Introduction
Eicosapentaenoic acid (EPA) and docosahexaenoic acid
(DHA) are essential fatty acids belonging to the group of ω-3 fatty
acids, which are primarily found in coldwater fish. These
polyunsaturated fatty acids (PUFAs) have an important role in the
functions of the body and are converted into hormone-like
substances known as prostaglandins and leukotrienes (1). EPA has been shown to provide health
benefits in patients with coronary heart disease, high blood
pressure, mental health conditions, such as schizophrenia, and
inflammatory disorders, including rheumatoid arthritis (2–6). DHA
is a key component of brain tissue and the retina of the eye
(7,8). Low levels of DHA cause reduced
serotonin levels in the brain, which may result in depression,
attention deficit hyperactivity disorder, cognitive decline and
Alzheimer’s disease (9–11).
It is well established that the key factors
associated with cholesterol gallstone (CG) formation are biliary
cholesterol hypersaturation and gallbladder mucin hypersecretion.
The hypersaturation of cholesterol in the bile may result from a
high-cholesterol diet or hepatic cholesterol overproduction. The
elevation of biliary cholesterol concentration leads to the
hypersecretion of mucin and the aggregation of cholesterol crystals
(12,13). Several mucin (MUC) genes, including
MUC2, MUC5AC, MUC5B and MUC6, are expressed in the gallbladder
mucosa (14). The upregulation of
these MUC genes leads to increased mucin concentrations, which
increase bile viscosity and lead to the formation of a gel matrix
that can entrap cholesterol crystals in the gallbladder (15–16).
The only established medical treatment for CG is
ursodeoxycholic acid (UDCA). However, a number of studies have
demonstrated that the therapeutic efficacy exhibited by UDCA lacks
consistency and is not entirely satisfactory (17–19).
Despite numerous attempts to develop novel CG drugs, there have
been no satisfactory findings. In our previous study (20), it was found that a medical
combination of ω-3 PUFAs originating from fish oil had a preventive
effect against CG formation. This medical combination consisted
primarily of EPA and DHA. Therefore, the present study aimed to
investigate which of these two ω-3 PUFAs is responsible for the
anti-lithogenic effect observed on CG formation using a mouse
model.
Materials and methods
Materials
EPA (90.2%) and DHA (80.9%) were obtained from
Chemport, Inc. (Naju, Korea). The lithogenic diet (LD) was
purchased from Dyets, Inc. (cat no. 102136; Bethlehem, PA,
USA).
Animals and diets
Male 12-week-old C57BL/6J mice were purchased from
Central Lab Animal, Inc. (Seoul, Korea) and bred in a laboratory
animal breeding room at the College of Veterinary Medicine of
Konkuk University (Seoul, Korea). The mice were divided into the
following four groups of 10 mice: A) LD, B) LD plus EPA, C) LD plus
DHA and D) LD plus EPA plus DHA. The acclimation period was four
weeks. Subsequent to being fed the LD for four weeks, EPA and/or
DHA was orally administered to the mice at a dose of 70 mg/kg/day
for eight weeks. The LD feeding was continued during this period.
The LD contained 1.0% cholesterol and 0.5% cholic acid. EPA (70
mg/kg/day) and DHA (70 mg/kg/day) were diluted in 0.75% Tween-80
and administered orally by gavage for eight weeks. The LD group was
treated with 0.75% Tween-80 as a vehicle control. The animal
experiments were approved by the Institutional Animal Care and Use
Committee of Konkuk University. Mouse blood was collected from the
vena cava and separated serum samples were stored in a −80°C
biofreezer. The liver and gallbladder were isolated and frozen in
liquid N2 until required for analysis.
Blood chemical analysis
The plasma levels of aspartate aminotransferase
(AST), alanine aminotransferase (ALT), total cholesterol,
high-density lipoprotein (HDL)-cholesterol, phospholipids and
triglycerides were determined using an automated Hitachi Clinical
Analyzer (model 7020; Hitachi, Ltd., Kobe, Japan).
Stone formation assessment
Gallstone formation was assessed using a six-point
system as follows: 0, clear bile; 1, little biliary sludge; 2,
widespread biliary sludge; 3, high levels of biliary sludge; 4, a
few small stones; 5, several stones; and 6, full of stones. The
average scores of each group were compared.
