Introduction
Ischemia/reperfusion (I/R) injury of the intestine
is a common type of intraoperative tissue and organ damage that is
secondary to vascular disease of the gut and its mesentery, as well
as to a variety of clinical pathophysiological processes of the
disease, including acute blood loss, shock, severe trauma, small
bowel transplantation and sepsis, among others (1,2). I/R
injury is characterized by a high mortality rate of up to 60–80%
(3) and causes gut barrier
disruption, oxygen free radical generation and endotoxin entry into
the circulation, thereby leading to severe systemic inflammation
and eventual multiple organ failure of the lungs, liver and kidneys
(4–7). In fact, acute lung injury (ALI) is a
common complication following intestinal I/R and tends to develop
as acute respiratory distress syndrome, which is a major
contributor to its high mortality rate (8–11).
Specific mechanisms underlying ALI, however, are
highly complex and involve the production of reactive oxygen
species, the activation of cytokines and inflammatory mediators,
and the initiation of cell apoptosis (12,13).
A previous study reported that the antioxidant manganese superoxide
dismutase (MnSOD) and the apoptosis-associated molecule B-cell
lymphoma 2 (Bcl-2) have important roles in the pathogenesis of
I/R-induced ALI in the small intestine, in which the above
molecules are mediated by the regulatory factor forkhead box
protein O3 (FOXO3) (14).
Furthermore, the sirtuin 1 (SIRT1)/FOXO3 signaling pathway is
indispensable to the antioxidative and anti-apoptotic properties of
I/R disease (15).
SIRT1, a nicotinamide adenine dinucleotide
(NAD)-dependent class III histone deacetylase (HDAC), is a
mammalian SIR2 homologue in the yeast Saccharomyces
cerevisiae. Among the seven sirtuin genes (SIRT1-7) in mammals,
SIRT1 is the most widely studied and was found to be mostly
localized to the nucleus, where it has a vital role in
transcriptional repression via histone deacetylation (16–18).
Recent studies have identified that SIRT1 is important in DNA
damage repair, apoptosis inhibition, oxidative stress resistance
and cell lifespan extension (19).
According to a study by Hsu et al (20), SIRT1 expression in myocardial I/R
injury may confer a protective effect. For example, SIRT1 regulates
a diverse set of proteins, including p53 and the transcription
factors nuclear factor-kappa B (NF-κB) and FOXO, and promotes cell
survival in response to severe stress (21–24).
Under ischemic or hypoxic conditions, FOXO3 may enhance cellular
resistance to oxidative stress through SIRT1 deacetylation in a
process that is mainly completed by MnSOD, which is able to
scavenge reactive oxygen species (ROS) (21,25).
In addition, a study by Motta et al (24) demonstrated that mammalian SIRT1
deacetylated FOXO3 and attenuated its pro-apoptotic abilities.
Furthermore, Brunet et al (21) revealed that SIRT1 deacetylation
increased the ability of FOXO3 to induce cell cycle arrest and
oxidative stress resistance; however, this inhibited the ability of
FOXO3 to induce cell death. Therefore, to the best of our
knowledge, the present study assessed, for the first time, whether
SIRT1 may possess protective effects on intestinal I/R-induced ALI
by regulating the FOXO3 signaling pathway.
A variety of natural products are involved in SIRT1
regulation, including polyphenols and flavonoids, among others.
Resveratrol is a representative polyphenolic compound that is known
to have a role in SIRT1 regulation. Icariin (ICA) is a flavonoid
extracted from Epimedium, a genus of flowering plants in the
family Berberidaceae, which is used in Traditional Chinese
Herbal Medicine as an aphrodisiac. A recent study by Wang et
al (26) found that ICA
upregulated SIRT1 expression, which resulted in the enhancement of
neuronal viability and the suppression of neuronal death in
response to oxygen and glucose deprivation. Furthermore, regulation
of the SIRT1 signaling pathway by ICA has been demonstrated to
ameliorate oxidative stress in the treatment of central nervous
system disorders (27,28). However, to the best of our
knowledge, no studies have reported ICA regulation of the
SIRT1/FOXO3 signaling pathway in intestinal I/R injury.
Therefore, the aims of the present study were i) to
determine whether ICA-mediated SIRT1/FOXO3 activation had a
protective effect on intestinal I/R-induced ALI by inhibition of
alveolar cell apoptosis and increased antioxidant enzyme activity
and ii) to determine whether ICA was able to increase SIRT1 levels
to reduce the content of acetylated FOXO3 and regulate the
antioxidant MnSOD and apoptosis-associated factors, Bcl-2 and Bcl-2
interacting mediator of cell death (Bim).
Materials and methods
Animals and reagents
Male Sprague-Dawley rats (aged 5 weeks) weighing
160–180 g were obtained from the Animal Center of Dalian Medical
University (Dalian, China) and maintained in a
temperature-controlled (25°C) facility with a 12-h light/12-h dark
cycle with free access to a standard laboratory diet and water. The
rats were acclimatized for one week prior to the experiments. All
procedures were conducted according to the Institutional Animal
Care Guidelines and were approved by the Institutional Ethics
Committee of Dalian Medical College (Dalian, China). ICA (>98%
of purity) was purchased from Shanghai Winherb Medical S&T
Development Co., Ltd. (Shanghai, China).
Intestinal I/R model and experimental
design
The rat intestinal I/R model was established as
previously described with several small modifications (29). Briefly, the superior mesenteric
artery (SMA) was identified through a midline laparotomy and
occluded gently by an atraumatic microvascular clamp for 60 min.
The occlusion was confirmed when the mesenteric pulsations ceased
and the intestines became pale. A 120-min reperfusion was then
performed. In the control groups, the rats underwent surgical
preparation, including isolation of the SMA but without occlusion.
All of the animals were randomly divided into five groups, with
eight rats in each of the following: i) Control group; ii)
intestinal I/R group; iii) control+ICA group, where the mice were
pretreated with ICA (60 mg/kg by intragastric administration for
three consecutive days); iv) I/R+ICA group, where the mice were
pretreated with ICA as above and then surgery was performed in
analogy to that in the I/R group and v) I/R+ischemia
preconditioning (IPC) group, where the rats were pretreated with
reperfusion for 5 min following occlusion of the SMA for 5 min,
which was repeated three times and then surgery was performed in
analogy to that in the I/R group. All of the animals were
sacrificed at the end of the reperfusion, and the tissues and blood
samples were obtained for analysis.
