Introduction
A glioma is a type of malignant brain tumor that
mainly arises from glial cells. The most common site of gliomas is
in the brain (1). Gliomas
represent ~30% of tumors of the brain and central nervous system
and 80% of malignant tumors in the brain (2). The main types of glioma are
ependymomas, astrocytic gliomas, oligodendrogliomas, brainstem
gliomas, optic nerve gliomas and mixed gliomas (3,4). At
present, due to the characteristics of glioma, including a rapid
growth rate, unclear boundaries and frequent relapse following
surgery, it remains one of the most intractable diseases in the
field of neurosurgery (5).
Therefore, it is particularly important to identify and develop new
and effective treatment methods for glioma. Investigation of the
targets of key molecules in the tumor signal transduction system
has provided new hope for patients with gliomas.
The Notch signaling pathway is well known and is
highly conserved in the majority of multicellular organisms
(6). It has been confirmed that
the occurrence of a variety of diseases, including cardiovascular
disease and cancer, is closely associated with the abnormal
activation of the Notch signaling pathway (7,8). The
abnormal activation of Notch is found in several types of cancer,
including lung cancer(9), colon
cancer (10), cervical cancer
(11) and pancreatic cancer
(12). Notch 2 is expressed in
normal neuroblasts and controls neuronal differentiation (13). It is also one of the receptors with
a single-pass transmembrane receptor protein (13). Notch signaling enhances the
proliferative effects during neurogenesis in mammals (14,15).
The present study aimed to clarify the effect of the
Notch 2 protein on the proliferation of glioma cells. Initially,
specimens from glioma tissues and normal brain tissues were
obtained and the expression of Notch 2 was compared between them.
Subsequently, RNA interference technology was used to knock down
the expression of the Notch 2 protein in the human glioma cell line
U251. Cell proliferation and cell cycle arrest were detected using
an MTT assay and fluorescence-activated cell sorting (FACS).
Additionally, proteins associated with the cell cycle and cell
apoptosis were detected and compared using western blot analysis.
Therefore, the present study aimed to provide new information to
assist in the therapy of human gliomas.
Materials and methods
Specimens
A total of 32 glioma tumor samples and 20 normal
tissue samples were obtained by surgical resection of traumatized
brain tissue following traumatic brain injury. All the specimens
were obtained from the Institute of Neurosurgery, Nanfang Hospital
Affiliated to Southern Medical University (Guangzhou, China). All
the experiments were performed on patients in compliance with the
Helsinki Declaration and study approval was obtained from the
Ethics Committee of Nanfang Hospital Affiliated to Southern Medical
University. The patients and their families were well informed of
the details and written informed consent was obtained prior to the
study.
Immunohistochemical staining
The specimens, obtained from the glioma tumor and
normal brain tissues, were fixed in neutral buffered
paraformaldehyde and processed for hematoxylin and eosin staining.
The process of immunohistochemical staining was performed, as
described previously (16,17). The primary antibody used was rabbit
polyclonal anti-Notch 2 (cat no. NB600-879; Novus Biologicals,
Littleton, CO, USA), which was diluted (1:200) for use in the
immunohistochemical analysis.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to detect the Notch 2 mRNA
expression levels in the glioma and normal brain tissues. Firstly,
RNA was reverse transcribed into cDNA and the resulting cDNA was
used as templates for PCR amplification. The primer sequences were
as follows: Upstream, 5′-ATGACTGCCCTAACCACAGG-3′ and downstream,
5′-TGCAGTCATCTCCACTCCAG-3′ for Notch 2; upstream,
5′-AGAGCTACGAGCTGCCTGAC-3′ and downstream,
5′-AGCACTGTGTTGGCGTACAG-3′ for β-actin. Here, β-actin was used as
the internal control. A PCR kit (Invitrogen Life Technologies) was
used and the cycle conditions were an initial step (hot start) of
95°C for 10 min prior to amplification cycles, followed by the PCR
conditions of denaturation (95°C for 15 sec), annealing (59°C for
20 sec) and elongation (72°C for 15 sec) for a total of 25
cycles.