Quantitative polymerase chain reaction
(qPCR) analysis for MUC genes, low-density lipoprotein receptor
(LDLR) and 3-hydroxy-3-methylglutaryl-coenzyme A reductase
(HMGCR)
Total RNA was extracted from mouse gallbladder
tissue using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and dissolved in RNase-free water.
Reverse transcription was performed using superscript III reverse
transcriptase (Invitrogen Life Technologies). The mRNA levels of
MUC2, -5AC, -5B and -6, and LDLR and HMGCR were quantified using
TaqMan® PCR with GAPDH as an internal control. qPCR was
performed using a Chromo4 Real-Time PCR Detection System (BioRad,
Hercules, CA, USA). The relative abundances of the target genes
were calculated using the comparative threshold cycle method. The
qPCR primers and fluorogenic probes were purchased from Metabion
(Martinsried, Germany) and were as follows: MUC2, NM_023566;
MUC5AC, NM_010844; MUC5B, NM_028801; MUC6, NM_181729; LDLR,
NM_010700; HMGCR, NM_008255 and GAPDH, NM_008084.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Student’s t-tests were performed to compare the treatment groups. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of EPA and DHA on the liver
weight-to-body weight ratio and plasma aminotransferase and lipid
levels
To assess the effects of EPA and DHA on the liver,
the liver weight-to-body weight ratio and plasma aminotransferase
