Introduction
Several studies have demonstrated that flavonoids
are preventive in cancer (1,2). The
most common nonmutagenic flavonoid, 4′,5,7-trihydroxyflavone
(apigenin), has demonstrated marked effects in inhibiting cancer
cell growth in vitro and in vivo (2–4).
Apigenin has also been found to possess anti-inflammatory and
antioxidant properties (5,6) and inhibit tumor cell invasion,
metastasis (7), mitogen-activated
protein kinases and downstream oncogenes (8). Our previous study demonstrated that
apigenin was able to affect the number of glioma stem-like cells
(GSLCs) derived from U251 cells (9) However, the effect of apigenin on the
self-renewal capacity of cervical cancer stem-like cells (CCSLCs)
remains to be elucidated.
The protein kinase casein kinase 2 (CK2) is a highly
conserved serine/threonine kinase with a broad spectrum of
substrates. CK2 is a multifunctional protein kinase that has been
demonstrated to be involved in almost every aspect of cell
proliferation and survival (10–13).
The expression and activity of CK2 are frequently elevated in
cancer cells, including cervical cancer (14,15).
Previous studies by Zhang et al demonstrated that the
function of CK2α is involved in the activation of Hedgehog (Hh) and
Notch pathways and in the maintenance of cancer stem cell
properties (16,17). Whether or not targeting CK2 by the
selective CK2 kinase inhibitor apigenin leads to self-renewal
inhibition of cervical cancer stem-like cells remains to be
elucidated.
The present study was performed to examine whether
apigenin inhinited the self-reneweal capactiy of sphere-forming
cells (SFCs) of the cervical cancer HeLA cell line and its
underlying mechanisms, which aimed to assess the possibility for
its use in the treatment of human cervical cancer by targeting
cancer stem cells.
Materials and methods
Reagents
Apigenin was obtained from Sigma (St. Louis, MO,
USA) and was dissolved in dimethyl sulfoxide to a final
concentration of 0.1% in media without causing cytotoxicity.
Anti-β-actin and CK2α antibodies were obtained from Calbiochem (La
Jolla, CA, USA).
Cell culture
The HeLa human cervical cell line was maintained in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carslbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco), 100 units/ml
penicillin (Gibco) and 100 μg/ml streptomycin (Gibco). In all
experiments, the cells were maintained at 37°C, in a 5%
CO2 and 95% air atmosphere. All the experiments were
performed on cultures that were 70% confluent. The present study
was approved by the ethics committee of Hunan Normal University
(Changsha, China).
Tumorsphere culture
Single cell suspensions were suspended at a density
of 5,000 cells/ml in serum-free DMEM/F12 supplemented with 100
IU/ml penicillin, 100 μg/ml streptomycin, 20 ng/ml human
recombinant epidermal growth factor, 10 ng/ml human recombinant
basic fibroblast growth factor, 2% B27 supplement without vitamin A
and 1% N2 supplement (Invitrogen Life Technologies, Carlsbad, CA,
USA) and seeded into ultra low attachment 6-well plates (Corning
Inc., Corning, NY, USA). Suspension cultures were continued for 6
days until tumorspheres were formed. In order to propagate spheres
in vitro, the sphere cells were collected by centrifugation
(1,000 g for 5 min), dissociated into single-cell suspensions and
cultured to permit the regeneration of spheres. Third-generation
spheres were used for all subsequent experiments.
To investigate the percentage of single cells
capable of regenerating new spheres, cells were plated at a density
of 1,000 cells/ml in a 6-well plate in order to obtain new spheres.
The total number of tumor spheres was counted after 6 days of
culture. Sphere formation efficiency was calculated using the
following formula: (Total number of spheres formed / total number
of live cells seeded) × 100.
Limiting dilution analysis
The third-generation spheres were dissociated, as
described above, and 100 cells were plated in 150 μl of growth
medium in a 96-well culture plate to obtain a single cell per well.