Assessment of lung histopathology
The isolated right middle lobe of the lung was fixed
in 4% formaldehyde. After being embedded in paraffin, the tissues
were sectioned and stained with hematoxylin and eosin (H&E).
The specimens were examined under light microscopy (Leica DM4000,
Wetzlar, Germany). The pathological scores of lung damage were
examined according to the previously described methods (30).
Measurement of serum cytokine levels
Blood samples were collected from the abdominal
aorta and allowed to coagulate for 2 h on ice. The serum was then
isolated as the supernatant fraction following centrifugation at
3,000 × g for 20 min. Tumor necrosis factor-α (TNF-α) and
interleukin 6 (IL-6) levels were measured with ELISA kits (Wuhan
Boster Biological Technology, Ltd., Wuhan, China), according to the
manufacturer’s instructions.
Lung malondialdehyde assay (MDA),
glutathione (GSH) and GSH peroxidase (GSH-PX) activity assay
The lung tissues were weighed, minced and
transferred into a centrifuge tube. The 1:9 (w/v) volume of cold
isotonic sodium chloride solution was added and the mixtures were
homogenized by a tissue disperser (IKA, Staufen, Germany) four
times at 4°C, each for 10 sec. The homogenates were centrifuged at
3,000 × g for 15 min to remove the debris. The MDA, GSH and GSH-PX
activity in the supernatants were determined using an assay kit
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China),
according to the manufacturer’s instructions.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
The TUNEL assays were performed with the TMR red In
Situ Cell Death Detection kit (supplemented with 0.1% sodium
citrate; Hoffmann-La Roche Inc., Nutley, NJ, USA), according to the
manufacturer’s instructions. The lung samples were treated as
mentioned in the histopathological analysis. Briefly, the lung
tissue sections were pretreated with 0.1% Triton X-100 for 8 min,
washed with PBS, then stained by TUNEL reaction mixture (label and
enzyme solutions) for 1 h at 37°C. The fluorescein
isothiocyanate-labeled TUNEL-positive cells were imaged under a
fluorescent microscope (DMI 4000 B; Leica, Wetzlar, Germany) using
488 nm excitation and 530 nm emission. The cells exhibiting green
fluorescence were defined as apoptotic cells.
Western blot analysis of SIRT1, FOXO3,
MnSOD, Bim and Bcl-2 in lung tissue
Nuclear and cytosolic proteins were extracted from
the lung tissues with a protein extraction kit (Nanjing KeyGen
Biotech. Co. Ltd., Nanjing, China) according to the manufacturer’s
instructions. The protein concentrations were determined using an
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Jiangsu, China). Equal amounts of nuclear and
cytosolic protein were used to determine SIRT1, FOXO3, MnSOD, Bim
and Bcl-2 protein concentrations. The sample proteins (50 μg/lane)
were separated by 12% SDS-PAGE (Bio-Rad, Hercules, CA, USA) and
then transferred to a polyvinylidene difluoride membrane
(Millipore, Bedford, MA, USA). Following blocking, the membranes
were probed with the following primary antibodies: Anti-SIRT1
(mouse, mAb, 1:4000, Abcam Ltd., Cambridge, UK), anti-FOXO3
(rabbit, pAb, 1:2000, Abcam Ltd.), anti-MnSOD (mouse, mAb, 1:1000,
Abcam Ltd.), anti-Bcl-2 (rabbit, pAb, 1:1000, Beyotime Institute of
Biotechnology) and anti-β-actin (mouse, mAb, 1:1000, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C.
Following washing in tris-buffered saline containing Tween 20
(TTBS) three times, the membranes were incubated with the relevant
horseradish peroxidase-conjugated secondary antibodies for 2 h at
37°C. Following adequate washing with TTBS, the membranes were
exposed to chemiluminescence-plus reagents. The blots were then
developed with a BioSpectrum-410 multispectral imaging system with
a Chemi HR camera 410 and the density of the bands was determined
using Gel-Pro Analyzer Version 4.0 (Media Cybernetics, Bethesda,
MD, USA).
Immunoprecipitation assay
The protein samples were extracted from the lung
tissues, mixed with non-specific rabbit immunoglobulin G and the
fully resuspended Protein A+G Agarose (Beyotime Institute of
Biotechnology) and slowly shaken at 4°C for 2 h, 1,000 × g for 5
min and the supernatant was used for subsequent
immunoprecipitation. Rabbit anti-human polyclonal acetylated lysine
antibody (Abcam Ltd.) was added at 4°C, the mixture was slowly
agitated for 18 h and then fully resuspended in Protein A+G Agarose
with 4°C with gentle agitation for 2 h, 1,000 × g for 5 min, and
then the precipitation of all of the acetylated protein was
performed. Finally, acetylated FOXO3 proteins were detected using
western blot analysis with the previously mentioned antibodies
against FOXO3 (Abcam Ltd.).
RNA isolation and polymerase chain
reaction (PCR) analysis
Total RNA was extracted from the frozen lung using
RNA iso Plus (Takara Biotechnology (Dalian) Co., Ltd., Dalian,
China) following the manufacturer’s instructions. RNA was dissolved
in diethylpyrocarbonate-treated deionized water, and the RNA
concentrations were analyzed using a nanodrop spectrophotometer
(UV1102II, Shanghai, China) to determine quality and quantity.
Reverse transcription was performed using a Takara RNA PCR kit
(AMV) version 3.0 (Takara Bio, Inc., Shiga, Japan). For PCR
analysis, the primers and their sequences were as follows: SIRT1
(forward, F) 5′-GCAACAGCATCTTGCCTGAT-3′ and (reverse, R)
5′-GTGCTACTGGTCTCACTT-3′; β-actin, F 5′-AGAGGGAAATCGTGCGTGAC-3′ and
R 5′-CAATAGTGATGACCTGGCCGT-3′. Two-step PCR was conducted according
to the instructions of the Takara RNA PCR Kit (AMV) version 3.0.