Cell line and short hairpin (sh)RNA
The human glioma cell line U251 was cultured in
Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum
(Hyclone Laboratories, Inc., Logan, UT, USA), penicillin and
streptomycin (100 U/ml; Gibco-BRL, Carlsbad, CA, USA) at 37°C in 5%
CO2. Notch 2 shRNA (human) was obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA; cat no. sc-40135-V) and
the control shRNA of Lentiviral Particles-A was obtained from Santa
Cruz Biotechnology, Inc.; cat no. sc-108080).
Western blot analysis
The U251 cells were seeded into 48-well plates.
After 3 days, the whole-cell extracts were prepared and the
proteins were separated by PAGE, as previously described (18–20).
MTT assay
An MTT assay was performed, as described previously
(21–23). Human glioma U251 cells
(1×105) were seeded into a 48-well plate. Following
culture for different time periods (1–7 days), the plates were read
on a microplate reader (Corning, Inc., Acton, MA, USA) with a test
wavelength of 490 nm.
Flow cytometric analysis
The apoptosis of human glioma U251 cells was
determined using annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) dual staining according to the
manufacturer’s instructions (Santa Cruz Biotechnology, Inc.) and
cell cycle analysis was performed using PI staining, as described
previously (24,25). Cells were then subjected to FACS
analysis and >10,000 events were recorded in every example. The
experiment was performed at least three independent times.
Statistical analysis
Basic statistical analyses were performed using the
statistical software, SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). All
results are expressed as the mean ± standard deviation. P<0.01
was considered to indicate a statistically significant
difference.
Results
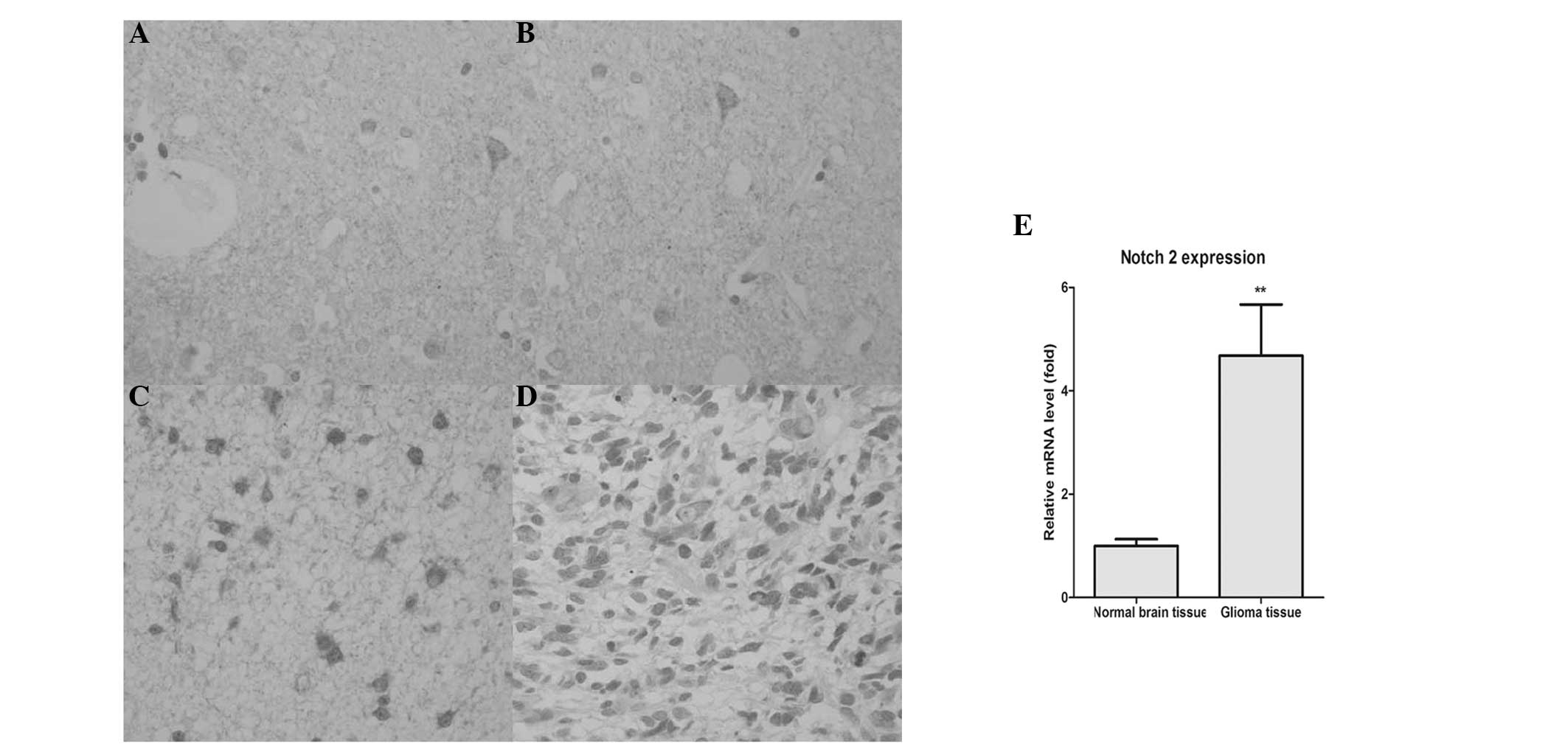
Positive Notch 2 staining is observed in
the majority of gliomas
In order to confirm the expression of Notch 2 in
gliomas and in normal brain tissues, immunohistochemical staining
analysis was performed. As shown in Fig. 1A–D, positive staining for Notch 2
appeared as brown granules, which were predominantly located in the
cell membrane, nucleus and cytoplasm. However, only a small
quantity of visible staining, indicating expression of Notch 2, was
observed in normal brain tissues.
