Introduction
Autophagy is a regulated intracellular degradation
process in lysosomes for recycling damaged organelles and toxic
proteins to maintain cell homeostasis (1). Under cellular stresses, including
nutrient deprivation, the promotion of autophagy protects cells
from pathways of cell death (2–5).
Autophagy-deficient mice died within 1 day following birth due to a
shortage of nutrients (6).
However, this survival mechanism also assists cancer cells in
overcoming starvation and hypoxia. Several studies have
demonstrated that autophagy is involved in the progression of
cancer (7–9). In the past decade, studies focusing
on autophagy have advanced following the identification of a large
number of autophagy-related genes in yeast, a significant
proportion of which are conserved in humans (10,11).
However, the regulation of autophagy is complex and the molecular
mechanisms in humans, particularly in cancer cells, remain to be
elucidated. The function of autophagy-related 2 (ATG2) is not well
characterized in humans and only one study has demonstrated that
the homologous ATG2A and ATG2B genes are involved in autophagosome
formation in HeLa cells (12). It
has previously been reported that microRNA (miR)-130a is able to
target ATG2B to inhibit autophagic flux and induce cell
death in human chronic lymphocytic leukemia (CLL) (5).
microRNAs (miRNAs) are a group of small, non-coding
RNAs. They are important in diverse biological processes, including
development, differentiation, the cell cycle and apoptosis
(13,14), through post-transcriptional gene
regulation. Previous studies have demonstrated that miRNAs are
involved in the regulation of autophagy (15,16).
At present, ~20 miRNAs have been reported to target ATG-related
genes (15,16), among which include the core
components of autophagy (15).
However, the post-transcriptional regulation of autophagy by miRNAs
remains to be fully elucidated. It is necessary to investigate the
complicated regulatory mechanisms of autophagy by miRNAs, which may
have the potential to be developed in cancer therapy.
Lung cancer is the leading cause of cancer mortality
worldwide and non-small cell lung cancer (NSCLC) accounts for ~80%
of lung cancer. The five-year survival rate of NSCLC is only 15%
and the majority of patients with NSCLC succumb to this disease due
to ineffective therapy and poor diagnostic tools during its early
stages (17). It has been reported
that miR-143 acts as a tumor suppressor by inhibiting cell
proliferation in NSCLC (18).
Materials and methods
Cell culture
All cell lines were cultured at 37°C in a humidified
atmosphere containing 5% CO2. The NSCLC cell lines A549,
H1299, Spca-1, HCC827 and Beas-2B were obtained from the American
Type Culture Collection (Manassas, VA, USA) and cultured according
to the manufacturer’s instructions. H23 and 95-D were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). H23 and 95-D were maintained in RPMI-1640 medium with 10%
fetal bovine serum (FBS). The medium and FBS of all cell lines were
purchased from Invitrogen Life Technologies (Gaithersburg, MD,
USA). Lipofectamine 2000 (Invitrogen Life Technologies) was used
for cell transfection according to the manufacturer’s
instructions.
A549, HCC827, Spca-1, 95-D, HEK293T and Beas-2B
cells were obtained from the Cell Bank of the Chinese Academy of
Sciences. H1299 and H23 cells were purchased from the American Type
Culture Collection. A549, HCC827, H1299, H23 and Spca-1 cell lines
belong to the NSCLC cell line. A549 cells were cultured in F-12K
medium (Gibco-BRL, Gaithersburg, MD, USA). HCC827, H1299 and Spca-1
cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-BRL) with the exception of the A549 cells. H23 and 95-D were
maintained in RPMI-1640 medium. The Beas-2B cell line was isolated
from normal human bronchial epithelium. Beas-2B cells were cultured
in LHC-9 medium, including epidermal growth factor (EGF),
hydrocortisone and adrenaline. All media were supplemented with 10%
FBS, 100 U/ml penicillin and 100 μg/ml streptomycin (Sangon
Biotech, Shanghai, China). Lipofectamine 2000 (Invitrogen Life
Technologies) was used for cell transfection according to the
manufacturer’s instructions.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
Total RNA for RT-qPCR was extracted using TRIzol
(Sangon Biotech, Shanghai, China) according to the manufacturer’s
instructions. For detection of miR-143, reverse transcription was
performed using a One step PrimeScript® miRNA cDNA
Synthesis kit (Takara Bio, Inc., Shiga, Japan). For analysis of
coding gene expression, the cDNA was synthesized by reverse
transcription using a PrimeScript™ 1st Strand cDNA Synthesis kit
(Takara Bio, Inc.). RT-qPCR was performed using SYBR Green Reagents
(Bio-Rad, Hercules, CA, USA) on the iQ5 Real-Time PCR Detection
system (Bio-Rad). Expression of ATG2B and HK2 relative to 18S and
miR-143 relative to U6 was calculated using the 2−ΔΔCt
method. miR-143 was amplified using the forward primer
5′-TGAGATGAAGCACTGTAGCTC-3′ and the reverse Uni-miR RT-qPCR primer
(Takara Bio, Inc.). The ATG2B and HK2 primers for RT-qPCR were used
according to the previous design (5,18).