The conditions for reverse transcription were 42°C for 30 min, 99°C
for 5 min and then 5°C for 5 min to terminate the reverse
transcription. The PCR was performed as follows: 94°C for 30 sec
for denaturation; annealing, 55°C for 30 sec and 72°C for 90 sec
for extension. The products were then separated by electrophoresis
using 1.5% agarose gels. The bands were visualized using the
BioSpectrum-410 multispectral imaging system with a Chemi HR camera
410 (UVP, Upland, CA, USA).
Statistical analysis
The mean ± standard deviation values were calculated
to summarize all of the measurements. A one-way analysis of
variance was used to compare the means of the five groups. The
Student-Newman-Keuls’/Least Significant Difference test was
performed to compare all pair means. A value of P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were run with the SPSS version 17 Statistical
Software Package (SPSS, Inc., Chicago, IL, USA).
Results
Effects of ICA on intestinal I/R-induced
ALI and systemic inflammation
To determine whether ICA protects against intestinal
I/R-induced ALI, the histopathological properties of lung tissue,
serum TNF-α and IL-6 levels, as well as GSH and GSH-PX activity in
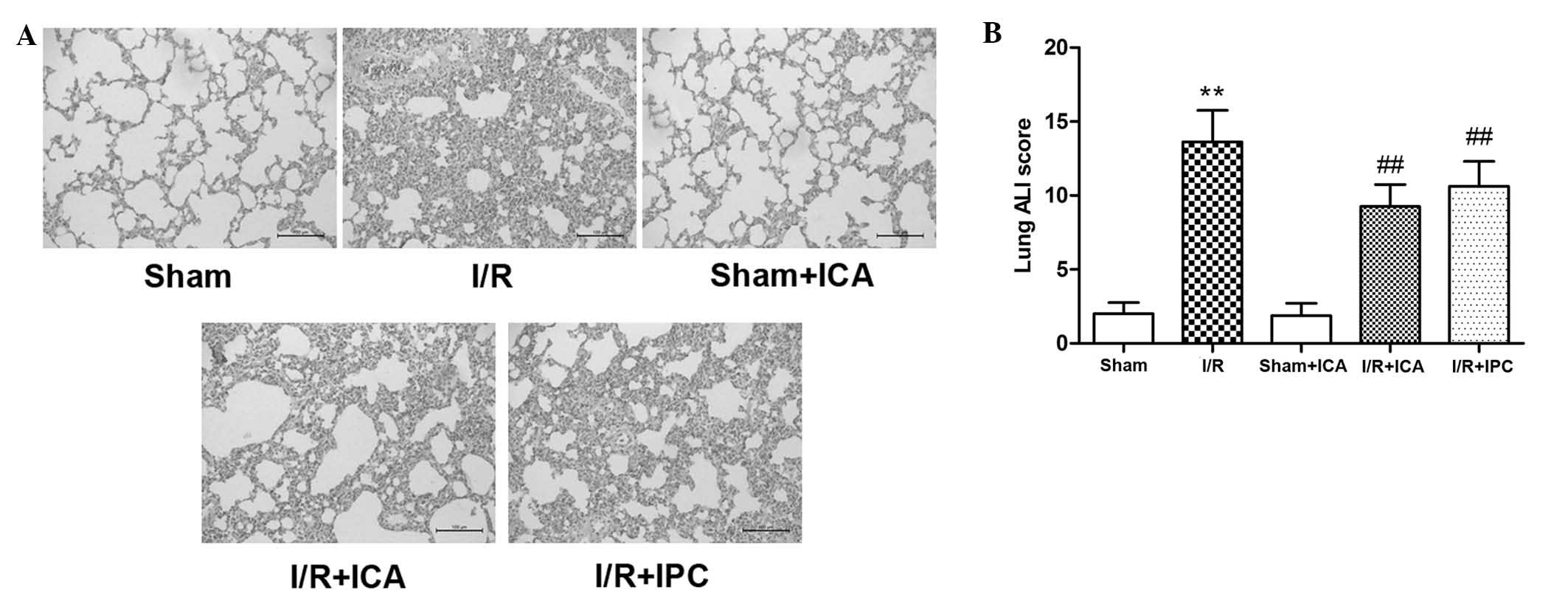
the lung were examined. As demonstrated in Fig. 1A, in the I/R group, the lung tissue
was markedly damaged, as characterized by severe congestive
alveoli, alveolar wall telangiectasia, increased inflammatory cell
infiltration, thickened alveolar walls and hyaline membrane
formation. In conjunction with this damage, serum TNF-α and IL-6
levels were significantly increased, while lung GSH and GSH-PX
activities were decreased (Table
I). By contrast, in the I/R+ICA and IPC groups, the results
demonstrated that ICA and IPC attenuated inflammatory infiltration,
improved lung histopathology scores (Fig. 1B), reduced serum TNF-α and IL-6
levels, and increased lung GSH and GSH-PX activities (Table I). These results indicated that ICA
offered effective protection against intestinal I/R-induced
ALI.
 | Table IGSH, GSH-PX, TNF-α and IL-6
expression levels in intestinal tissues from the different groups
(mean ± standard deviation, n=8). |
Table I
GSH, GSH-PX, TNF-α and IL-6
expression levels in intestinal tissues from the different groups
(mean ± standard deviation, n=8).