Growth medium (20 μl) was added to each well every 3 days. The
number of clonal tumor spheres in each 96-well culture plate was
evaluated after 6 days of culture.
MTT assay
SFCs from the HeLa cell line and the parental cells
were seeded in 96-well plates precoated with Matrigel (Gibco-BRL)
at a density of 5,000 cells per well. Cells were exposed to
increasing concentrations (10, 20 and 40 μmol/l) of apigenin. After
48 h, MTT reagent (Sigma) was added to each well according to the
manufacturer’s instructions. Absorbance was measured at 570 nm
using an automated microplate reader (Bio-RAD 550; Bio-Rad,
Hercules, CA, USA).
Overexpression of the CK2α protein
The pcDNA3.1-CK2α or control pcDNA3.1-LacZ plasmid
vectors were transfected into HeLa cells or the SFCs of HeLa cells
(0.5 μg/ml in a 24-well plate) using Lipofectamine 2000
transfection reagent (Invitrogen Life Technologies), according to
the manufacturer’s instructions. The cells were resuspended in
complete medium (DMEM supplemented with 10% fetal bovine serum;
Gibo-BRL) for 48 h. The cells were harvested and western blotting
was performed using mouse monoclonal antibodies against βactin and
CK2α.
Western blot analysis
Western blot analysis was performed, as previously
described (18). Cells were lysed
by incubating in lysis buffer for 20 min at 4°C. The protein
concentration was determined using the Bio-Rad assay system
(Bio-Rad, Hercules, CA, USA). Total proteins were fractionated
using SDS-PAGE and transferred onto a polyvinylidene fluoride
membrane (Millipore, Billerica, MA, USA). Signals were detected
using an ECL Advance western blot analysis system (Amersham
Pharmacia Biotech, Inc., Piscataway, NJ, USA).
Statistical analysis
Statistical analysis and database management was
performed using SPSS (version 15.0) software (SPSS, Inc., Chicago,
