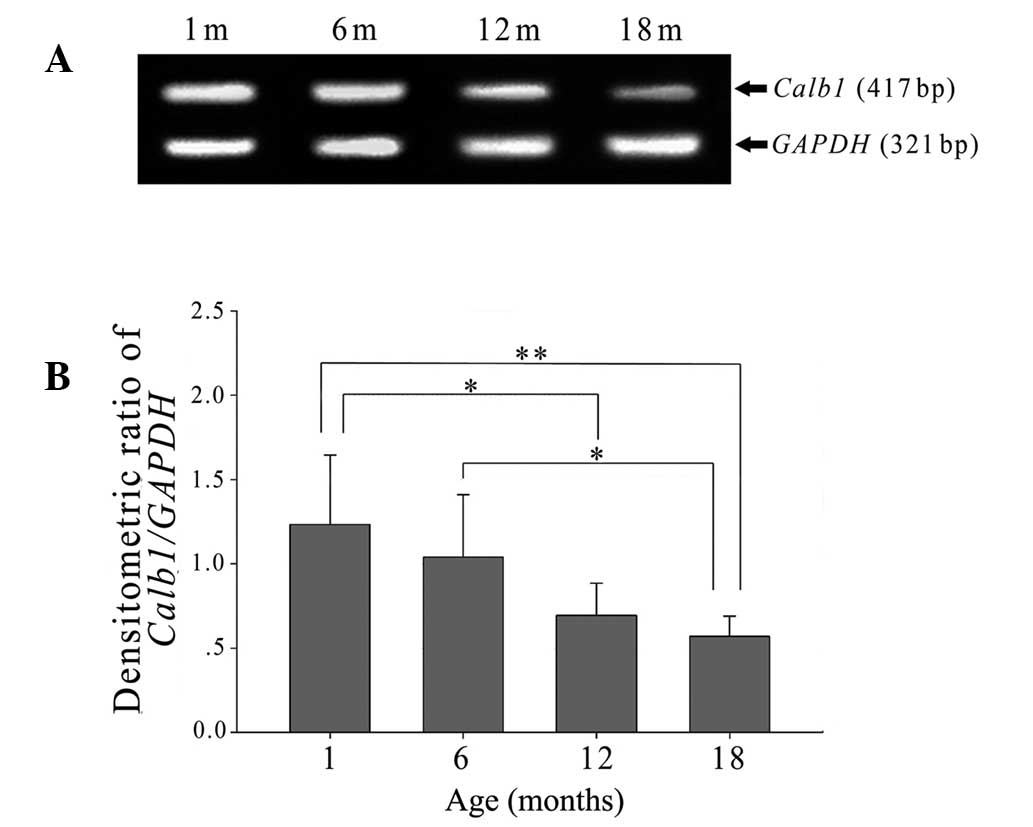
Calb1 mRNA levels were also lower relative to GAPDH in
lenses obtained from older rats than those from younger rats
(Fig. 4A). In parallel with the
protein measures, there was no significant difference between
Calb1 mRNA levels of lenses from 1- and 6-month old rats,
whilst levels observed in lenses from 12- and 18-month-old rats
were significantly reduced in comparison to their younger
counterparts (Fig. 4B).
Discussion
The primary role of the ocular lens is to focus
light onto the retina. To support this role, the lens has evolved
into a highly specialized avascular tissue with a single layer of
epithelial cells on the anterior surface. Cataracts, in which
reduced lens optical homogeneity or transparency are observed
(24), are linked to a number of
factors, including genetics, diabetes, smoking, nutrition,
radiation, ultraviolet exposure and changes in endocrine or
enzymatic equilibria (25–28). In the lens, an increase in internal
calcium can be induced by several processes. These include
oxidation, either of external or internal sulphydryl groups
(4); removal of external glucose
(5); reduction of external calcium
(29); and aging (7). Reactive oxygen species reduce lens
homeostasis and elevate Ca2+ concentration (30,31),
increasing calpain activity (32–35),
which denatures lens proteins and reduces lens transparency
(25,36). Consistent with this model, the
Ca2+ content of cataractous lenses of inherited
cataract/f rats is ~10-fold that of lenses from Wistar rats
(37–39). In the lenses of UPL rats, a
hereditary rat cataract model, decreased ATP levels lead to a
reduction in Ca2+-ATPase function, resulting in the
elevation of lens Ca2+ levels. This process may
contribute to cataract development (40). In human senile cataracts, an ionic
imbalance in the lens with increased levels of Ca2+ has
been suggested to be involved in cataract formation (25,41).