| Group | GSH (mg/g
protein) | GSH-PX (U/g
protein) | TNF-α (pg/ml) | IL-6 (pg/ml) |
|---|
| Sham | 6.32±0.51 | 87.51±6.54 | 63.96±10.12 | 165.72±21.47 |
| I/R | 2.54±0.31a | 36.27±3.78a |
117.05±15.31a |
257.88±34.97a |
| Sham+ICA | 6.48±0.42 | 95.74±7.78 | 62.31±10.77 | 151.89±18.25 |
| I/R+ICA | 4.53±0.57b | 65.27±10.53b | 83.81±9.69b |
205.96±21.87b |
| I/R+IPC | 3.70±0.46b | 55.53±8.30b | 99.70±12.26b |
200.73±20.91b |
Effects of ICA on the SIRT1-FOXO3 pathway
activation in rat lung tissues
To determine whether intestinal I/R, ICA
administration and IPC affected SIRT1 mRNA expression in the lung,
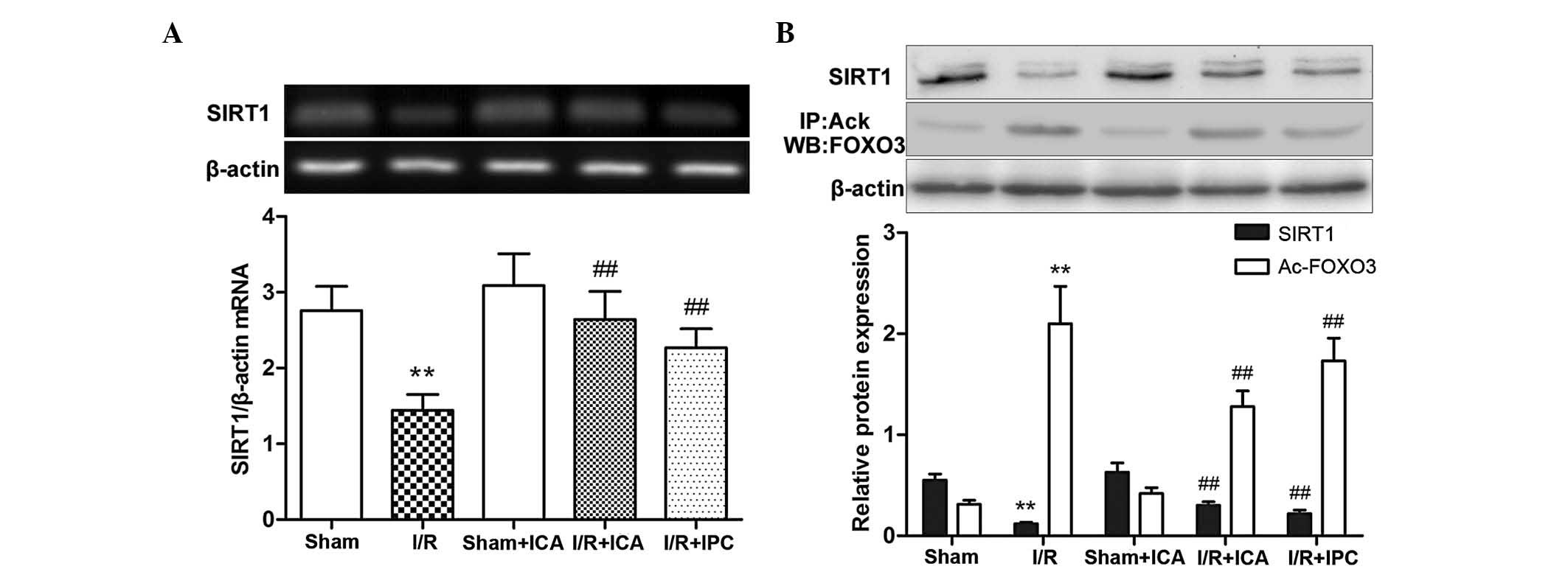
PCR analysis of SIRT1 was performed. As demonstrated in Fig. 2A, following intestinal ischemia for
60 min followed by reperfusion for 120 min, SIRT1 mRNA expression
in the injured lung tissue was significantly decreased compared
with that in the control tissues. Following ICA administration or
IPC, SIRT1 mRNA expression was significantly increased compared
with that in the I/R group. These results suggested that ICA
administration or IPC increased SIRT1 mRNA expression in the I/R
group.
To determine whether intestinal I/R, ICA
administration or IPC affected the expression of proteins of the
SIRT1-FOXO3 pathway in lung tissues, including SIRT1 and acetylated
FOXO3, western blot analysis for SIRT1 and immunoprecipitation of
acetylated FOXO3 were performed. Brunet et al (21) reported that in response to
oxidative stress, the deacetylase SIRT1 forms a protein complex
with the forkhead transcription factor FOXO3 that contributes to
FOXO3 deacetylation and then steers FOXO3-dependent responses away
from cell death and towards stress resistance. As demonstrated in
Fig. 2B, following intestinal
ischemia for 60 min and subsequent reperfusion for 120 min, SIRT1
protein expression in the lung tissue was significantly decreased
compared with that in the controls, while acetylated FOXO3 was
significantly increased. Following ICA administration or IPC, SIRT1
expression was increased, whereas acetylated FOXO3 expression was
significantly decreased compared with the I/R group. Collectively,
these results suggested that both ICA administration and IPC
suppressed intestinal I/R-induced ALI via upregulation of SIRT1
expression, thereby contributing to FOXO3 deacetylation.
In conclusion, ICA administration and IPC promoted
SIRT1 mRNA and protein expression, which contributed to FOXO3
deacetylation in intestinal I/R-induced ALI in rats.
Effects of ICA-mediated SIRT1-FOXO3
pathway activation on the expression of apoptosis-associated
factors in rat lung tissue
Since it is well-established that intestinal
I/R-induced ALI causes alveolar cell apoptosis, the TUNEL assay was
performed to assess the extent of apoptosis. As revealed in
Fig. 3A, marked alveolar cell
apoptosis was observed in the lungs of rats in the intestinal I/R
group, whereas in the ICA and IPC groups, alveolar cell apoptosis
was markedly attenuated.