Subsequently, RT-qPCR was performed to further
confirm the expression of Notch 2 in the glioma and normal brain
tissues. As shown in Fig. 1E, the
expression of Notch 2 in the glioma tissues was increased
significantly compared with the normal brain tissues
(P<0.01).
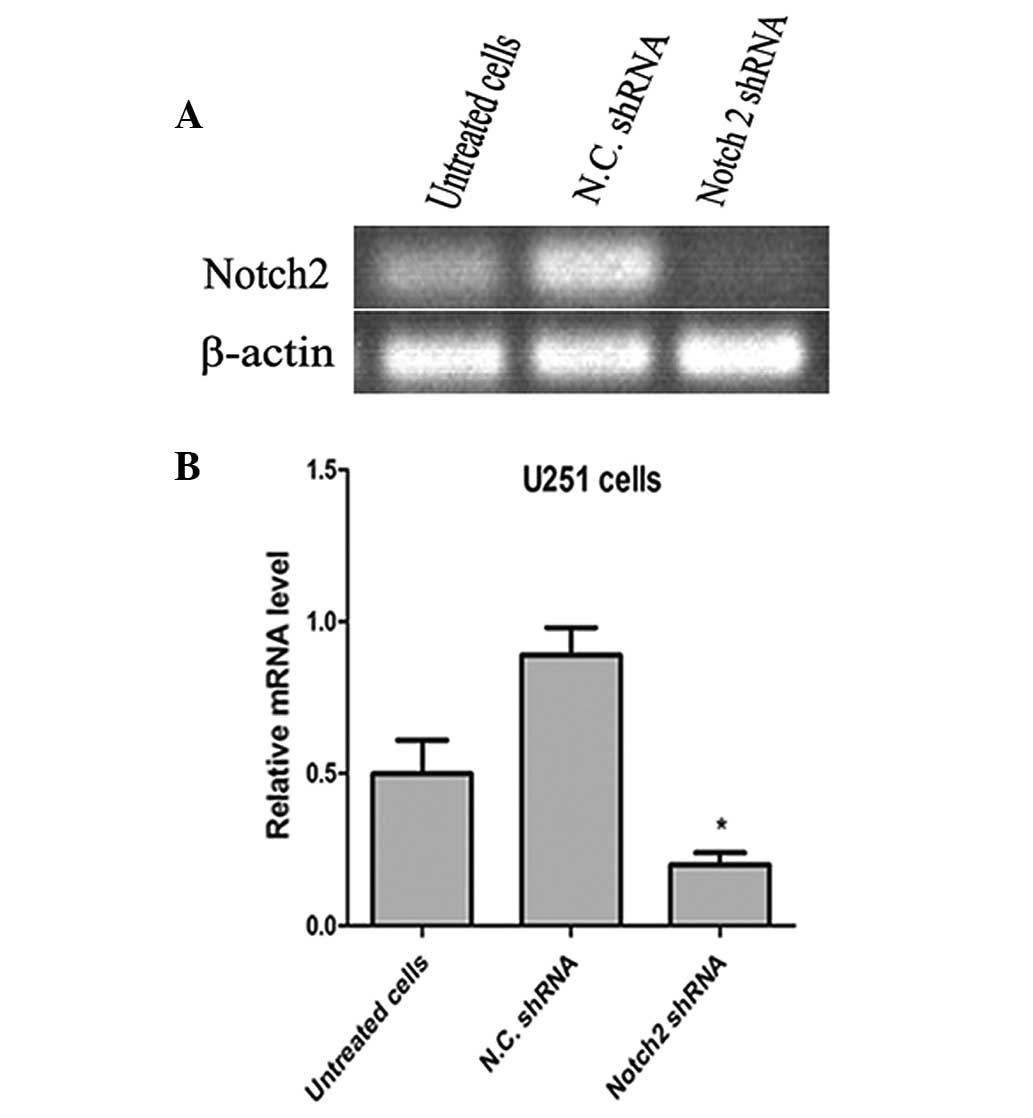
Notch 2 shRNA decreases the expression of
Notch 2 in U251 cells
Notch 2 shRNA was used for interference of
endogenous Notch 2 gene expression. The silencing effect was then
determined using RT-qPCR. As shown in Fig. 2, the Notch 2 shRNA effectively
interfered with the expression of Notch 2. The control shRNA was
used as a negative control.