CALB1 is a member of the calcium-binding protein
super family (42). CALB1 has high
affinity for Ca2+. It buffers Ca2+ quickly,
preventing Ca2+-induced impairment of mitochondria and
also preventing the release of cytochrome c (43). The majority of the literature
regarding CALB1 focuses on the nervous system. For example, spinal
motor neurons that lack CALB1 are sensitive to
Ca2+-induced injury (15). When examined across the age range,
neuronal expression of Calb1 declines. It is also known to
be reduced in neurodegenerative disorders. In these conditions,
decreased or absent CALB1 may contribute to free radical stress
altering the normal distribution of Ca2+ (44). However, the correlation between
Ca2+ and CALB1 in the lens has not yet been
reported.
The present study demonstrated that CALB1 is
expressed in the SD rat lens, where it is primarily localized to
the cortex lentis. In addition, it was shown that CALB1 levels
decreased with age in the SD rat lens. This reduction did not
reflect a reduction in the number of lenticular cells. In fact,
although CALB1 levels declined between 1 and 6 months of age, the
number of lens cells increased between these time points. Thus, the
reduction in CALB1 reflected a true decrease in Calb1
expression. There is no earlier information concerning Calb1
expression in the rat lens, although prior studies have focused on
multiple tissues, including the retina (17). We hypothesize that the age-related
reduction in CALB1 may contribute to the observed increases in
Ca2+ levels in lenses obtained from older animals. This
may in turn increase lenticular oxidative damage. However, the
mechanisms underlying CALB1 downregulation remain unclear. In
addition to addressing this issue, evaluation of changes in CALB1
levels and Calb1 expression with age in the human lens is
required. These studies are important in understanding the function
of CALB1 in the lens and its possible role in cataract
development.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81270980) and the
Fundamental Research Funds of State Key Laboratory of Ophthalmology
(grant no. 303060202400444).
References
|
1
|
Xu W, Yao K, Sun ZH, Wang KJ and Shentu
XC: Expression and proteolytic activity of calpain in lens
epithelial cells of oxidative cataract. J Zhejiang Univ Sci.
5:743–748. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Obara Y: The oxidative stress in the
cataract formation. Nihon Ganka Gakkai Zasshi. 99:1303–1341.
1995.(In Japanese).
|
|
3
|
Duncan G and Wormstone IM: Calcium cell
signalling and cataract: role of the endoplasmic reticulum. Eye
(Lond). 13:480–483. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanderson J and Duncan G: pCMPS-induced
changes in lens membrane permeability and transparency. Invest
Ophthalmol Vis Sci. 34:2518–2525. 1993.PubMed/NCBI
|
|
5
|
Duncan G and Jacob TJ: Calcium and the
physiology of cataract. Ciba Found Symp. 106:132–152.
1984.PubMed/NCBI
|
|
6
|
Delamere NA, Paterson CA and Holmes DL:
Hypocalcemic cataract. I An animal model and cation distribution
study. Metab Pediatr Ophthalmol. 5:77–82. 1981.PubMed/NCBI
|
|
7
|
Duncan G, Hightower KR, Gandolfi SA,
Tomlinson J and Maraini G: Human lens membrane cation permeability
increases with age. Invest Ophthalmol Vis Sci. 30:1855–1859.