levels were analyzed. The liver weight-to-body weight ratio was not
significantly different among the different treatment groups.
Fig. 1 shows that the serum ALT
and AST levels were unchanged by treatment with EPA or DHA. In
addition, no significant differences were observed in the levels of
plasma lipids, including HDL-cholesterol, phospholipids and
triglycerides, among the treatment groups. Although the level of
cholesterol was significantly increased in the LD plus DHA group
relative to that in the control LD group, this difference was
<20%.
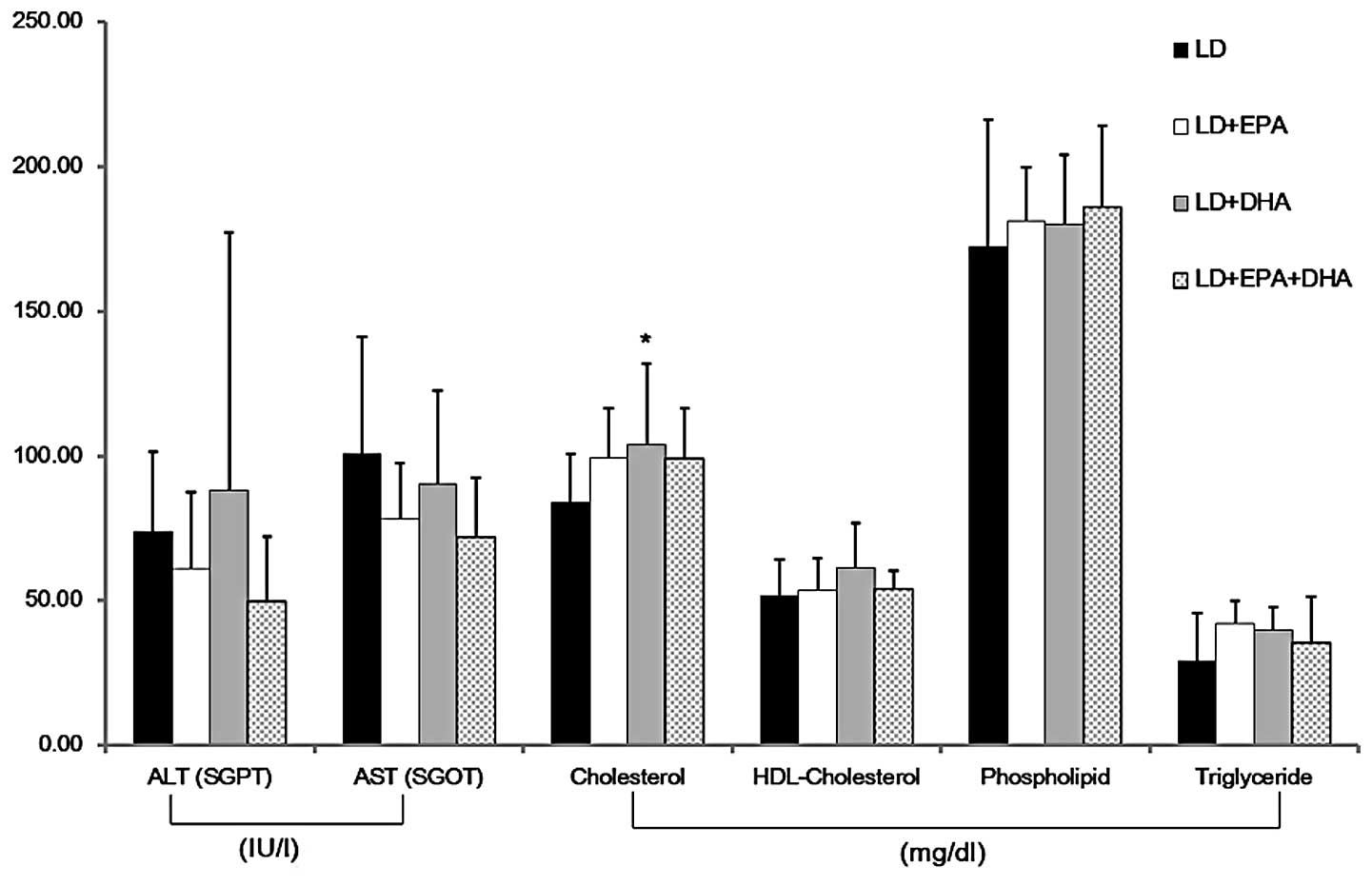 | Figure 1Effects of EPA and DHA on the levels
of aminotransferases and lipids in the plasma. The level of
cholesterol was significantly increased in the LD+DHA treatment
group. The levels of ALT, AST, HDL-cholesterol, phospholipid and
triglyceride showed no significant differences among the treatment
groups. Data are presented as the mean ± standard deviation (n=5).
*P<0.05 versus the LD group. LD, lithogenic diet;
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ALT, alanine
aminotransferase; SGPT, serum glutamic pyruvic transaminase; SGOT,
serum glutamic oxaloacetic transaminase; AST, aspartate
aminotransferase; HDL, high-density lipoprotein. |
Effects of EPA and DHA on gallstone
formation
Gallstone formation was observed in the different