IL, USA). Data are expressed as the mean ± standard deviation.
Multiple group comparisons were made using one-way analysis of
variance and pairwise comparison was performed using the least
squares difference method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sphere formation and self-renewal in the
HeLa cell line
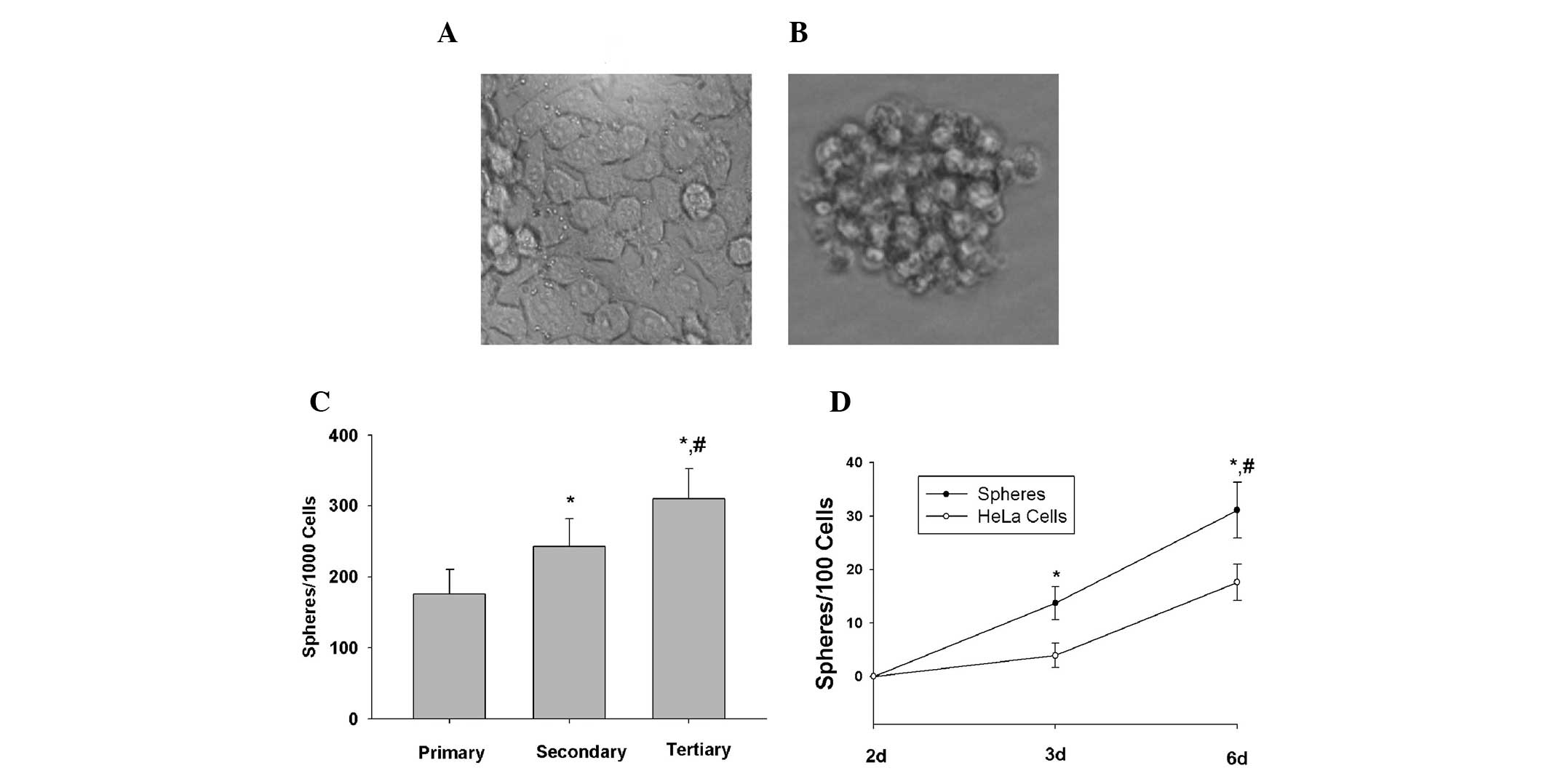
HeLa cells were plated in stem cell-conditioned
culture medium in 6-well plates at a density of 10,000 cells/well,
which enabled the formation of discrete colonies. Under these
conditions, cells grew as non-adherent, three-dimensional sphere
clusters. The HeLa cella maintained in DMEM medium supplemented
with 10% fetal bovine serum (monolayer adhere growth cells) and
anchorage-independent spheres formed in the HeLa cell line are
shown in Fig. 1A and Fig 1B, respectively. The spheres were
passaged after 6 days once they had reached ~50 μm in diameter. The
HeLa sphere-derived cells were serially passaged for >12
generations, indicating that HeLa sphere-derived cells were fully
capable of self-renewal in vitro.
To determine the percentage of CCSLCs in HeLa
third-generation spheres, a limiting dilution assay was used to
examine the ability of single cells from third-generation spheres
to produce new spheres. After 6 days of culture, 31% of the single
cells had generated new spheres (Fig.
1C). By contrast, a lower percentage of single cells derived
from the HeLa cells could regenerate spheres when compared with the
single cells derived from third-generation spheres (Fig. 1D). These results demonstrated that
a considerable percentage of single cells derived from
third-generation spheres were self-renewing cells that were able to
be expanded and maintained in culture as tumor spheres.