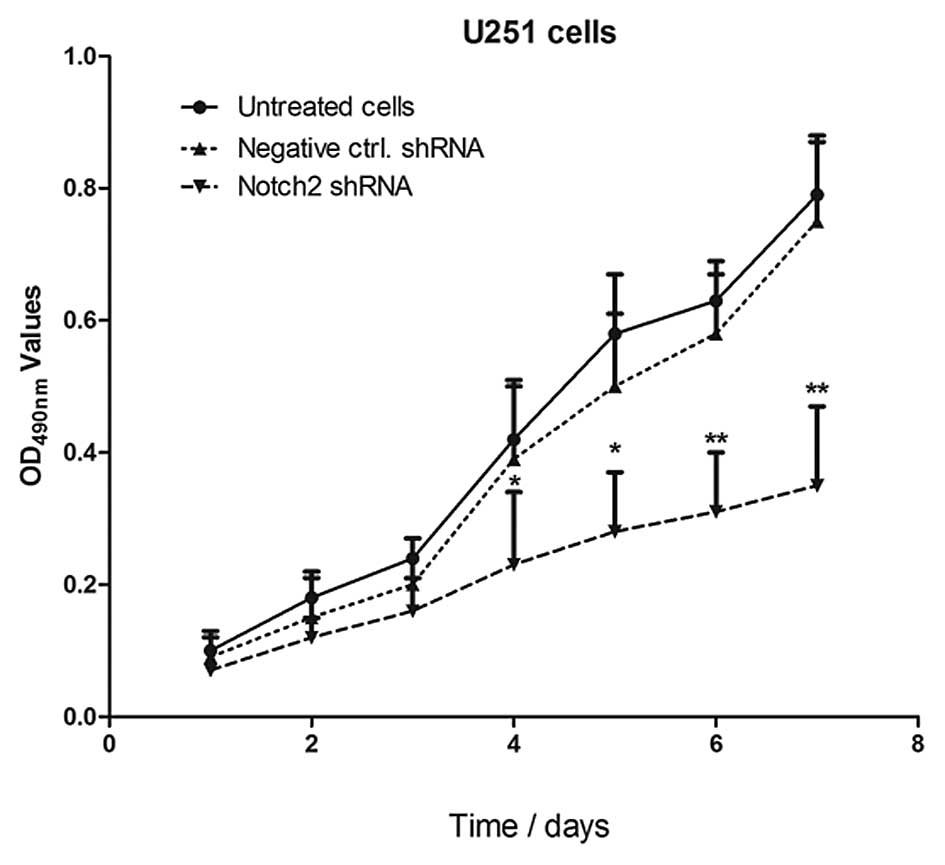
Knocking down the expression of Notch 2
inhibits the growth of the glioma cell line U251
In order to determine whether Notch 2 knockdown
inhibited the growth of U251 cells, an MTT assay was performed. As
shown in Fig. 3, 4 days after
transfection with shRNA, the optical density (OD)490 values in the
U251 cells transfected with Notch 2 shRNA were lower compared with
the control cells (*P<0.05 and
**P<0.01). Additionally, inhibition of U251 cell
proliferation occurred in a time-dependent manner.