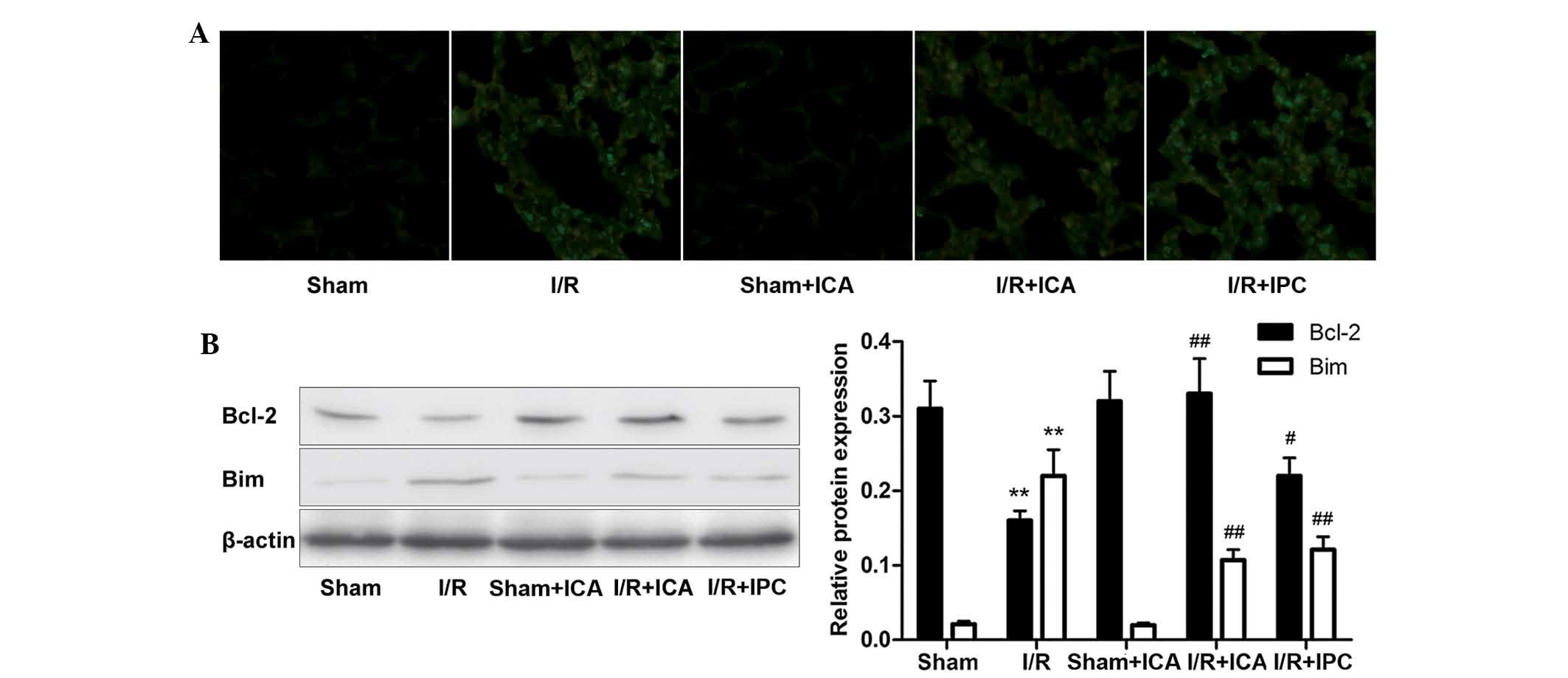 | Figure 3Effects of ICA-mediated SIRT1-FOXO3
pathway activation on the expression of apoptosis-associated
factors in rat lung tissues. (A) ICA attenuated I/R-induced
alveolar cell apoptosis in vivo, as confirmed using the
TUNEL assay. The content of TUNEL-positive cells was equal to the
amount of green fluorescenct in the image (magnification, ×400).
(B) Western blot analysis of Bim and Bcl-2 protein in lung tissue
samples from each group. ICA pretreatment or IPC reduced Bim
expression and enhanced Bcl-2 expression in the lung tissue samples
following intestinal I/R. Values are presented as the mean ±
standard deviation (n=3). **P<0.01 vs. the control
group; #P<0.05, ##P<0.01 vs. the I/R
group. I/R, ischemia/reperfusion; ICA, icariin; IPC, ischemia
preconditioning; SIRT1, sirtuin 1; FOXO3, forkhead box protein O3;
TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling; Bcl-2, B-cell lymphoma 2; Bim, Bcl-2-interacting mediator
of cell death. |
The pro-apoptotic factor Bim and anti-apoptotic
factor Bcl-2 have crucial roles in the regulation of cellular
responses to apoptosis. According to a study by Brunet et al
(21), SIRT1 inhibited the ability
of FOXO3 to induce cell death, whereas Motta et al (24) and Dijkers et al (31) demonstrated that SIRT1 deacetylated
and repressed the activity of the forkhead transcription factor
FOXO3a, which, in turn, reduced the expression of the
forkhead-dependent apoptotic factor Bim. To determine whether
intestinal I/R, ICA administration and IPC affected Bim and Bcl-2
expression in ALI, western blot analysis was performed. As revealed
in Fig. 3B, following intestinal
ischemia for 60 min and subsequent reperfusion for 120 min, the
expression of Bim was elevated and Bcl-2 was significantly
decreased in the injured lung tissues compared with those in the
controls. Following ICA administration or IPC, Bim levels were
decreased and Bcl-2 levels were increased compared with those in
the I/R group. Along with the conclusions mentioned above, these
phenomena indicated that ICA administration or IPC upregulated
SIRT1 expression and the latter deacetylated FOXO3, thereby
lowering the pro-apoptotic factor Bim, while enhancing the
anti-apoptotic factor Bcl-2, which suggested that the ICA-mediated
SIRT1-FOXO3 activation produced an anti-apoptotic effect in
intestinal I/R-induced ALI.