treatment groups. Gross analysis of the gallbladder revealed that
the mice in the EPA treatment groups (groups B and D) exhibited
significantly lower stone formation (P<0.01) than those in the
LD plus DHA treatment group (group C), which exhibited only
slightly suppressed stone formation (Fig. 2). Combined EPA and DHA treatment
(group D) showed no significant synergistic effect on preventing
gallstone formation. The mean scores for the stone formation grades
were 4.38, 1.75, 3.0 and 2.13 for the LD, LD plus EPA, LD plus DHA
and LD plus EPA plus DHA groups, respectively.
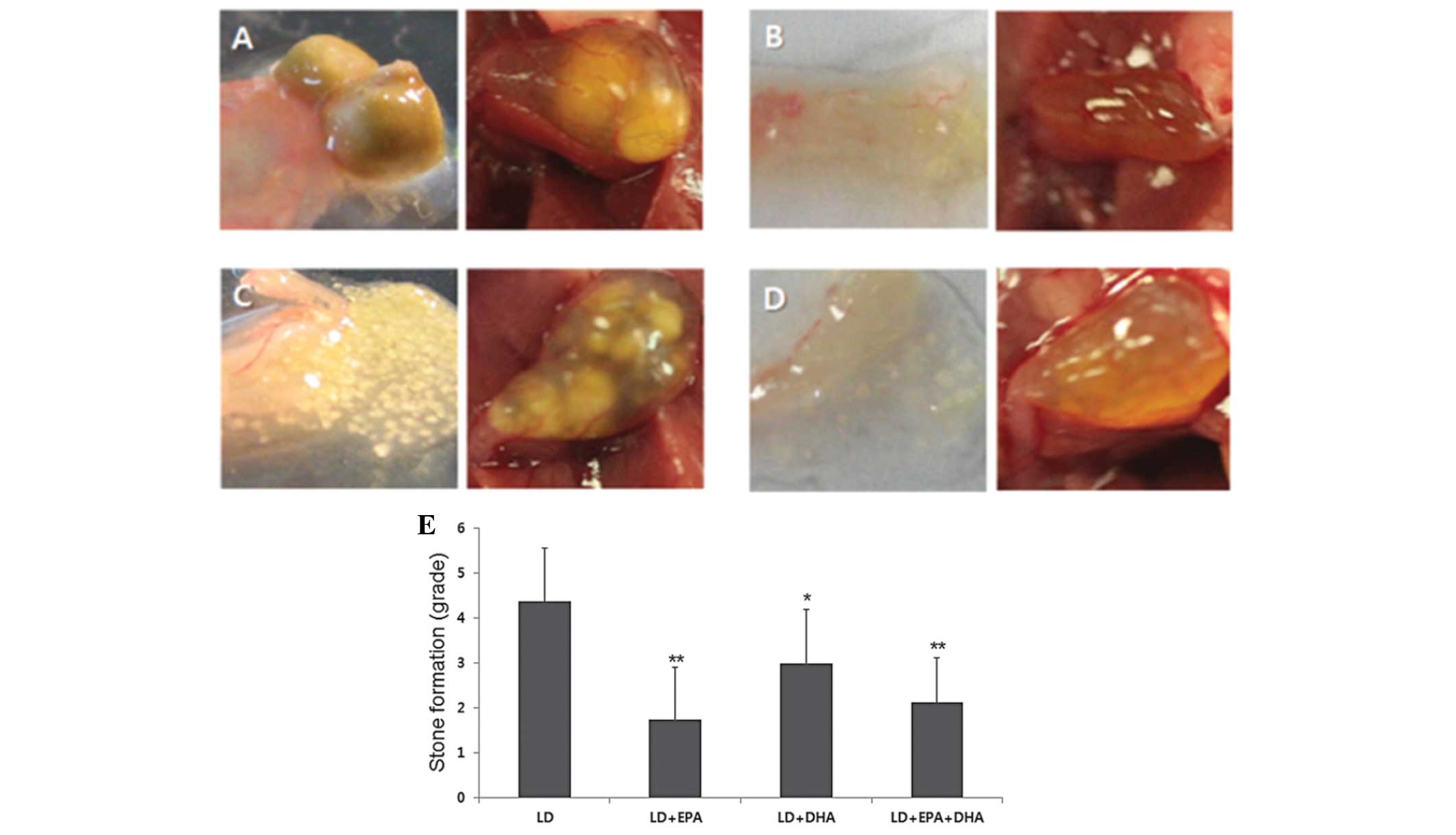 | Figure 2Gross findings for the gallbladder and
gallstones. (A) LD, (B) LD+EPA, (C) LD+DHA and (D) LD+EPA+DHA
groups (Left image: Postseparation; magnification, ×20. Right
image: Preseparation, magnification, ×10). Mice fed EPA exhibited
significantly fewer gallstones than those in the LD+DHA group. (E)
Grading of gallstone formation. Mice in the LD+EPA and LD+EPA+DHA
treatment groups showed significantly lower gallstone formation
than those in the LD+DHA treatment group. Mice were graded
according to the following scale: 0, clear bile; 1, little biliary
sludge; 2, widespread biliary sludge; 3, high levels of biliary
sludge; 4, a few small stones, 5, several stones and 6, full of
stones. Data are presented as the mean ± standard deviation (n=10).