18S RNA was detected using the following primers: forward
5′-TGAGATGAAGCACTGTAGCTC-3′ and reverse 5′-TAGTAGCGACGGGCGGTGTG-3′.
The RT-qPCR primers for U6 were as follows: forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′.
Western blot analysis
Proteins were extracted from cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and separated using sodium dodecyl
sulfate polyacrylamide gel electrophoresis. Subsequently, proteins
were transferred from the gel onto a polyvinylidene difluoride
membrane Merck Millipore□Billerica, MA, USA. The primary antibodies
used in the assay were rabbit anti-ATG2B (1:1000) and rabbit
anti-GAPDH (1:1000). The secondary antibodies were goat-anti-rabbit
(1:10000). Following incubation with the specific antibody
overnight followed by washing and incubating with a secondary
antibody, protein expression was detected using a Pierce™ ECL
Western Blotting substrate (Thermo Fisher Scientific, Inc.,
Rockford, IL, USA) and images were captured using a ChemiDoc XRS
(Bio-Rad). GAPDH rabbit antibody was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) and ATG2B antibody produced in
rabbit was purchased from Abcam (Cambridge, UK).
Luciferase reporter assay
The 3′ untranslated region (3′UTR) of ATG2B was
amplified using the following primers: forward
5′-CGGAATTCTGGCTTGGAACTGACAGTGT-3′ and reverse
5′-AAACTGCAGCACATTCCTAACAAGCCTGC-3′. The DNA fragment was ligated
into the pGL3 vector (Promega GmbH, Mannheim, Germany) using two
restriction enzymes, EcoRI and PstI. A mutant 3′UTR
of ATG2B was generated using a QuikChange® Site-Directed
Mutagenesis kit (Stratagene, La Jolla, CA, USA). Each reporter
construct was co-transfected into 293T cells in 24-well plates with
the thymidine kinase Renilla luciferase plasmid (pRL-TK) for use in
a Dual-Luciferase reporter assay, together with miR-143 mimic or
negative control RNA (Riobo, Guangzhou, China). The cells were
harvested 48 h after transfection and the luciferase activity was
measured using a Dual-Luciferase reporter assay system (Promega,
Madison, WI, USA) and normalized to the pRL-TK control.
Cell proliferation assay
Cells were seeded into 96-well plates at 2,000
cells/well after 6 h of transfection. The Cell Counting kit-8
(CCK-8; Dojindo Laboratories, Kumamoto, Japan) was used to detect
the relative cell proliferation for 4 days. Prior to detection, 5
μl CCK-8 agent was added to the media for 3 h at 37°C and light
absorbance was then measured at 450 nm. The siRNA used for
knockdown of HK2 and ATG2B was performed as previously described
(5,18).
Statistical analysis
Student’s t-test was performed to compare the data
of two experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Target prediction and functional analysis
of miR-143
Previous studies have demonstrated that miR-143 is
important in various types of cancer via different biological
pathways (19–22). The diverse functions of miR-143
have been observed in various tumor systems, and prompted the
investigation of the complex regulatory mechanisms of miR-143
(18–26,30).
To determine the downstream signaling of miR-143, four miRNA target
prediction programs (miRDB, TargetScan5.2, DIANA-microT3.0 and
miRanda) were used to predict its putative targets. As results were
obtained using two or more independent programs, a total of 28 more
promising targets were selected from the hundreds of putative
targets of miR-143 (Table I).