Effects of ICA-mediated SIRT1-FOXO3
pathway activation on the expression of antioxidative factors in
rat lung tissues
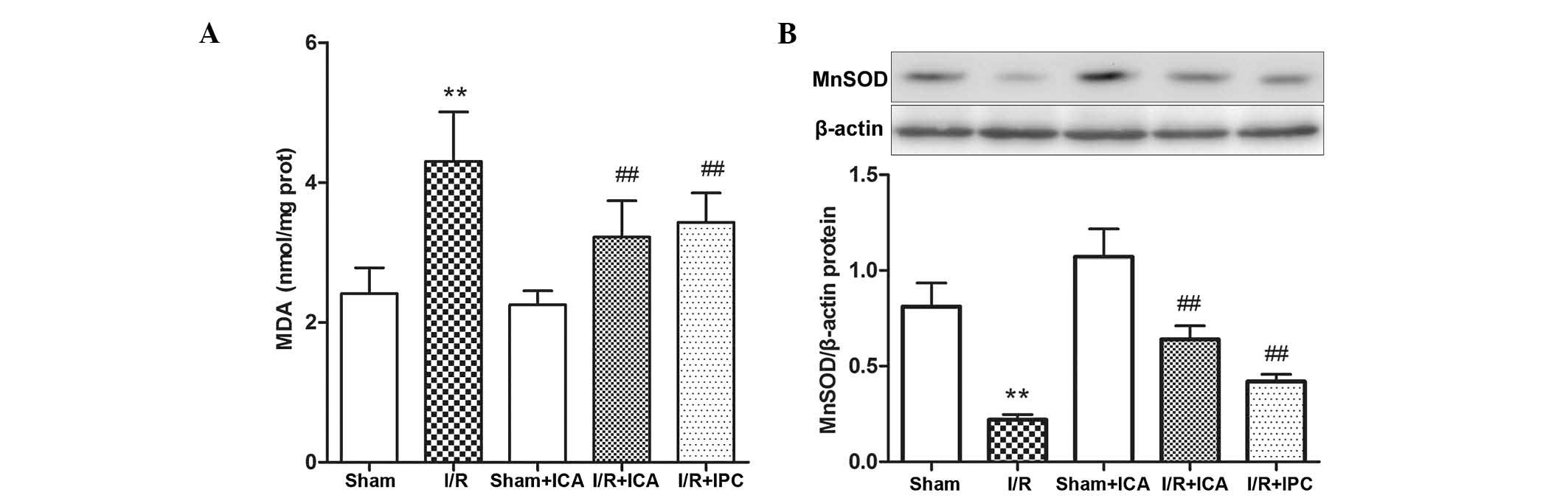
MDA is a degradation product of polyunsaturated
fatty acid peroxidation and arachidonic acid metabolism, and is
used as an indicator of oxidative stress in cells and tissues.
Therefore, serum MDA levels were analyzed to quantify oxidative
damage to alveolar cells. As revealed in Fig. 4A, lung MDA activity was increased
in the I/R group. By contrast, the results demonstrated that ICA
administration and IPC evidently attenuated MDA activity in the
I/R+ICA and IPC groups. These results indicated that ICA
administration and IPC effectively protected the alveolar cells
against oxidative stress in intestinal I/R-induced ALI.
To further assess the impact of ICA-mediated
SIRT1-FOXO3 pathway activation on the expression of antioxidative
factors, the role of MnSOD in intestinal I/R-induced ALI was
investigated. MnSOD is the primary mitochondrial ROS-scavenging
enzyme and is the target protein of the FOXO transcription factors
involved in the FOXO3-MnSOD network (21). As revealed in Fig. 4B, MnSOD protein expression in the
lung tissue samples was significantly decreased in the intestinal
I/R group compared with that in the control group, whereas ICA
administration or IPC demonstrated an efficient recovery of MnSOD
protein levels. These results indicated that ICA administration or
IPC upregulated SIRT1 protein expression, which subsequently
deacetylated and activated FOXO3, while the latter increased the
levels of the antioxidative molecule MnSOD, suggesting that the
ICA-mediated activation of the SIRT1-FOXO3 pathway had an
antioxdative role in intestinal I/R-induced ALI via MnSOD
induction.
Discussion
Intestinal I/R injury is considered a harbinger of
systemic inflammation and contributes to distant organ injury and
multiple organ dysfunction syndrome (32). The lung is the remote organ most
susceptible to injury following intestinal I/R, which explains why
ALI is a major cause of death (8,9,11).
Mechanisms underlying ALI are complex, but ultimately induce an
antioxidative stress imbalance and the initiation of apoptotic
signaling. Previous studies have demonstrated that the antioxidant
molecule MnSOD and anti-apoptotic Bcl-2 family of molecules
attributed to small intestine I/R-induced ALI (33–35).
SIRs were first identified in yeast as silent information
regulators and grouped as class III HDACs. SIRs function by
removing acetyl groups from lysine residues via NAD consumption.
There are seven human SIR homologs (SIRT1-7), each of which
exhibits different enzymatic activities and functions, although
SIRT1 is the most widely studied (36,37).
SIRT1 enhances cellular responses to exogenous stress and survival
via regulation of various proteins, including p53, NF-κB and the
FOXO transcription factors (25,38–40).
FOXO3 enhances cellular responses to oxidative stress and apoptosis
under ischemic or hypoxic conditions (41). In addition, in tissues and organs,
IPC has a protective effect via activation of endogenous
mechanisms; however, the delayed protection of IPC limits its
clinical application. Therefore an ideal method would be to
administer drugs, including ICA, which exerted positive effects in
the present study, to achieve the effects of IPC.
Following ICA and IPC pretreatment, levels of SIRT1
and acetylated FOXO3 were altered in small intestinal I/R-induced
ALI, as well as the regulation of downstream antioxidant factors
and apoptosis-associated factors. The present study demonstrated
that i) in small intestinal I/R-induced ALI, SIRT1 levels were
significantly lower and acetylated FOXO3 levels were significantly
higher; ii) following ICA administration or IPC, SIRT1 levels were
significantly increased, while acetylated FOXO3 levels were
significantly reduced and iii) the protective role of ICA and IPC
in this model was associated with the activation of downstream
FOXO3-associated molecules, including the antioxidant factor MnSOD,
the pro-apoptotic protein Bim and the anti-apoptotic protein Bcl-2.
These results suggested that the regulation of the SIRT1/FOXO3
signaling pathway by ICA may be beneficial in intestinal
I/R-induced ALI.