*P<0.05 and **P<0.01 versus the LD
group. LD, lithogenic diet; EPA, eicosapentaenoic acid; DHA,
docosahexaenoic acid. |
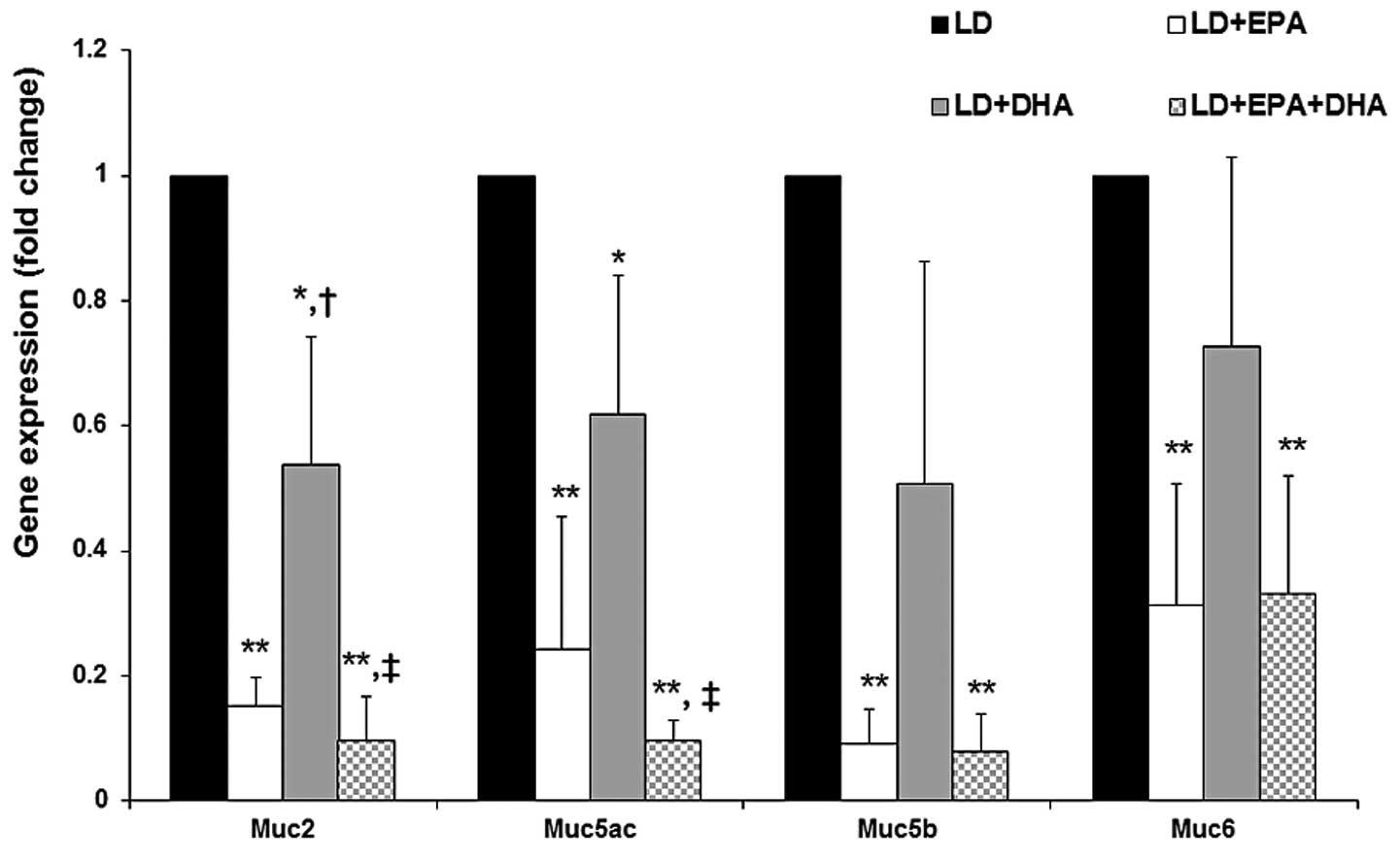
Effects of EPA and DHA on the expression
levels of MUC genes, LDLR and HMGCR
It has been suggested that the downregulation of MUC
gene expression causes a decrease in bile viscosity in the
gallbladder, which attenuates CG formation (15,17).