These genes were further categorized into the various pathways and
biological processes involved in tumorigenesis using the University
of California Santa Cruz Genome browser (genome.ucsc.edu),
including CREBZF in the transforming growth factor-β signaling
pathway, cysteine-rich with EGF-like domain protein 1 and
Kruppel-like factor 5 in the EGF signaling pathway, insulin-like
growth factor-binding protein-5 in the insulin-like growth factor
signaling pathway, cyclin-dependent kinase 1 in the cell cycle and
ATG2B in autophagy regulation.
 | Table IList of potential target genes of
hsa-143. |
Table I
List of potential target genes of
hsa-143.
| Gene name | miRDB | Target Scan5.2 |
DIANA-microT3.0 | miRanda |
|---|
| MAP3K7 | √ | √ | | √ |
| CREB1 | | | √ | √ |
| KLF5 | √ | √ | | √ |
| MAPK7 | √ | √ | √ | √ |
| MYO6 | | √ | √ | √ |
| ABL2 | √ | √ | √ | |
| ETV6 | √ | √ | | √ |
| IGFBP5 | √ | √ | √ | √ |
| SIX4 | √ | √ | | √ |
| WDR7 | √ | | √ | |
| SIRT5 | √ | | | √ |
| ITGA6 | √ | √ | | |
| TUB | √ | √ | √ | √ |
| KLF5 | √ | √ | | √ |
| CDK1 | √ | | | √ |
| TET1 | √ | √ | | √ |
| STAC | √ | √ | | |
| CREBZF | √ | √ | | √ |
| VAPB | √ | √ | √ | |
| PC | √ | √ | | √ |
| ETV6 | √ | √ | | √ |
| ITGB8 | √ | | | √ |
| ATG2B | √ | √ | √ | |
| CRELD1 | √ | √ | √ | |
| ARHGAP26 | | √ | √ | |
| MAF | √ | | | √ |
| SSH2 | | √ | √ | |
| MY09A | | | √ | √ |
ATG2B, a novel target of miR-143
ATG2B, an autophagy related gene, was identified due
to its prediction by three single prediction programs (Table I). The seeding region of miR-143
was located in the 3′UTR of ATG2B (TargetScan5.2), which is