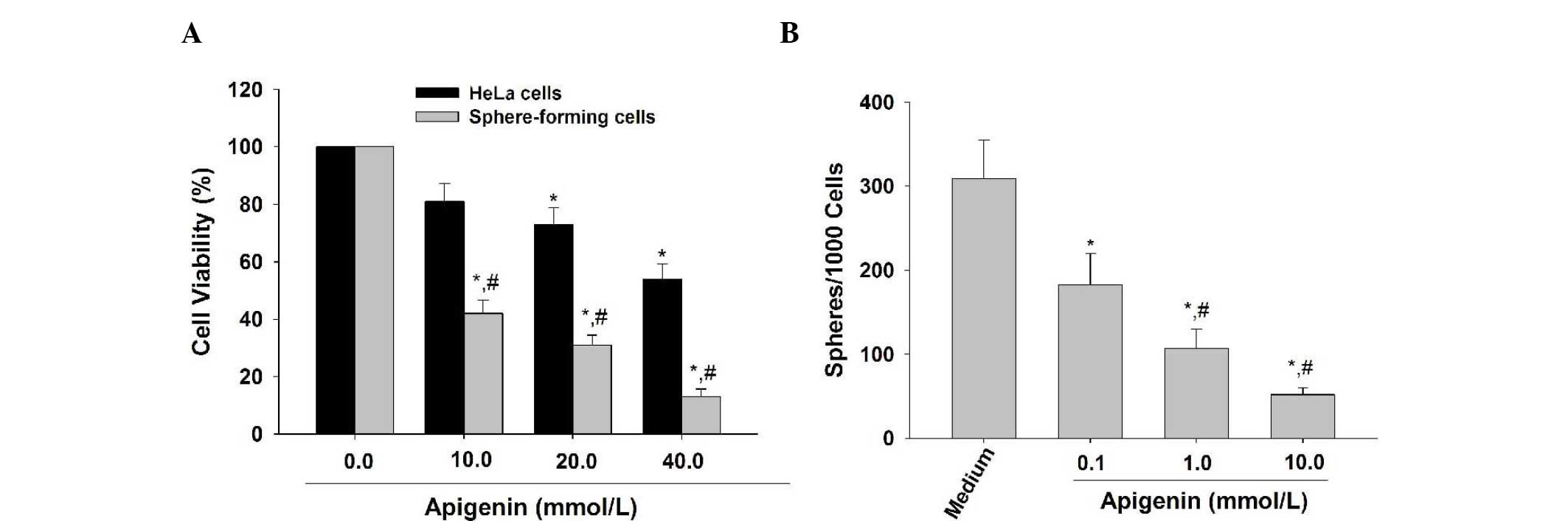
Apigenin inhibits the proliferation and
self-renewal capacity of the HeLa sphere-derived cells
It has been reported that cancer stem cells have the
characteristics of extensive proliferation and apigenin has been
demonstrated to inhibit the proliferative activity of glioma cancer
stem-like cells (9). In the
present study, the MTT results demonstrated that apigenin (10, 20
and 40 μmol/l) preferentially inhibited the proliferation of SFCs
derived from HeLa cells (Fig. 2A),
suggesting that apigenin is able to preferentially suppress the
proliferative ability of CCSLCs.
Apigenin (10, 20 and 40 μmol/l) reduced the number
of spheroids formed in the SFCs of HeLa cells in a
concentration-dependent manner (Fig.
2B). These results suggest that apigenin can suppress the
self-renewal capacity of CCSLCs.