Intestinal I/R initiates a series of inflammatory
and oxidative stress responses (42). In the present study, it was
identified that following intestinal I/R, the intestinal and lung
tissues exhibited histological damage and serum TNF-α and IL-6
levels were markedly increased in association with significantly
decreased levels of tissue GSH and GSH-PX, and the lung MDA
activity was elevated compared with the control group. These
changes were parallel to the decrease SIRT1 and MnSOD levels and
increased acetylated FOXO3 protein expression. Following ICA
pretreatment, SIRT1 mRNA and protein levels were elevated, which
induced the reduction of acetylated FOXO3 protein levels, which
then induced MnSOD production. More importantly, the indicators of
injury mentioned above were reversed, which suggested that the
SIRT1/FOXO3 signaling pathway had a crucial role in antioxidative
stress amelioration following intestinal I/R-induced ALI. Several
studies have suggested a functional link between SIRT1 and FOXO3
regulation of the MnSOD-mediated antioxidant pathway regarding ROS
production (41,43). In the present study, MnSOD levels
were evidently attenuated by intestinal I/R. By contrast, following
ICA administration, acetylated FOXO3 expression levels were reduced
and MnSOD was upregulated, which was attributed to the increasing
levels of deacetylated FOXO3. Therefore, intestinal I/R appeared to
activate a cascade resulting in ROS accumulation, SIRT1 reduction,
FOXO3 acetylatation and MnSOD downregulation. The regulation of the
SIRT1-FOXO3 signaling pathway by ICA upregulated MnSOD expression,
thereby strengthening cellular antioxidant capability and
survival.
To identify the potential mechanisms of the
SIRT1-FOXO3 signaling pathway associated with cell apoptosis in
intestinal I/R-induced ALI, the functional link between FOXO3 and
apoptosis by evaluating Bim and Bcl-2 expression was determined
using the TUNEL assay to measure the extent of apoptosis. The Bcl-2
family, which has both pro- and anti-apoptotic members, including
Bim and Bcl-2, has a critical role in the regulation of cell
survival. A study by Dijkers et al (31) suggested that the activation of
FOXO3 was sufficient to induce Bim expression. Furthermore, the
authors proposed that Bcl-2 suppressed the ability of FOXO3 to
induce apoptosis. In addition, a study by Motta et al
(24) demonstrated that SIRT1
deacetylated and repressed the activity of FOXO3 to reduce
forkhead-dependent apoptosis. The present study demonstrated that
Bim expression was increased and Bcl-2 expression was significantly
decreased in the intestinal I/R group; however, ICA or IPC
pretreatment markedly decreased Bim expression, increased Bcl-2
expression and reduced FOXO3 acetylation in response to SIRT1
upregulation. Therefore, consistent with the results of previous
studies, Bcl-2 overexpression via the SIRT1-FOXO3 signaling pathway
protected lung epithelial cells from intestinal I/R-induced
apoptosis and subsequent ALI. The TUNEL assay revealed marked
alveolar cell apoptosis in the lung tissues of rats in the
intestinal I/R group, while alveolar cell apoptosis was markedly
attenuated in the ICA and IPC groups.
In conclusion, the present study demonstrated that
ICA-mediated SIRT1-FOXO3 signaling regulated oxidative stress
injury and cell apoptosis in intestinal I/R-induced ALI. ICA or IPC
pretreatment protected the experimental rats from intestinal
I/R-induced ALI via activation of the SIRT1-FOXO3 signaling
pathway. However, this protective mechanism may be mediated by
MnSOD upregulation and enhanced Bcl-2 expression. Manipulation of
SIRT1 upregulation may offer a new therapeutic approach to
alleviate ALI in intestinal I/R patients. It is therefore important
to further elucidate the regulation mechanisms of ICA that induced
SIRT1 expression and FOXO3 deacetylation. At present, a study is
underway in our group to determine the precise mechanisms
underlying ICA-mediated regulation in SIRT1 expression.
Acknowledgements
This study was supported by grants of the Chinese
National Natural Science Foundation (no. 81171850) and a
Specialized Research Fund by the Doctoral Program of Higher
Education of China (no. 20122105110001).
References
|
1
|
Pierro A and Eaton S: Intestinal ischemia
reperfusion injury and multisystem organ failure. Semin Pediatr
Surg. 13:11–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Higuchi S, Wu R, Zhou M, et al: Gut
hyperpermeability after ischemia and reperfusion: attenuation with
adrenomedullin and its binding protein treatment. Int J Clin Exp
Pathol. 1:409–418. 2008.PubMed/NCBI
|
|
3
|
Tendler DA: Acute intestinal ischemia and
infarction. Semin Gastrointest Dis. 14:66–76. 2003.PubMed/NCBI
|
|
4
|
Li C and Jackson RM: Reactive species
mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol
Cell Physiol. 282:C227–C241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion - from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cui T, Miksa M, Wu R, et al: Milk fat
globule epidermal growth factor 8 attenuates acute lung injury in
mice after intestinal ischemia and reperfusion. Am J Respir Crit
Care Med. 181:238–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu R, Dong W, Ji Y, et al: Orexigenic
hormone ghrelin attenuates local and remote organ injury after
intestinal ischemia-reperfusion. PLOS One. 3:e20262008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sorkine P, Szold O, Halpern P, et al: Gut
decontamination reduces bowel ischemia-induced lung injury in rats.
Chest. 112:491–495. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mura M, Andrade CF, Han B, et al:
Intestinal ischemia-reperfusion induced acute lung injury and
oncotic cell death in multiple organs. Shock. 28:227–238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu TL, Zhang WT, Zhang L, et al:
L-arginine administration ameliorates serum and pulmonary cytokine
response after gut ischemia-reperfusion in immature rats. World J
Gastroenterol. 11:1070–1072. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moraes LB, Murakami AH, Fontes B, et al:
Gut ischemia/reperfusion induced acute lung injury is an alveolar
macrophage dependent event. J Trauma. 64:1196–1200. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ben DF, Yu XY, Ji GY, et al: TLR4 mediates
lung injury and inflammation in intestinal ischemia-reperfusion. J
Surg Res. 174:326–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biswas SK and Rahman I: Environmental
toxicity, redox signaling and lung inflammation: the role of
glutathione. Mol Aspects Med. 30:60–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marfe G, Tafani M, Pucci B, et al: The
effect of marathon on mRNA expression of anti-apoptotic and
pro-apoptotic proteins and sirtuins family in male recreational
long-distance runners. BMC Physiol. 10:72010. View Article : Google Scholar
|
|
15
|
Lai L, Yan L, Gao S, et al: Type 5
adenylyl cyclase increases oxidative stress by transcriptional
regulation of MnSOD via the sirt1/foxo3a pathway. Circulation.