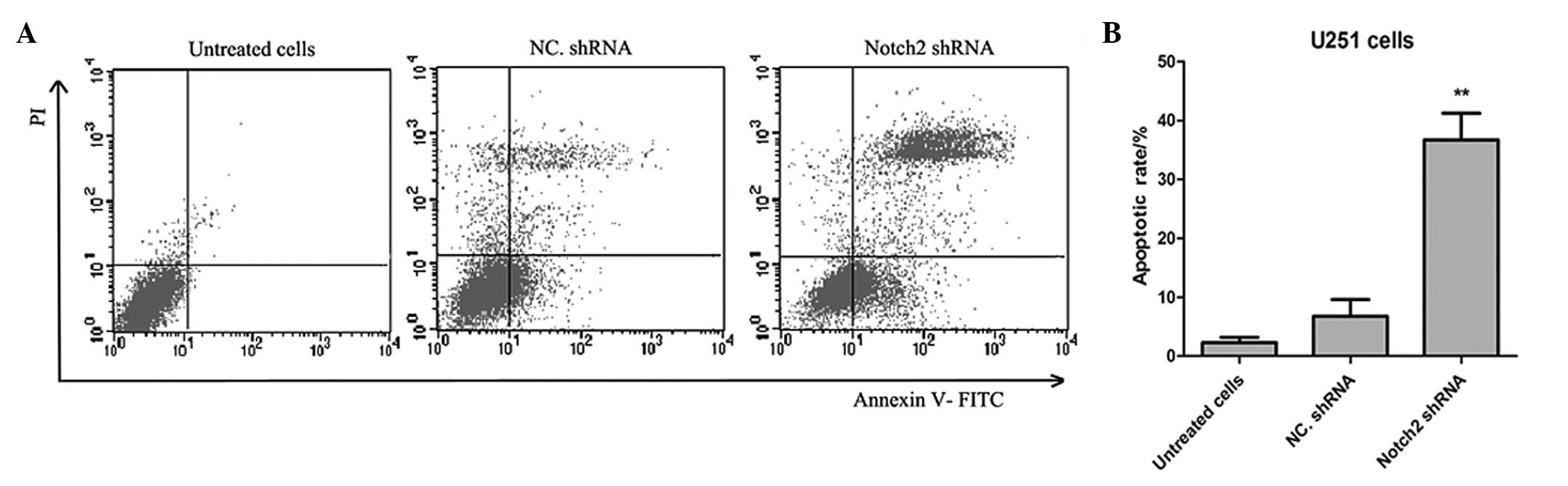
Transfection of Notch 2 shRNA induces the
apoptosis of U251 cells
The present study then aimed to assess whether
treating cancer cells with Notch 2 shRNA further induced apoptosis.
As shown in Fig. 4, Annexin
V-FITC/PI dual staining was used to detect the apoptotic rate of
the U251 cells. After 5 days, the results demonstrated that the
apoptotic rate of the cells transfected with Notch 2 shRNA was
significantly higher in the Notch 2 shRNA group compared with the
negative control shRNA group (36.7±4.5% and 6.8±2.8%, respectively;
n=5; P=0.0027).