The expression of MUC2, -5AC, -5B and -6 was significantly reduced
in the gallbladders of the mice in the EPA groups (70–90%) and the
LD plus DHA treatment group (30–50%), compared with that in the
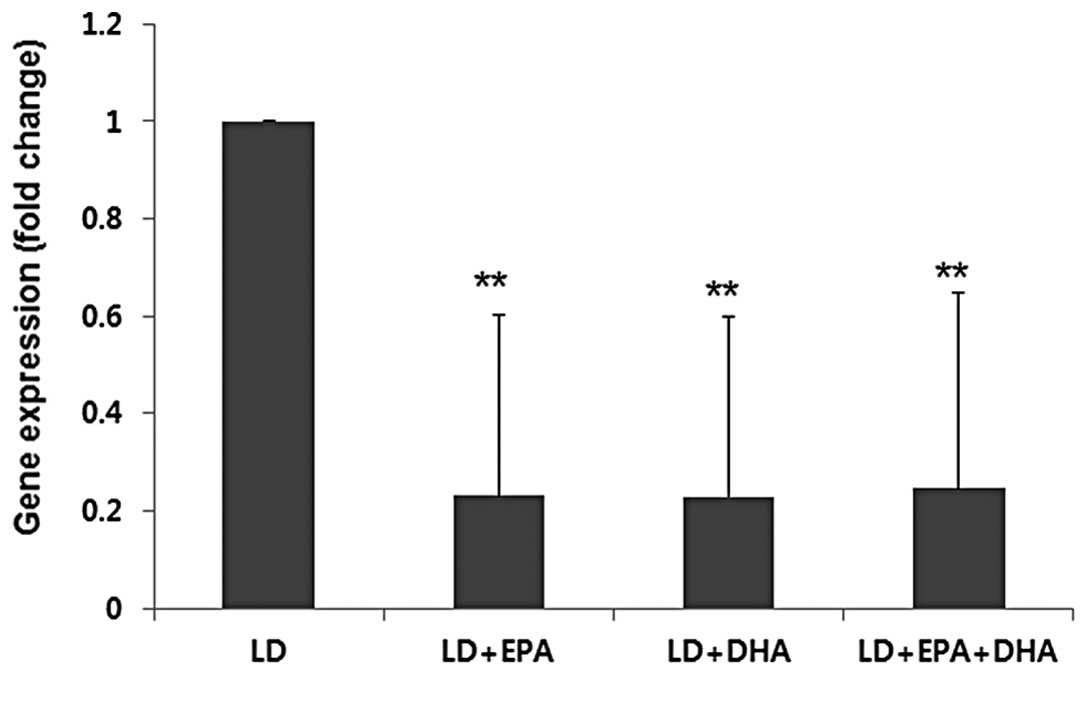
gallbladders of the mice in the LD group (Fig. 3). No significant differences were
observed in the mRNA expression of LDLR in the liver among the mice
in the different treatment groups (data not shown). As shown in
Fig. 4, the mRNA expression of
HMGCR was significantly decreased in the livers of the mice in the
LD plus EPA, LD plus DHA and LD plus EPA plus DHA treatment groups
compared with that in the mice in the LD group (P<0.01). HMGCR
expression is an index of cholesterol uptake and synthesis in the
liver.
Discussion
EPA from fish oil has a number of biological
effects, including the reduction of the severity of cardiovascular
diseases, such as atherosclerosis, through modulating lipid
metabolism, as well as the inhibition of inflammatory processes
(21,22). In the present study, EPA was found
to have a dominant anti-lithogenic effect in C57BL/6J mice.
Previous animal studies (24–26)
have attempted to develop ω-3 PUFAs as dominant anti-lithogenic
agents. Magnuson et al (23) reported that dietary fish oil
inhibited solid cholesterol crystal precipitation and gallstone
formation in prairie dogs. Furthermore, Scobey et al
(24) demonstrated that fish oil
consumption led to a decrease in gallstone formation and the
cholesterol saturation index (CSI) in African green monkeys.
Mizuguchi et al (25) also
presented animal data indicating that the repeated administration
of EPA to hamsters decreased the incidence of cholesterol
crystallization and gallstone formation. However, the molecular
mechanisms responsible for the roles of ω-3 PUFAs and EPA in CG
formation are yet to be elucidated.
Gallstone formation is primarily caused by the
hypersaturation of cholesterol in the bile and the hypersecretion
of mucin in the gallbladder (26).
MUC gene expression is a well-established indicator of gallstone
nucleation. In the present study, the expression of secretory MUC
genes in the gallbladder was investigated, in order to assess mucin
hypersecretion. Treatment with EPA in the presence or absence of
DHA was found to significantly decrease the gene expression of
MUC2, -5AC, -5B and -6. This finding suggests that EPA can
attenuate CG formation, as it is well established that the
downregulation of secretory MUC genes results in a decrease in bile
viscosity in the gallbladder. The CSI was not able to be assessed
in the present study due to the low volume of bile available in
mice. However, Janowitz et al (27) reported that fish oil consumption
significantly decreased the CSI in humans.
The expression levels of LDLR and HMGCR are
considered to be major indicators of cholesterol uptake and
synthesis in the liver, respectively (28,29).
In the present study, both EPA and DHA were found to significantly
inhibit HMGCR expression, suggesting that these fatty acids may
affect the availability of cholesterol in the liver. However, no
significant difference was observed in the expression of LDLR and
HMGCR between mice treated with EPA and those treated with DHA.