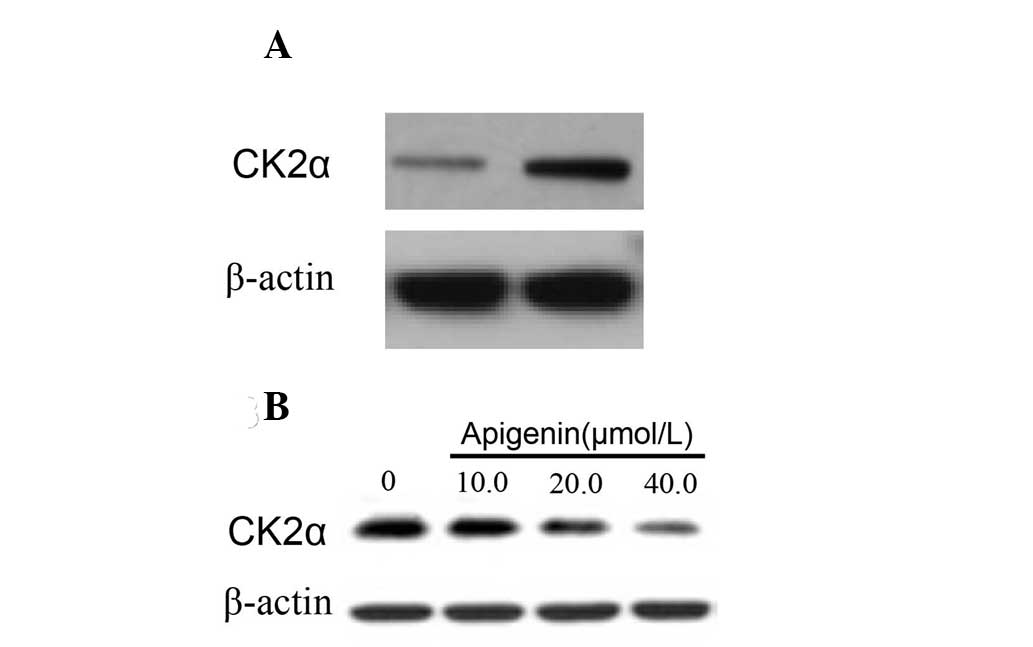
Apigenin downregulates the protein
expression of CK2α in HeLa sphere-derived cells
Previous studies have reported that CK2α is involved
in the activation of the Hh and Notch pathways and is associated
with the maintenance of cancer stem cell properties (16,17).
The present study aimed to compare the status of CK2α protein
expression in parental cells and in SFCs. The results demonstrated
that the expression of CK2α was higher in the SFCs than in the
parental cells (Fig. 3A). In
addition, CK2α expression in SFCs was downregulated by apigenin
(Fig. 3B).
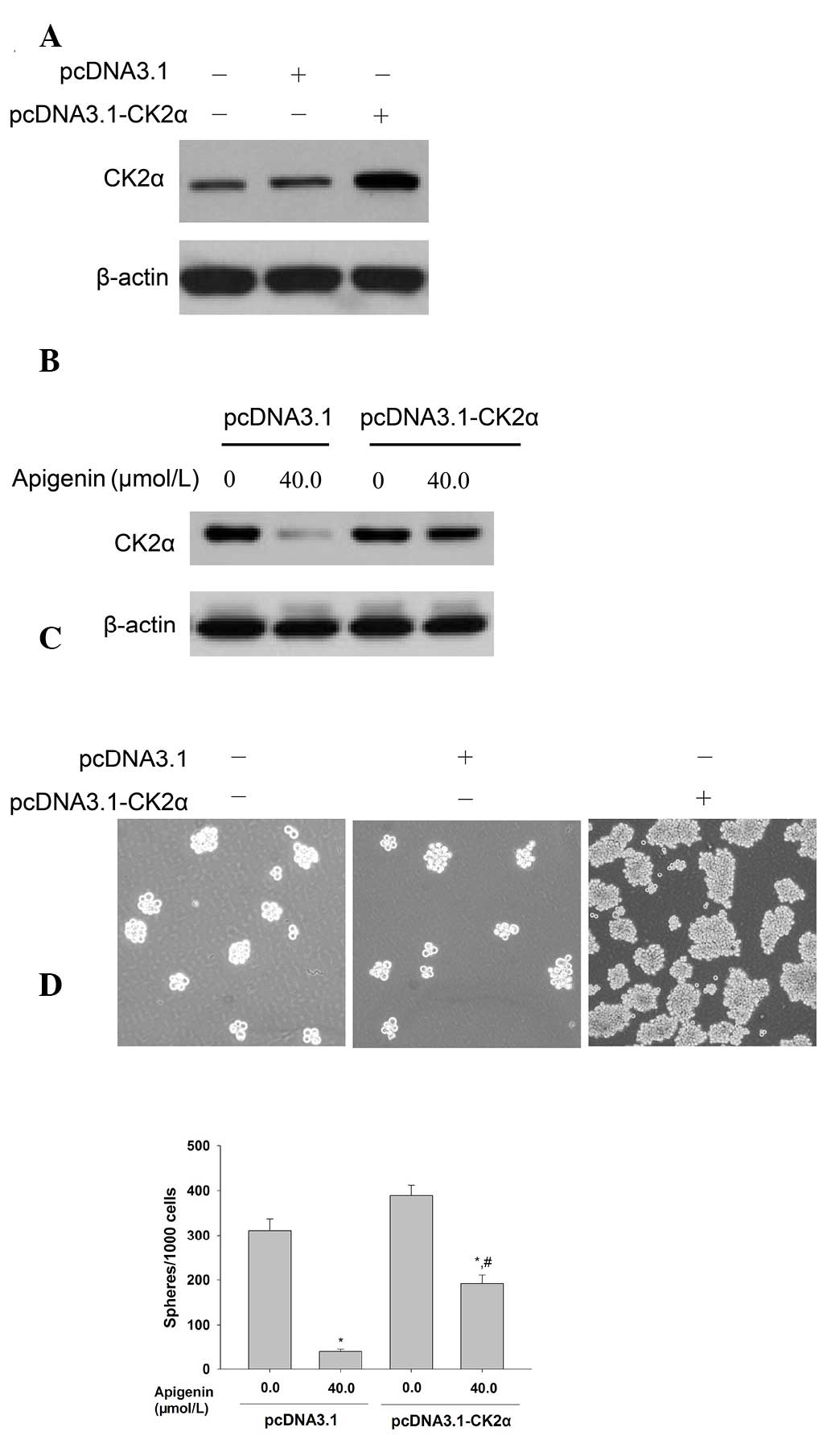
Overexpression of CK2α attenuates the
inhibitory effects of apigenin on the self-renewal capacity of HeLa
sphere-derived cells
Western blot analysis demonstrated that upregulation
of CK2α by pcDNA3.1-CK2α transfection resulted in overexpression of
the CK2α protein in the parental HeLa cells (Fig. 4A). The results from the tumorsphere
formation assay revealed that overexpression of CK2α attenuated
apigenin-induced downregulation of CK2α protein expression in the
HeLa SFCs (Fig. 4B) and also
revealed that overexpression of the CK2α protein increased the
self-renewal capacity of HeLa cells (Fig. 4C). In addition, . It also partially
reduced the inhibition of self-renewal of SFCs by apigenin
(Fig. 4D). These results provided