conserved across species, including human, chimpanzee, rhesus,
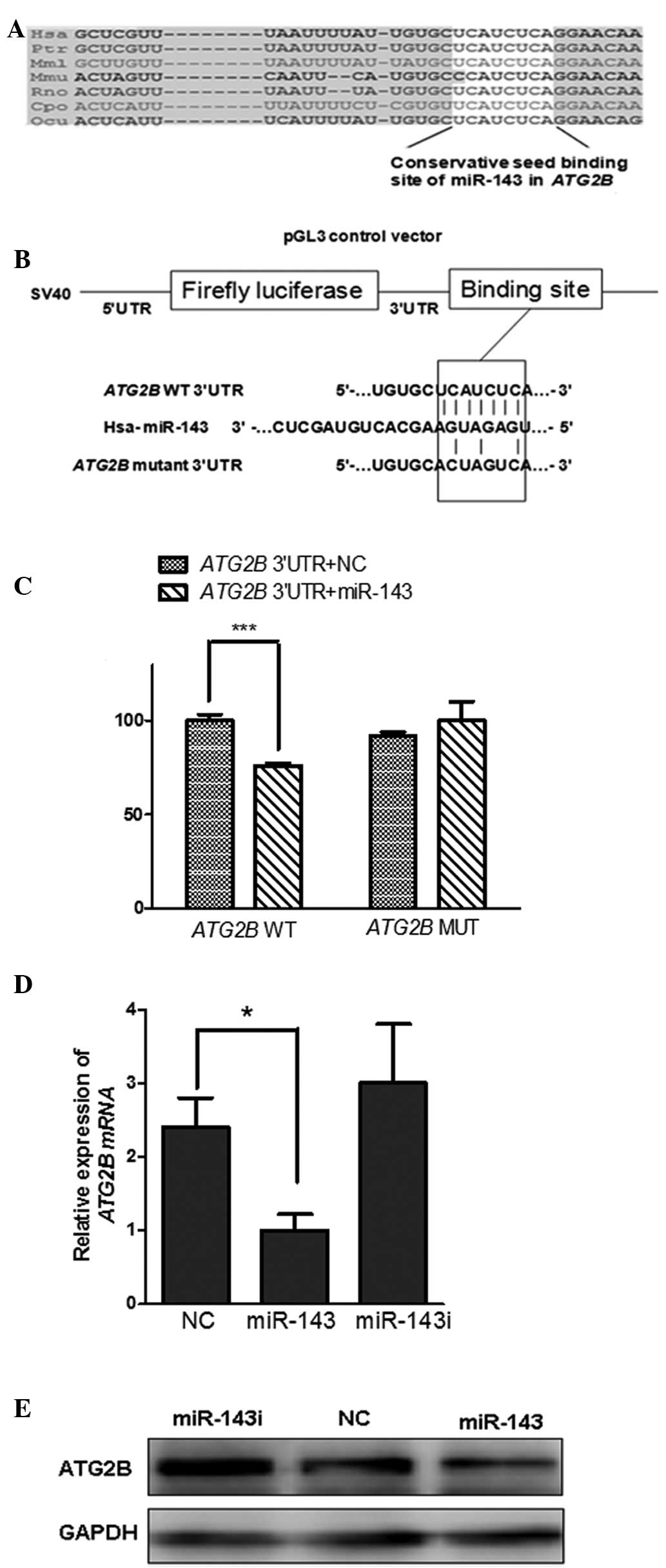
mouse, rat, guinea pig and rabbit (Fig. 1A). To verify whether miR-143
directly regulated ATG2B by binding to its 3′UTR, the full-length
3′UTR of ATG2B was constructed into the downstream of the firefly
luciferase gene in the luciferase reporter vector PGL3
(luci-ATG2B-3′UTR). In addition, mutation of the conservative
seeding region of ATG2B 3′UTR (luci-ATG2B-3′UTRmut) was
constructed as a negative control (Fig. 1B). A luciferase assay was performed
in the 293T cells. When co-transfected with luci-ATG2B-3′UTR, the
miR-143 mimics reduced the activity of luciferase to ~70% compared
with the mimic negative control (Fig.
1C). Notably, this significant inhibition by miR-143 was
eliminated on the conservative seeding region of the mutated
plasmid luci-ATG2B-3′UTRmut, (Fig. 1C). Taken together, these results
demonstrated that miR-143 directly binds to the conservative seed
sequence of ATG2B 3′UTR to negatively regulate the expression of
ATG2B.
Negative regulation of ATG2B by miR-143
in NSCLC
It is well established that autophagy is involved in
the development of cancer (7).
ATG2B, the miR-143 target verified in the present study, is a key
component involved in autophagosome formation (12). Inhibition of ATG2B-related
autophagic flux induces cell death in CLL (5). However, the contribution of autophagy
in NSCLC, particularly in autophagy regulated by miRNA in NSCLC,
remains to be elucidated. In the present study, miR-143/ATG2B was