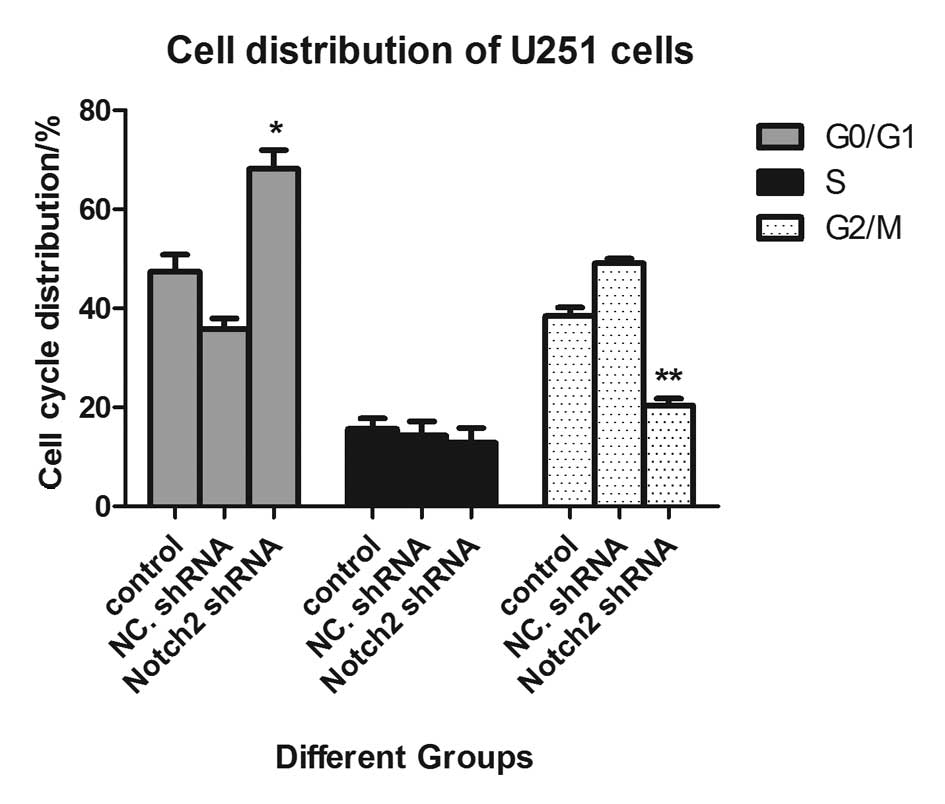
Notch 2 shRNA1 induces cell cycle arrest
at the G0/G1 phase in U251 cells
Subsequently, cell cycle distribution was assessed
in the different groups of cells. As shown in Fig. 5, the cell cycle was arrested at the
G0/G1 phase following transfection with the Notch 2 shRNA compared
with the negative control shRNA. The percentage of cells in the
G0/G1 phase was increased from 47.40 to 68.20%, however, the
percentage of cells in the G2/M phase was decreased from 38.40 to
20.40% (P<0.01).
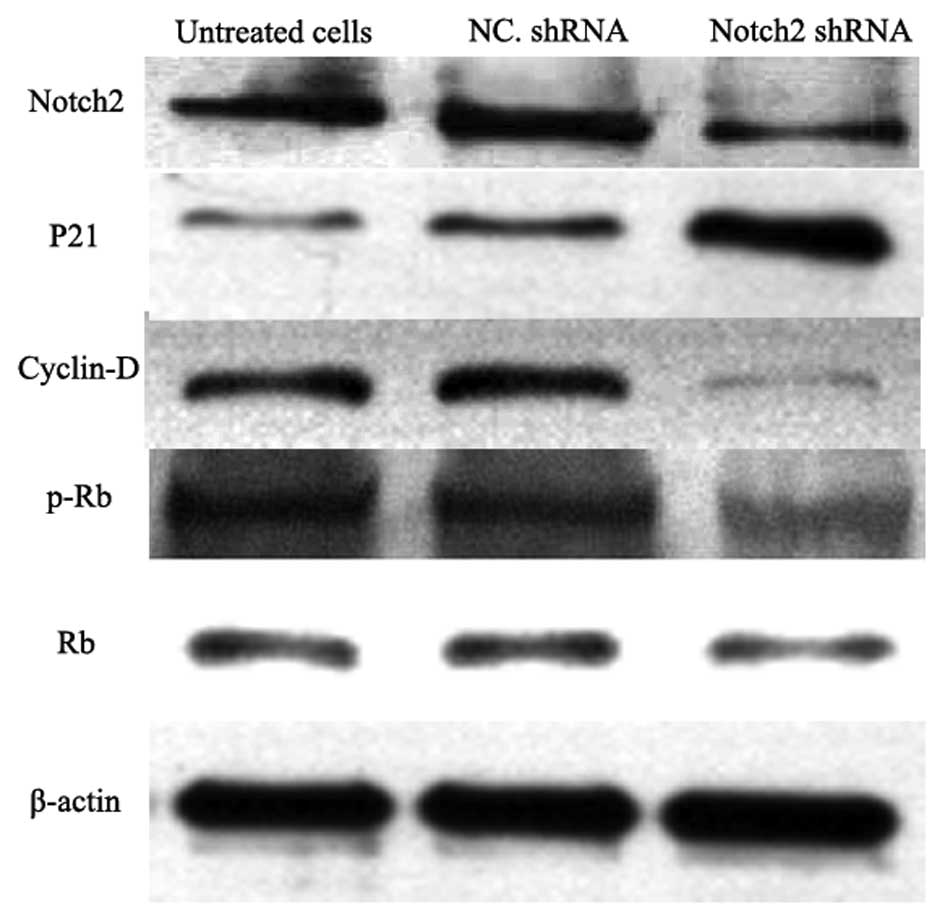
Detection of proteins associated with
cell proliferation, the cell cycle and apoptosis
The expression levels of proteins associated with
cell proliferation, the cell cycle and apoptosis were determined
using western blot analysis. As shown in Fig. 6, the expression of Notch 2 was
effectively suppressed in the Notch 2 shRNA-transfected U251 cells.