In our previous study (20), it was reported that a medical
combination of ω-3 PUFAs originating from fish oil that contained
two major types of PUFAs, EPA and DHA, had a preventive effect
against CG formation in C57BL/6J mice. The present study found that
EPA has a significantly higher anti-lithogenic effect than DHA in
C57BL/6J mice. This anti-lithogenic activity of EPA may be due to
the inhibition of nucleation and cholesterol synthesis.
Acknowledgements
This study was supported by Konkuk University in
2012.
Abbreviations:
|
EPA
|
eicosapentaenoic acid
|
|
DHA
|
docosahexaenoic acid
|
|
PUFAs
|
polyunsaturated fatty acids
|
|
CG
|
cholesterol gallstone
|
|
LD
|
lithogenic diet
|
|
HMGCR
|
3-hydroxy-3-methylglutaryl-coenzyme A
reductase
|
|
MUC
|
mucin
|
|
UDCA
|
ursodeoxycholic acid
|
References
|
1
|
Drevon CA: Marine oils and their effects.
Nutr Rev. 50:38–45. 1992. View Article : Google Scholar
|
|
2
|
Song C and Zhao S: Omega-3 fatty acid
eicosapentaenoic acid. A new treatment for psychiatric and
neurodegenerative diseases: a review of clinical investigations.
Expert Opin Investig Drugs. 16:1627–1638. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yokoyama M and Origasa H; JELIS
Investigators. Effects of eicosapentaenoic acid on cardiovascular
events in Japanese patients with hypercholesterolemia: rationale,
design, and baseline characteristics of the Japan EPA Lipid
Intervention Study (JELIS). Am Heart J. 146:613–620. 2003.
View Article : Google Scholar
|
|
4
|
Miyajima T, Tsujino T, Saito K and
Yokoyama M: Effects of eicosapentaenoic acid on blood pressure,
cell membrane fatty acids, and intracellular sodium concentration
in essential hypertension. Hypertens Res. 24:537–542. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peet M, Brind J, Ramchand CN, Shah S and
Vankar GK: Two double-blind placebo-controlled pilot studies of
eicosapentaenoic acid in the treatment of schizophrenia. Schizophr
Res. 49:243–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calder PC: Session 3: Joint Nutrition
Society and Irish Nutrition and Dietetic Institute Symposium on
‘Nutrition and autoimmune disease’ PUFA, inflammatory processes and
rheumatoid arthritis. Proc Nutr Soc. 67:409–418. 2008.
|
|
7
|
Singh M: Essential fatty acids, DHA and
human brain. Indian J Pediatr. 72:239–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pifferi F, Perret M, Guesnet P, Aujard F
and Alessandri JM: Fatty acid composition of the brain, retina,
liver and adipose tissue of the grey mouse lemur (Microcebus
murinus, primate). Lipids. 47:793–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cunnane SC, Plourde M, Pifferi F, Bégin M,
Féart C and Barberger-Gateau P: Fish, docosahexaenoic acid and
Alzheimer’s disease. Prog Lipid Res. 48:239–256. 2009.
|
|
10
|
Fotuhi M, Mohassel P and Yaffe K: Fish
consumption, long-chain omega-3 fatty acids and risk of cognitive
decline or Alzheimer disease: a complex association. Nat Clin Pract
Neurol. 5:140–152. 2009. View Article : Google Scholar
|
|
11
|
McNamara RK, Hahn CG, Jandacek R, Rider T,
Tso P, Stanford KE and Richtand NM: Selective deficits in the
omega-3 fatty acid docosahexaenoic acid in the postmortem
orbitofrontal cortex of patients with major depressive disorder.
Biol Psychiatry. 62:17–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SP, LaMont JT and Carey MC: Role of
gallbladder mucus hypersecretion in the evolution of cholesterol
gallstones. J Clin Invest. 67:1712–1723. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang DQ, Paigen B and Carey MC: Phenotypic
characterization of Lith genes that determine susceptibility to
cholesterol cholelithiasis in inbred mice: physical-chemistry of
gallbladder bile. J Lipid Res. 38:1395–1411. 1997.
|
|
14
|
Vilkin A, Nudelman I, Morgenstern S,
Geller A, Bar Dayan Y, Levi Z, Rodionov G, Hardy B, Konikoff F,
Gobbic D and Niv Y: Gallbladder inflammation is associated with
increase in mucin expression and pigmented stone formation. Dig Dis
Sci. 52:1613–1620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chuang SC, Hsi E and Lee KT: Mucin genes
in gallstone disease. Clin Chim Acta. 413:1466–1471. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KT and Liu TS: Mucin gene expression
in gallbladder epithelium. J Formos Med Assoc. 101:762–768.