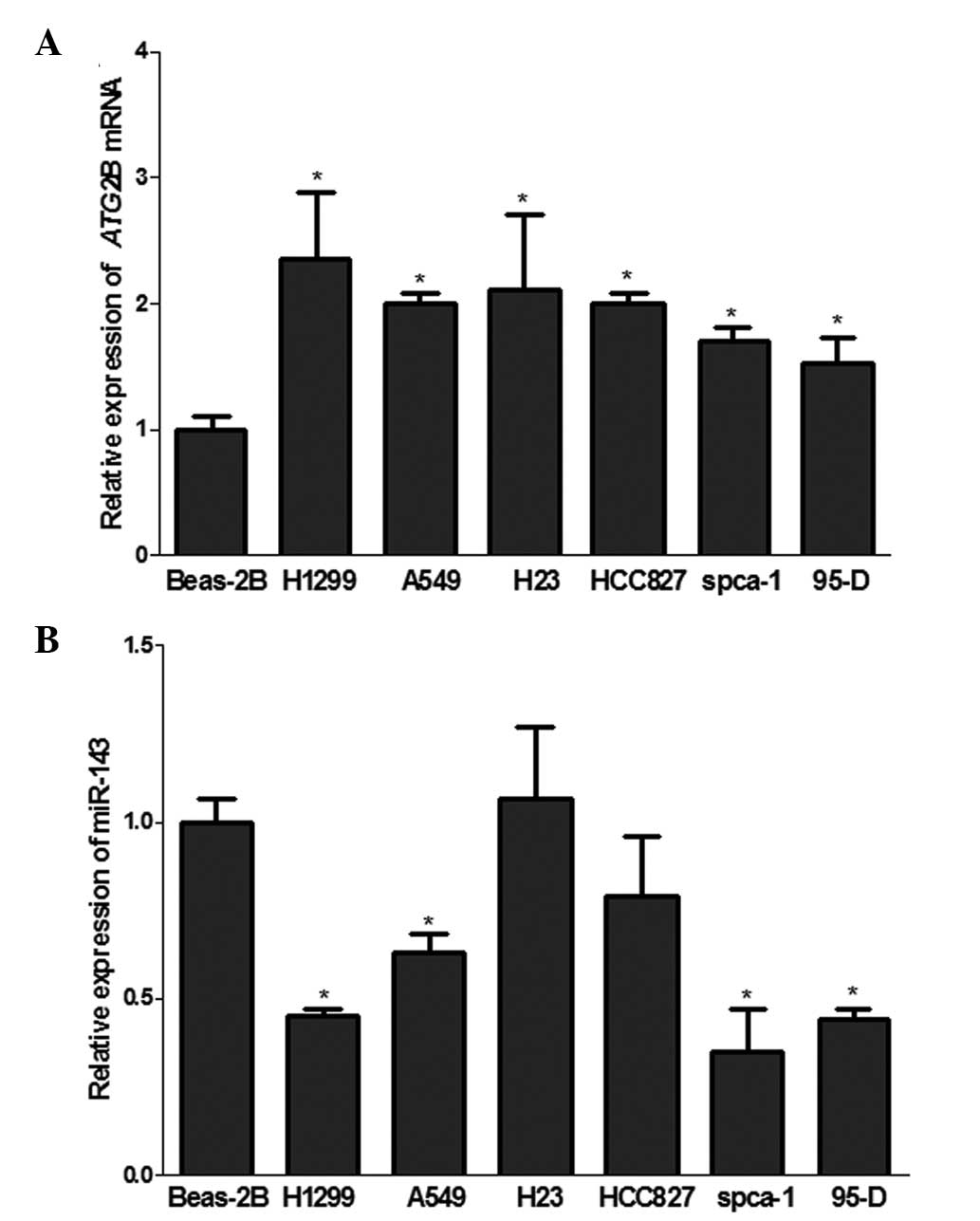
evaluated in NSCLCs. The results from the RT-qPCR of miR-143 in
NSCLC cell lines H1299, A549, spca-1 and 95-D (Fig. 2B) were consistent with previous
studies that miR-143 was downregulated in NSCLC cell lines.
Notably, the ATG2B mRNA was significantly upregulated in all six
NSCLC cell lines compared with the normal lung BEAS-2B cells
(Fig. 2A), which demonstrated
reverse correlation with the expression of miR-143.
To determine whether the upregulation of ATG2B
results from the loss of miR-143 in NSCLCs, the level of ATG2B was
monitored in differing levels of miR-143 expression. The
upregulated expression of ATG2B in H1299 cells was restored by
transfection with miR-143 mimics, while upregulation of ATG2B was
identified in cells transfected with the miR-143 inhibitor
(Fig. 1D and E). These data
indicated that miR-143 negatively regulated ATG2B in the NSCLC
cells and suppression by miR-143 decreased the level of ATG2B mRNA
(Fig. 1D).
Growth inhibition of NSCLC cells by
miR-143 via co-ordinate silencing of ATG2B and HK2
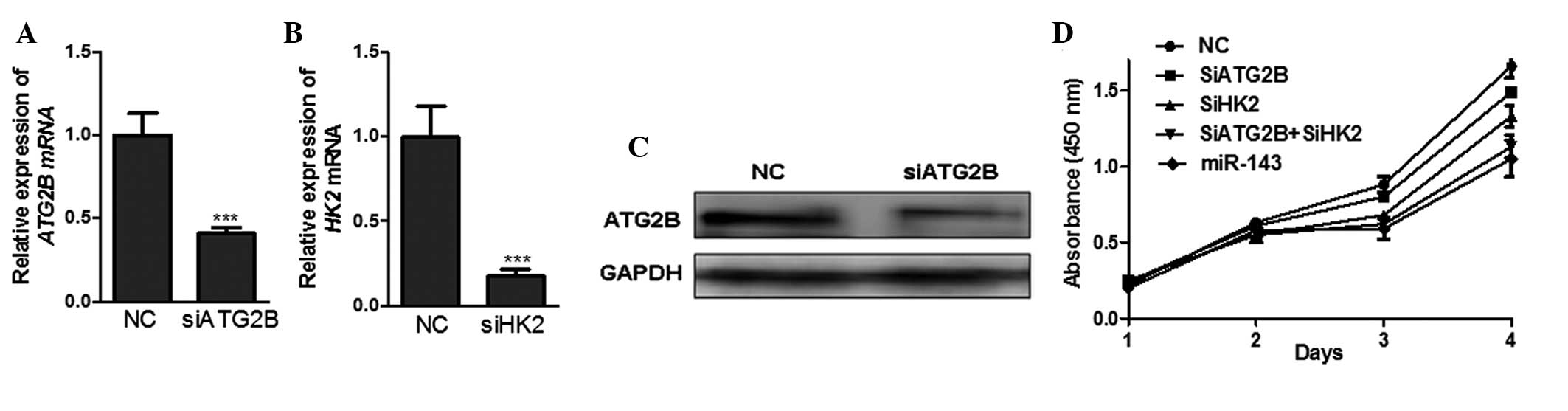
To investigate the contribution of ATG2B, the novel
target of miR-143, to growth inhibition, the expression of ATG2B
was knocked down by siRNA-mediated silencing. (Fig. 3A and C). The results demonstrated
that the proliferation of H1299 cells transfected with siATG2B
decreased by ~10% compared with the cells transfected with the
negative control siRNA (Fig. 3D),
which suggested that miR-143 inhibited NSCLC cell proliferation, at
least partially, via its negative regulation of ATG2B.