mechanistic evidence that apigenin-inhibited self-renewal was, in
part, due to inactivation of CK2α in SFCs derived from HeLa
cells.
Discussion
The present study demonstrated that a natural
dietary flavonoid, apigenin, inhibited the proliferation and
self-renewal capacity of HeLa sphere-derived cells and inhibited
the protein expression of CK2α. The inhibition of the CK2-mediated
self-renewal capacity in HeLa sphere-derived cells by apigenin may
be the primary mechanism mediating the anticancer activities of
apigenin.
Previous studies have reported that the function of
CK2α is involved in the activation of Hh and Notch pathways and in
the maintenance of cancer stem cell properties (16,17).
In order to investigate the potential mechanism through which CK2
positively regulates the self-renewal capacity of CCSLSCs, the
present study performed a tumorsphere formation assay in the
parental HeLa cells following transfection with pcDNA3.1-CK2α. The
results indicated that forced overexpression of CK2α resulted in an
increase in sphere formation rate in HeLa cells. Furthermore,
apigenin, a small molecule CK2α inhibitor, significantly inhibited
sphere formation of HeLa sphere-derived cells. Taken together,
these results suggest that CK2α is important in facilitating the
self-renewal capacity of CCSLCs. Further investigation is required
to elucidate the precise mechanisms.