In addition, the expression of P21 was also markedly increased
compared with the untreated and negative control shRNA-transfected
U251 cells, however, cyclin D and phosphorylated retinoblastoma
(p-Rb) were significantly inhibited in the Notch 2
shRNA-transfected U251 cells.
Discussion
Gliomas are the most common and malignant type of
tumor in the central nervous system (26). Glioma is particularly difficult to
treat, with high rates of recurrence and low median survival rates.
However, the treatment of gliomas is constantly improving and
combination therapy, surgical and concurrent radiotherapy and
chemotherapy are used. Despite this, the rate of recurrence is
almost 100% (27,28), with little improvement in efficacy
and a poor prognosis. The present study demonstrated that the Notch
2 protein exhibited abnormal and high expression of the Notch 2
protein in human glioma tissues. Following interference of Notch 2,
the proliferation of the glioma cell line U251 was significantly
inhibited, the cell cycle was arrested at the G0/G1 phase and
apoptotic rates were markedly increased. These results suggested
that the Notch 2 protein may be an effective target molecule in the
therapy of gliomas. Additionally, it is key in several important
pathways in tumor development and progression.
Purow et al (8) successfully reduced the expression of
Notch 1 protein in the human glioma cell line U251 using RNA
interference technology. The cells were then implanted into nude
mice, which significantly prolonged the survival rate of the mice
compared with the control group. Several studies have also
demonstrated that activation of the Notch signaling pathway can
stimulate cell proliferation in acute T lymphoblastic leukemia,
breast cancer, renal epithelial tumor with transitional cells and
pancreatic cancer (12,29–33).
In the present study, the effects of depletion of Notch 2 by RNA
interference on glioma cell cycle progression was investigated. The
results revealed that loss of Notch 2 led to cell cycle arrest at
the G0/G1 phase in U251 cells, with a significant decrease in the
proportion of U251 cells in the G2/M phase. In addition, cell cycle
arrest at the G0/G1 phase was accompanied by an accumulation of p21
and a decrease in cyclin D and p-Rb. All these results were
consistent with each other.
In conclusion, the results of the present study
demonstrated that interference of Notch 2 may provide a useful
therapeutic approach in the treatment of human brain tumors and, to
a certain extent, provides theoretical support for the use of gene
therapy in the treatment of human gliomas.
References
|
1
|
Mamelak AN and Jacoby DB: Targeted
delivery of antitumoral therapy to glioma and other malignancies
with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv.
4:175–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar
|
|
3
|
Tobias A, Ahmed A, Moon KS and Lesniak MS:
The art of gene therapy for glioma: a review of the challenging
road to the bedside. J Neurol Neurosurg Psychiatry. 84:213–222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carpentier AF, Auf G and Delattre JY:
CpG-oligonucleotides for cancer immunotherapy: review of the
literature and potential applications in malignant glioma. Front
Biosci. 8:e1151272003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gotz I and Grosu AL: ([18)F]FET-PET
Imaging for treatment and response monitoring of radiation therapy
in malignant glioma patients - a review. Front Oncol.
3:1042013.
|
|
6
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao Q, Lu J, Kaur C, et al: Expression of
Notch-1 receptor and its ligands Jagged-1 and Delta-1 in amoeboid
microglia in postnatal rat brain and murine BV-2 cells. Glia.