A previous study demonstrated that miR-143 targets
HK2, a key enzyme in the high glycolysis (Warburg effect) of a
tumor, to inhibit the proliferation of the NSCLC cell line H1299
(18). The results of the present
study also demonstrated that the mRNA expression of HK2 was
markedly decreased and the cell proliferation was significantly
inhibited in the H1299 cells transfected with HK2 siRNA (siHK2)
compared with those transfected with negative control siRNA
(Fig. 3B). Furthermore, the
inhibition of cell proliferation was more significant in the H1299
cells co-transfected with siATG2B and siHK2 compared with those
transfected with either siATG2B or siHK2 alone, which was similar
to the potent inhibitory effect of miR-143 (Fig. 3D). From these data, it is possible
to conclude that miR-143 targeted ATG2B and HK2 to suppress the
growth of NSCLC cells more efficiently. Interruption of autophagy
and the Warburg effect provides a potentially efficient therapeutic
strategy against NSCLC.
Discussion
Emerging studies have demonstrated the effects of
miR-143 in various types of human cancer (18–26,30).
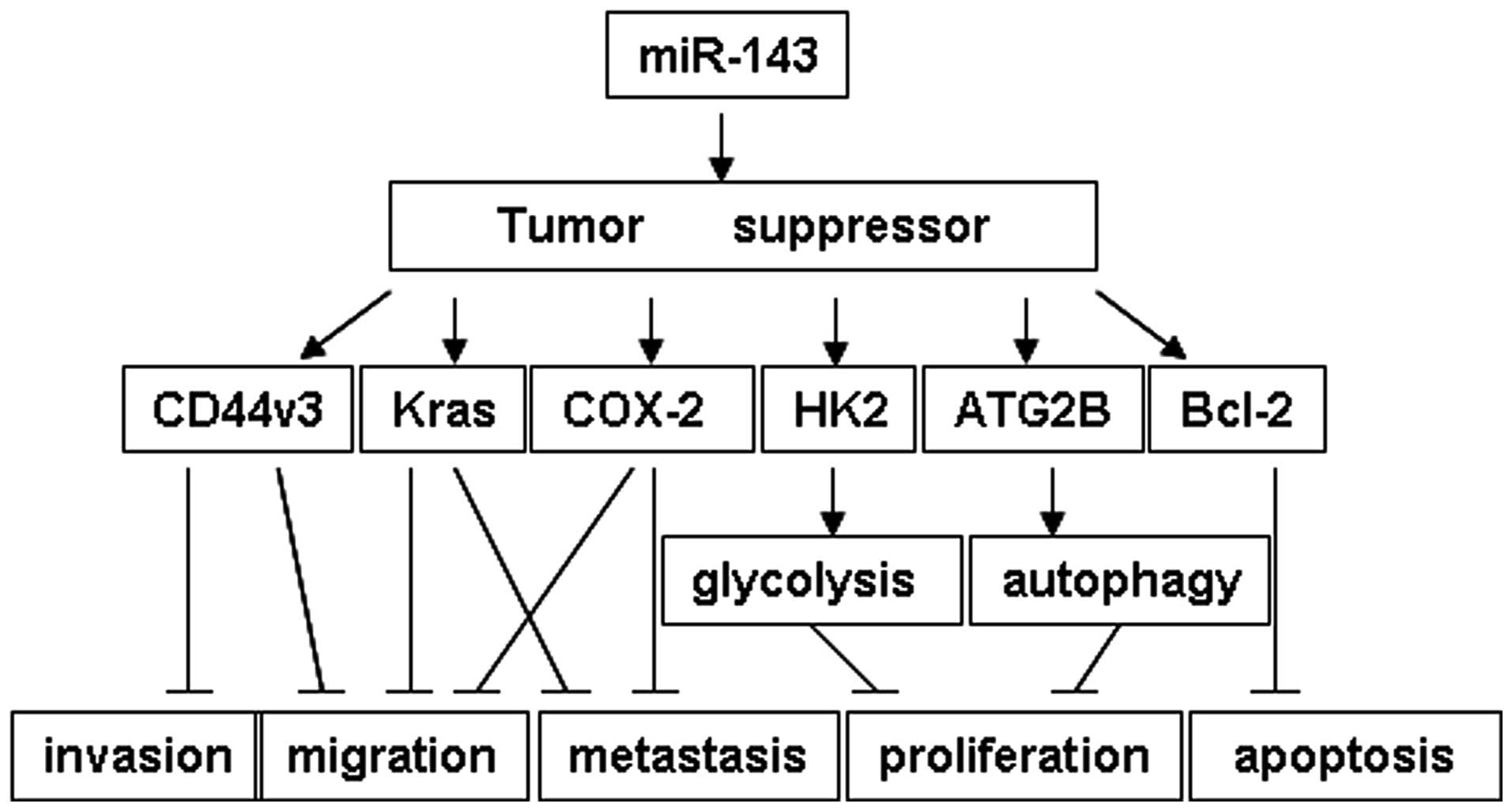
miR-143 functions as a tumor suppressor through targeting different
oncogenes in various pathways (Fig.
4) in pancreatic cancer (21),
bladder cancer (23), colorectal
cancer (20), breast cancer
(24), esophageal squamous cell
carcinoma (25), osteosarcoma
(26) and lung cancer (18,19).
In NSCLC, the loss of miR-143 promotes tumor proliferation,
migration and invasion through targeting the Kras,
CD44v3 and HK2 genes (18,19).
For the first time, to the best of our knowledge, the present study
linked miR-143 to the autophagy pathway. ATG2B, an autophagy
related gene, was demonstrated to be a target of miR-143 (Fig. 4).
There have been several studies suggesting that
autophagy is important in NSCLC. A549 cells present higher
sensitivity to the chemotherapeutic chemical 5-fluorouracil when
the autophagic response is attenuated by knockdown of the