1989.PubMed/NCBI
|
|
8
|
Christakos S, Gabrielides C and Rhoten WB:
Vitamin D-dependent calcium binding proteins: chemistry,
distribution, functional considerations, and molecular biology.
Endocr Rev. 10:3–26. 1989. View Article : Google Scholar
|
|
9
|
Wasserman RH and Taylor AN: Vitamin
d3-induced calcium-binding protein in chick intestinal mucosa.
Science. 152:791–793. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bronner F and Stein WD: CaBPr facilitates
intracellular diffusion for Ca pumping in distal convoluted tubule.
Am J Physiol. 255:F558–F562. 1988.PubMed/NCBI
|
|
11
|
Johnson JA and Kumar R: Renal and
intestinal calcium transport: roles of vitamin D and vitamin
D-dependent calcium binding proteins. Semin Nephrol. 14:119–128.
1994.PubMed/NCBI
|
|
12
|
Christakos S, Barletta F, Huening M, et
al: Vitamin D target proteins: function and regulation. J Cell
Biochem. 88:238–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt H, Schwaller B and Eilers J:
Calbindin D28k targets myo-inositol monophosphatase in spines and
dendrites of cerebellar Purkinje neurons. Proc Natl Acad Sci USA.
102:5850–5855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iacopino AM and Christakos S: Specific
reduction of calcium-binding protein (28-kilodalton calbindin-D)
gene expression in aging and neurodegenerative diseases. Proc Natl
Acad Sci USA. 87:4078–4082. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waldvogel HJ, Faull RL, Williams MN and
Dragunow M: Differential sensitivity of calbindin and parvalbumin
immunoreactive cells in the striatum to excitotoxins. Brain Res.
546:329–335. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baimbridge KG, Miller JJ and Parkes CO:
Calcium-binding protein distribution in the rat brain. Brain Res.
239:519–525. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ellis JH, Richards DE and Rogers JH:
Calretinin and calbindin in the retina of the developing chick.
Cell Tissue Res. 264:197–208. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baimbridge KG, Celio MR and Rogers JH:
Calcium-binding proteins in the nervous system. Trends Neurosci.
15:303–308. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hermsdorf CL and Bronner F: Vitamin
D-dependent calcium-binding protein from rat kidney. Biochim
Biophys Acta. 379:553–561. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rhoten WB and Christakos S:
Immunocytochemical localization of vitamin D-dependent calcium
binding protein in mammalian nephron. Endocrinology. 109:981–983.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhoten WB, Bruns ME and Christakos S:
Presence and localization of two vitamin D-dependent calcium
binding proteins in kidneys of higher vertebrates. Endocrinology.
117:674–683. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lian JB, Hauschka PV and Gallop PM:
Properties and biosynthesis of a vitamin K-dependent calcium
binding protein in bone. Fed Proc. 37:2615–2620. 1978.PubMed/NCBI
|
|
23
|
Pochet R, Pipeleers DG and Malaisse WJ:
Calbindin D-27 kDa: preferential localization in non-B islet cells
of the rat pancreas. Biol Cell. 61:155–161. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye J and Zadunaisky JA: Study of the
Ca2+/Na+ exchange mechanism in vesicles
isolated from apical membranes of lens epithelium of spiny dogfish
(Squalus acanthias) and bovine eye. Exp Eye Res. 55:243–250.
1992.
|
|
25
|
Dilsiz N, Olcucu A and Atas M:
Determination of calcium, sodium, potassium and magnesium
concentrations in human senile cataractous lenses. Cell Biochem
Funct. 18:259–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cekic O and Bardak Y: Lenticular calcium,
magnesium, and iron levels in diabetic rats and verapamil effect.
Ophthalmic Res. 30:107–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saygili EI, Aksoy SN, Gurler B, Aksoy A,
Erel O and Ozaslan M: Oxidant/antioxidant status of patients with
diabetic and senile cataract. Biotechnol Biotec Eq. 24:1648–1652.