Zhao et al (19) found that apigenin inhibits
proliferation and induces apoptosis in human multiple myeloma cells
through the regulation of CK2, Cdc37 and Hsp90 expression.
Feliciano et al (20)
indicated that miR-125b is inversely correlated with glutamyl
aminopeptidase and CK2α expression in breast tumorigenesis and that
CK2α overexpression is associated with the presence and number of
lymph node metastases. Our previous study demonstrated that
apigenin preferentially inhibited the proliferation of GSLCs
derived from U251 cells (9). The
results of the present study demonstrated that apigenin
significantly inhibited the proliferation and tumorsphere formation
of HeLa sphere-derived cells. Furthermore, forced overexpression of
CK2α effectively attenuated the apigenin inhibited tumorsphere
formation of HeLa sphere-derived cells. These results suggested
that apigenin-inhibited self-renewal is partly due to the
inactivation of CK2α in SFCs derived from HeLa cells.
In conclusion, the present study demonstrated that
CK2 is a positive regulator in the self-renewal of CCSLCs and
apigenin, inhibiting the self-renewal capability, is involved in
the downregulation of CK2α protein expression. The findings of the
present study provide important evidence for the potential benefits
of CK2 inhibitors in the treatment of human cervical cancer by
targeting cancer stem cells.
Acknowledgements
The authors would like to thank Professor Jian-Guo
Cao and Dr Xi-Yun Deng, Medical College, Hunan Normal University,
(Changsha, Hunan, China) for their input into the scientific
content of this study. The present study was supported by the
Construct Program of the Key Discipline of Basic Medicine in
Hunan.
References
|
1
|
Birt DF, Hendrich S and Wang W: Dietary
agents in cancer prevention: flavonoids and isoflavonoids.
Pharmacol Ther. 90:157–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benavente-García O and Castillo J: Update
on uses and properties of citrus flavonoids: new findings in
anticancer, cardiovascular, and anti-inflammatory activity. J Agric
Food Chem. 56:6185–6205. 2008.PubMed/NCBI
|
|
3
|
Arango D, Morohashi K, Yilmaz A, Kuramochi
K, Parihar A, Brahimaj B, Grotewold E and Doseff AI: Molecular
basis for the action of a dietary flavonoid revealed by the
comprehensive identification of apigenin human targets. Proc Natl
Acad Sci USA. 110:e2153–e2162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oishi M, Iizumi Y, Taniguchi T, Goi W,
Miki T and Sakai T: Apigenin sensitizes prostate cancer cells to
Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS
One. 8:e559222013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pham H, Chen M, Takahashi H, King J, Reber
HA, Hines OJ, Pandol S and Eibl G: Apigenin inhibits NNK-induced
focal adhesion kinase activation in pancreatic cancer cells.
Pancreas. 41:1306–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HK, Cheon BS, Kim YH, Kim SY and Kim
HP: Effects of naturally occurring flavonoids on nitric oxide
production in the macrophage cell line RAW 264.7 and their
structure-activity relationships. Biochem Pharmacol. 58:759–765.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raso GM, Meli R, Di Carlo G, Pacilio M and
Di Carlo R: Inhibition of inducible nitric oxide synthase and
cyclooxygenase-2 expression by flavonoids in macrophage J774A.1.
Life Sci. 68:921–931. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindenmeyer F, Li H, Menashi S, Soria C
and Lu H: Apigenin acts on the tumor cell invasion process and
regulates protease production. Nutr Cancer. 39:139–147. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He J, Xu Q, Wang M, Li C, Qian X, Shi Z,
Liu LZ and Jiang BH: Oral administration of apigenin inhibits
metastasis through AKT/P70S6K1/MMP-9 pathway in orthotopic ovarian
tumor model. Int J Mol Sci. 13:7271–7282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng X, Zhou Q, Liu C and Tao ML: Drug
screening study using glioma stem-like cells. Mol Med Rep.