ATG-related gene ATG7 (27). In the mouse model
(atg7−/−), an autophagic defect was demonstrated to
suppress the progression of K-ras-induced lung tumor (8). In addition, miR-130a inhibits the
autophagic flux and induces cell death in human CLL via direct
targeting to ATG2B (5), which
involves the formation of an autophagosome (12). The present study also demonstrated
that miR-143 was able to downregulate the expression of ATG2B and
suppress cell proliferation in H1299 cells. To the best of our
knowledge, this is the first study investigating miR-related
autophagy regulation in NSCLC. It is known that autophagy is not
only important in maintaining cell homeostasis by degrading damaged
organelles and proteotoxic waste, but it also functions in stress
and starvation conditions (2–5). The
upregulation of ATG2B mRNA in lung cancer patients and NSCLC
cell lines indicated that the progression of cancer requires
autophagy for survival more urgently than in normal cells.
The Warburg effect is the process of high
glycolysis, which occurs in the majority of cancer cells,
converting glucose into lactic acid even in the presence of oxygen
(28). This alternative metabolic
process enables tumor cells to obtain more efficient energy for
survival. HK2 is a key enzyme in glycolysis (22,29).
Studies have demonstrated that miR-143 targets HK2 to inhibit the
Warburg effect, resulting in the inhibition of cell proliferation
in colon cancer (22), breast
cancer (24), renal cell cancer
(30) and NSCLC (18). In the present study, the result of
HK2 knockdown by siRNA-mediated silencing in the H1299 cells was
consistent with previous studies (18). Furthermore, the results revealed
that cell survival was reduced more significantly following HK2 and
ATG2B knockdown by RNAi in the H1299 cells. Therefore, it can be
deduced that when cancer cells lack energy, the function of
autophagy becomes essential. The possible cross-talk between HK2
and ATG2B or between the Warburg effect and autophagy requires
investigation in future studies.