127:1692–1701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alcendor RR, Kirshenbaum LA, Imai S, et
al: Silent information regulator 2alpha, a longevity factor and
class III histone deacetylase, is an essential endogenous apoptosis
inhibitor in cardiac myocytes. Circ Res. 95:971–980. 2004.
View Article : Google Scholar
|
|
17
|
Kelly G: A review of the sirtuin system,
its clinical implications, and the potential role of dietary
activators like resveratrol: part 1. Altern Med Rev. 15:245–263.
2010.
|
|
18
|
Sauve AA, Wolberger C, Schramm VL and
Boeke JD: The biochemistry of sirtuins. Ann Rev Biochem.
75:435–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haiqis MC and Guarente LP: Mammalian
sirtuins - emerging roles in physiology, aging, and calorie
restriction. Gen Dev. 20:2913–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu CP, Zhai P, Yamamoto T, et al: Silent
information regulator 1 protects the heart from
ischemia/reperfusion. Circulation. 122:2170–2182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brunet A, Sweeney LB, Sturgill JF, et al:
Stress-dependent regulation of FOXO transcription factors by the
SIRT1 deacetylase. Science. 303:2011–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Langley E, Pearson M, Faretta M, et al:
Human SIR2 deacetylates p53 and antagonizes PML/p53-induced
cellular senescence. EMBO J. 21:2383–2396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeung F, Hoberg JE, Ramsey CS, et al:
Modulation of NF-kappaB-dependent transcription and cell survival
by the SIRT1 deacetylase. EMBO J. 23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Motta MC, Divecha N, Lemieux M, et al:
Mammalian SIRT1 represses forkhead transcription factors. Cell.
116:551–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Picone P, Giacomazza D, Vetri V, et al:
Insulin-activated Akt rescues Aβ oxidative stress-induced cell
death by orchestrating molecular trafficking. Aging Cell.
10:832–843. 2011.PubMed/NCBI
|
|
26
|
Wang L, Zhang L, Chen ZB, et al: Icariin
enhances neuronal survival after oxygen and glucose deprivation by
increasing SIRT1. Eur J Pharmacol. 609:40–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Huang S, Chen Y, et al: Icariin
inhibits hydrogen peroxide-mediated cytotoxicity by up-regulating
sirtuin type 1-dependent catalase and peroxiredoxin. Basic Clin
Pharmacol Toxicol. 107:899–905. 2010.PubMed/NCBI
|
|
28
|
Zhu HR, Wang ZY, Zhu XL, et al: Icariin
protects against brain injury by enhancing SIRT1-dependent
PGC-1alpha expression in experimental stroke. Neuropharmacology.
59:70–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang GZ, Yao JH, Jing HR, et al:
Suppression of the p66shc adapter protein by protocatechuic acid
prevents the development of lung injury induced by intestinal
ischemia reperfusion in mice. J Trauma Acute Care Surg.
73:1130–1137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mikawa K, Nishina K, Takao Y and Obara H:
ONO-1714, a nitric oxide synthase inhibitor, attenuates
endotoxin-induced acute lung injury in rabbits. Anesth Analg.
97:1751–1755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dijkers PF, Medema RH, Lammers JW, et al:
Expression of the pro-apoptotic Bcl-2 family member Bim is
regulated by the forkhead transcription factor FKHR-L1. Current
Biology. 10:1201–1204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hassoun HT, Kone BC, Mercer DW, et al:
Post-injury multiple organ failure: the role of the gut. Shock.
15:1–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kostopanagiotou G, Avgerinos E,
Costopanaqiotou C, et al: Acute lung injury in a rat model of
intestinal ischemia-reperfusion: the potential time depended role
of phospholipases A (2). J Surg Res. 147:108–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeh KY, Yeh M, Glass J, et al: Rapid
activation of NF-kappaB and AP-1 and target gene expression in
postischemic rat intestine. Gastroenterology. 118:525–534. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koo HC, Davis JM, Li Y, et al: Effects of
transgene expression of superoxide dismutase and glutathione
peroxidase on pulmonary epithelial cell growth in hyperoxia. Am J
Physiol Lung Cell Mol Physiol. 288:L718–L726. 2005.PubMed/NCBI
|
|
36
|
Blander G and Guarente L: The sir2 family
of protein deacetylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
North BJ and Verdin E: Sirtuins:
Sir2-related NAD-dependent protein deacetylases. Genome Biol.
5:2242004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okawa H, Motohashi H, Kobayashi A, et al:
Hepatocyte-specific deletion of the keapl gene activates Nrf2 and
confers potent resistance against acute drug toxicity. Biochem
Biophys Res Commun. 339:79–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reisman SA, Csanaky IL, Aleksunes LM and
Klaassen CD: Altered disposition of acetaminophen in Nrf2-null and
Keapl-knockdown mice. Toxicol Sci. 109:31–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhai X, Lin M, Zhang F, et al: Dietary
flavonoid genistein induces Nrf2 and phase II detoxification gene
expression via ERKs and PKC pathways and protects against oxidative
stress in Caco-2 cells. Mol Nutr Food Res. 57:249–259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Calvert JW, Jha S, Gundewar S, et al:
Hydrogen sulfide mediates cardioprotection through Nrf2 signaling.
Circ Res. 105:365–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Yao JH, Hu XW, et al: Inhibition of
Rho kinase by fasudil hydrochloride attenuates lung injury induced
by intestinal ischemia and reperfusion. Life Sci. 88:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tanaka J, Qiang L, Banks AS, et al: Foxo1
links hyperglycemia to LDL oxidation and endothelial nitric oxide
synthase dysfunction in vascular endothelial cells. Diabetes.
58:2344–2354. 2009. View Article : Google Scholar : PubMed/NCBI
|