2002.PubMed/NCBI
|
|
17
|
Fischer S, Müller I, Zündt BZ, Jüngst C,
Meyer G and Jüngst D: Ursodeoxycholic acid decreases viscosity and
sedimentable fractions of gallbladder bile in patients with
cholesterol gallstones. Eur J Gastroenterol Hepatol. 16:305–311.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Venneman NG, Besselink MG, Keulemans YC,
Vanberge-Henegouwen GP, Boermeester MA, Broeders IA, Go PM and van
Erpecum KJ: Ursodeoxycholic acid exerts no beneficial effect in
patients with symptomatic gallstones awaiting cholecystectomy.
Hepatology. 43:1276–1283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lioudaki E, Ganotakis ES and Mikhailidis
DP: Lipid lowering drugs and gallstones: a therapeutic option? Curr
Pharm Des. 17:3622–3631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JK, Cho SM, Kang SH, Kim E, Yi H, Yun
ES, Lee DG, Cho HJ, Paik YH, Choi YK, Haam SJ, Shin HC and Lee DK:
N-3 polyunsaturated fatty acid attenuates cholesterol gallstones by
suppressing mucin production with a high cholesterol diet in mice.
J Gastroenterol Hepatol. 27:1745–1751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krauss RM, Eckel RH, Howard B, Appel LJ,
Daniels SR, Deckelbaum RJ, Erdman JW Jr, Kris-Etherton P, Goldberg
IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K,
Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL and Bazzarre TL:
AHA Dietary Guidelines: revision 2000: A statement for healthcare
professionals from the Nutrition Committee of the American Heart
Association. Circulation. 102:2284–2299. 2000. View Article : Google Scholar
|
|
22
|
Kris-Etherton PM, Harris WS and Appel LJ;
American Heart Association. Nutrition Committee. Fish consumption,
fish oil, omega-3 fatty acids, and cardiovascular disease.
Circulation. 106:2747–2757. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Magnuson TH, Lillemoe KD, High RC and Pitt
HA: Dietary fish oil inhibits cholesterol monohydrate crystal
nucleation and gallstone formation in the prairie dog. Surgery.
118:517–523. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scobey MW, Johnson FL, Parks JS and Rudel
LL: Dietary fish oil effects on biliary lipid secretion and
cholesterol gallstone formation in the African green monkey.
Hepatology. 14:679–684. 1991.PubMed/NCBI
|
|
25
|
Mizuguchi K, Yano T, Kawano H, Abei M and
Tanaka N: Preventive effects of eicosapentaenoic acid (EPA) on
cholesterol gallstone formation in hamsters. Nihon Yakurigaku
Zasshi. 110(Suppl 1): 50P–55P. 1997.(In Japanese).
|
|
26
|
Womack NA: The development of gallstones.
Surg Gynecol Obstet. 133:937–945. 1971.
|
|
27
|
Janowitz P, Swobodnik W, Wechsler JG,
Janowitz A, Saal D and Ditschuneit H: Fish oil, enriched with
polyunsaturated fatty acids of the omega-3-type accelerates the
nucleation time in healthy subjects. Klin Wochenschr. 69:289–293.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weber LW, Boll M and Stampfl A:
Maintaining cholesterol homeostasis: sterol regulatory
element-binding proteins. World J Gastroenterol. 10:3081–3087.
2004.PubMed/NCBI
|
|
29
|
Trapani L, Segatto M, Simeoni V, Balducci
V, Dhawan A, Parmar VS, Prasad AK, Saso L, Incerpi S and Pallottini
V: Short- and long-term regulation of 3-hydroxy 3-methylglutaryl
coenzyme A reductase by a 4-methylcoumarin. Biochimie.
93:1165–1171. 2011. View Article : Google Scholar : PubMed/NCBI
|