Acknowledgements
This study was supported by grants from the National
Basic Research Program of China (no. 2011CBA01105), the National
Natural Science Foundation of China (nos. 31170750 and 31100570),
the Innovation Program of Shanghai Municipal Commission of the
Sciences and Technology (no. 11ZR141220) and from the State Key
Laboratory of Molecular Biology, Institute of Biochemistry and
Molecular Biology, Shanghai Institutes of Life Sciences, Chinese
Academy of Sciences.
References
|
1
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kroemer G, Mariño G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar
|
|
4
|
Altman BJ and Rathmell JC: Metabolic
stress in autophagy and cell death pathways. Cold Spring Harb
Perspect Biol. 4:a0087632012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kovaleva V, Mora R, Park YJ, et al:
miRNA-130a targets ATG2B and DICER1 to inhibit autophagy and
trigger killing of chronic lymphocytic leukemia cells. Cancer Res.
72:1763–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuma A, Hatano M, Matsui M, et al: The
role of autophagy during the early neonatal starvation period.
Nature. 432:1032–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: a double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo JY, Karsli-Uzunbas G, Mathew R, et al:
Autophagy suppresses progression of K-ras-induced lung tumors to
oncocytomas and maintains lipid homeostasis. Genes Dev.
27:1447–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie Z and Klionsky DJ: Autophagosome
formation: core machinery and adaptations. Nat Cell Biol.
9:1102–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Z and Klionsky DJ: Mammalian
autophagy: core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Velikkakath AK, Nishimura T, Oita E,
Ishihara N and Mizushima N: Mammalian Atg2 proteins are essential
for autophagosome formation and important for regulation of size
and distribution of lipid droplets. Mol Biol Cell. 23:896–909.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flynt AS and Lai EC: Biological principles
of microRNA-mediated regulation: shared themes amid diversity. Nat
Rev Genet. 9:831–842. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ventura A and Jacks T: MicroRNAs and
cancer: short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frankel LB and Lund AH: MicroRNA
regulation of autophagy. Carcinogenesis. 33:2018–2025. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhai H, Fesler A and Ju J: MicroRNA: a
third dimension in autophagy. Cell Cycle. 12:246–250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang R, Xiao T, Fang Z, et al:
MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting
hexokinase 2 gene. J Biol Chem. 287:23227–23235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Q, Jiang Q, Pu Q, et al: MicroRNA-143
inhibits migration and invasion of human non-small-cell lung cancer
and its relative mechanism. Int J Biol Sci. 9:680–692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qian X, Yu J, Yin Y, et al: MicroRNA-143
inhibits tumor growth and angiogenesis and sensitizes
chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle.
12:1385–1394. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Y, Ou Y, Wu K, Chen Y and Sun W:
miR-143 inhibits the metastasis of pancreatic cancer and an
associated signaling pathway. Tumor Biology. 33:1863–1870. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gregersen LH, Jacobsen A, Frankel LB, Wen
J, Krogh A and Lund AH: MicroRNA-143 down-regulates Hexokinase 2 in
colon cancer cells. BMC Cancer. 12:2322012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song T, Zhang X, Wang C, et al: Expression
of miR-143 reduces growth and migration of human bladder carcinoma
cells by targeting cyclooxygenase-2. Asian Pac J Cancer Prev.
12:929–933. 2011.PubMed/NCBI
|
|
24
|
Jiang S, Zhang LF, Zhang HW, et al: A
novel miR-155/miR-143 cascade controls glycolysis by regulating
hexokinase 2 in breast cancer cells. EMBO J. 31:1985–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ni Y, Meng L, Wang L, et al: MicroRNA-143
functions as a tumor suppressor in human esophageal squamous cell
carcinoma. Gene. 517:197–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Cai X, Wang Y, Tang H, Tong D and
Ji F: microRNA-143, down-regulated in osteosarcoma, promotes
apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol
Rep. 24:1363–1369. 2010.PubMed/NCBI
|
|
27
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8:e566792013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the
‘Warburg effect’ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009.
|
|
30
|
Yoshino H, Enokida H, Itesako T, et al:
Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in
renal cell carcinoma. Cancer Sci. 104:1567–1574. 2013. View Article : Google Scholar : PubMed/NCBI
|