2010. View Article : Google Scholar
|
|
28
|
Wolf N, Penn P, Pendergrass W, et al:
Age-related cataract progression in five mouse models for
anti-oxidant protection or hormonal influence. Exp Eye Res.
81:276–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto K, Fujiwara H, Nishikiori J, et
al: Aging of the human lens and the mechanisms of the senile
cataract formation - about structural lens crystallin. Nihon Ganka
Gakkai Zasshi. 86:1859–1892. 1982.(In Japanese).
|
|
30
|
Williams DL: Oxidation, antioxidants and
cataract formation: a literature review. Vet Ophthalmol. 9:292–298.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hains PG and Truscott RJ: Proteomic
analysis of the oxidation of cysteine residues in human age-related
nuclear cataract lenses. Biochim Biophys Acta. 1784:1959–1964.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borchman D, Delamere NA and Paterson CA:
Ca-ATPase activity in the rabbit and bovine lens. Invest Ophthalmol
Vis Sci. 29:982–987. 1988.PubMed/NCBI
|
|
33
|
Borchman D, Paterson CA and Delamere NA:
Ca2+-ATPase activity in the human lens. Curr Eye Res.
8:1049–1054. 1989.
|
|
34
|
Borchman D, Paterson CA and Delamere NA:
Oxidative inhibition of Ca2+-ATPase in the rabbit lens.
Invest Ophthalmol Vis Sci. 30:1633–1637. 1989.
|
|
35
|
Ahuja RP, Borchman D, Dean WL, et al:
Effect of oxidation on Ca2+-ATPase activity and membrane
lipids in lens epithelial microsomes. Free Radic Biol Med.
27:177–185. 1999.
|
|
36
|
Shearer TR, David LL, Anderson RS and
Azuma M: Review of selenite cataract. Curr Eye Res. 11:357–369.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagai N, Ito Y, Takeuchi N, Usui S and
Hirano K: Comparison of the mechanisms of cataract development
involving differences in Ca2+ regulation in lenses among
three hereditary cataract model rats. Biol Pharm Bull.
31:1990–1995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagai N, Ito Y and Takeuchi N: Inhibitive
effects of enhanced lipid peroxidation on Ca(2+)-ATPase in lenses
of hereditary cataract ICR/f rats. Toxicology. 247:139–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagai N, Ito Y and Takeuchi N: Effect of
disulfiram eye drops on lipid peroxide formation via excessive
nitric oxide in lenses of hereditary cataract ICR/f rats. Biol
Pharm Bull. 31:981–985. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nabekura T, Tomohiro M, Ito Y and Kitagawa
S: Changes in plasma membrane Ca2+ -ATPase expression
and ATP content in lenses of hereditary cataract UPL rats.
Toxicology. 197:177–183. 2004.
|
|
41
|
Tang D, Borchman D, Yappert MC, Vrensen GF
and Rasi V: Influence of age, diabetes, and cataract on calcium,
lipid-calcium, and protein-calcium relationships in human lenses.
Invest Ophthalmol Vis Sci. 44:2059–2066. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gross M and Kumar R: Physiology and
biochemistry of vitamin D-dependent calcium binding proteins. Am J
Physiol. 259:F195–F209. 1990.PubMed/NCBI
|
|
43
|
Kojetin DJ, Venters RA, Kordys DR,
Thompson RJ, Kumar R and Cavanagh J: Structure, binding interface
and hydrophobic transitions of Ca2+-loaded
calbindin-D(28K). Nat Struct Mol Biol. 13:641–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Siklós L, Engelhardt JI, Alexianu ME,
Gurney ME, Siddique T and Appel SH: Intracellular calcium parallels
motoneuron degeneration in SOD-1 mutant mice. J Neuropathol Exp
Neurol. 57:571–587. 1998.PubMed/NCBI
|