6:1117–1120. 2012.PubMed/NCBI
|
|
11
|
Litchfield DW: Protein kinase CK2:
structure, regulation and role in cellular decisions of life and
death. Biochem J. 369:1–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trembley JH, Wang G, Unger G, Slaton J and
Ahmed K: Protein kinase CK2 in health and disease: CK2: a key
player in cancer biology. Cell Mol Life Sci. 66:1858–1867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmad KA, Wang G, Unger G, Slaton J and
Ahmed K: Protein kinase CK2 - a key suppressor of apoptosis. Adv
Enzyme Regul. 48:179–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piazza FA, Ruzzene M, Gurrieri C, Montini
B, Bonanni L, Chioetto G, Di Maira G, Barbon F, Cabrelle A,
Zambello R, Adami F, Trentin L, Pinna LA and Semenzato G: Multiple
myeloma cell survival relies on high activity of protein kinase
CK2. Blood. 108:1698–1707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Solares AM, Santana A, Baladrón I,
Valenzuela C, González CA, Díaz A, Castillo D, Ramos T, Gómez R,
Alonso DF, Herrera L, Sigman H, Perea SE, Acevedo BE and
López-Saura P: Safety and preliminary efficacy data of a novel
casein kinase 2 (CK2) peptide inhibitor administered
intralesionally at four dose levels in patients with cervical
malignancies. BMC Cancer. 9:1462009. View Article : Google Scholar
|
|
16
|
Perea SE, Baladron I, Garcia Y, Perera Y,
Lopez A, Soriano JL, Batista N, Palau A, Hernández I, Farina H,
Garcia I, Gonzalez L, Gil J, Rodriguez A, Solares M, Santana A,
Cruz M, Lopez M, Valenzuela C, Reyes O, López-Saura PA, González
CA, Diaz A, Castellanos L, Sanchez A, Betancourt L, Besada V,
González LJ, Garay H, Gómez R, Gómez DE, Alonso DF, Perrin P,
Renualt JY, Sigman H, Herrera L and Acevedo B: CIGB-300, a
synthetic peptide-based drug that targets the CK2 phosphoaceptor
domain. Translational and clinical research. Mol Cell Biochem.
356:45–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang S, Long H, Yang YL, Wang Y, Hsieh D,
Li W, Au A, Stoppler HJ, Xu Z, Jablons DM and You L: Inhibition of
CK2α down-regulates Notch1 signalling in lung cancer cells. J Cell
Mol Med. 17:854–862. 2013.
|
|
18
|
Zhang S, Wang Y, Mao JH, Hsieh D, Kim IJ,
Hu LM, Xu Z, Long H, Jablons DM and You L: Inhibition of CK2α
down-regulates Hedgehog/Gli signaling leading to a reduction of a
stem-like side population in human lung cancer cells. PLoS One.
7:e389962012.
|
|
19
|
Ren KQ, Cao XZ, Liu ZH, Guo H, Quan MF,
Liu F, Jiang L, Xiang HL, Deng XY and Cao JG:
8-bromo-5-hydroxy-7-methoxychrysin targeting for inhibition of the
properties of liver cancer stem cells by modulation of Twist
signaling. Int J Oncol. 43:1719–1729. 2013.PubMed/NCBI
|
|
20
|
Zhao M, Ma J, Zhu HY, Zhang XH, Du ZY, Xu
YJ and Yu XD: Apigenin inhibits proliferation and induces apoptosis
in human multiple myeloma cells through targeting the trinity of
CK2, Cdc37 and Hsp90. Mol Cancer. 10:1042011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feliciano A, Castellvi J, Artero-Castro A,
Leal JA, Romagosa C, Hernández-Losa J, Peg V, Fabra A, Vidal F,
Kondoh HY, Cajal S and Lleonart ME: miR-125b acts as a tumor
suppressor in breast tumorigenesis via its novel direct targets
ENPEP, CK2-α, CCNJ and MEGF9. PLoS One. 8:e762472013.PubMed/NCBI
|