56:1224–1237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Purow BW, Haque RM, Noel MW, et al:
Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baumgart A, Mazur PK, Anton M, Rudelius M,
Schwamborn K, Feuchtinger A, et al: Opposing role of Notch1 and
Notch2 in a KrasG12D-driven murine non-small cell lung cancer
model. Oncogene. Feb 10–2014.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Bertrand FE, Angus CW, Partis WJ and
Sigounas G: Developmental pathways in colon cancer: crosstalk
between WNT, BMP, Hedgehog and Notch. Cell Cycle. 11:4344–4351.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Li H, Yang B, Yang F, Zhang LL,
Kong QY, et al: Biological significance and therapeutic implication
of resveratrol-inhibited Wnt, Notch and STAT3 signaling in cervical
cancer cells. Genes Cancer. 5:154–164. 2014.PubMed/NCBI
|
|
12
|
Ristorcelli E and Lombardo D: Targeting
Notch signaling in pancreatic cancer. Expert Opin Ther Targets.
14:541–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brou C, Logeat F, Gupta N, et al: A novel
proteolytic cleavage involved in Notch signaling: the role of the
disintegrin-metalloprotease TACE. Mol Cell. 5:207–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hatakeyama J, Wakamatsu Y, Nagafuchi A,
Kageyama R, Shigemoto R and Shimamura K: Cadherin-based adhesions
in the apical endfoot are required for active Notch signaling to
control neurogenesis in vertebrates. Development. 141:1671–1682.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vilas-Boas F, Fior R, Swedlow JR, Storey
KG and Henrique D: A novel reporter of notch signalling indicates
regulated and random Notch activation during vertebrate
neurogenesis. BMC Biol. 9:582011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saleem M, Maddodi N, Abu Zaid M, et al:
Lupeol inhibits growth of highly aggressive human metastatic
melanoma cells in vitro and in vivo by inducing apoptosis. Clin
Cancer Res. 14:2119–2127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adhami VM, Siddiqui IA, Ahmad N, Gupta S
and Mukhtar H: Oral consumption of green tea polyphenols inhibits
insulin-like growth factor-I-induced signaling in an autochthonous
mouse model of prostate cancer. Cancer Res. 64:8715–8722. 2004.
View Article : Google Scholar
|
|
18
|
Nishitani H, Sugimoto N, Roukos V, et al:
Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1
for proteolysis. EMBO J. 25:1126–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng L, Xu Z, Zhou Y, Yang T, Liang ZQ and
Zhang M: Effect of rosiglitazone on cells cycle, apoptosis and
expression of Skp2 and p27Kip1 in hepatocellular carcinoma cell
line. Zhonghua Gan Zang Bing Za Zhi. 18:148–149. 2010.(In
Chinese).
|
|
20
|
Schulman BA, Carrano AC, Jeffrey PD, et
al: Insights into SCF ubiquitin ligases from the structure of the
Skp1-Skp2 complex. Nature. 408:381–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Huang W, Huang S, Du J and Huang C:
Screening of anti-cancer agent using zebrafish: comparison with the
MTT assay. Biochem Biophys Res Commun. 422:85–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarzaeem A, Zare Mirakabadi A, Moradhaseli
S, Morovvati H and Lotfi M: Cytotoxic effect of ICD-85
(venom-derived peptides) on HeLa cancer cell line and normal LK
cells using MTT assay. Arch Iran Med. 15:696–701. 2012.PubMed/NCBI
|
|
23
|
Sylvester PW: Optimization of the
tetrazolium dye (MTT) colorimetric assay for cellular growth and
viability. Methods Mol Biol. 716:157–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo LD, Chen XJ, Hu YH, Yu ZJ, Wang D and
Liu JZ: Curcumin inhibits proliferation and induces apoptosis of
human colorectal cancer cells by activating the mitochondria
apoptotic pathway. Phytother Res. 27:422–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang CJ, Gu LG and Yu HT: Antagonism of
baicalin on cell cyclical distribution and cell apoptosis in A549
cells infected with influenza A (H1N1) virus. Bing Du Xue Bao.
27:108–116. 2011.(In Chinese).
|
|
26
|
You G, Yan W, Zhang W, Li S, Li G and
Jiang T: Isolated angiitis of the central nervous system with
tumor-like lesion, mimicking brain malignant glioma: a case report
and review of the literature. World J Surg Oncol. 9:972011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lakomý R, Fadrus P, Slampa P, et al:
Multimodal treatment of glioblastoma multiforme: results of 86
consecutive patients diagnosed in period 2003–2009. Klin Onkol.
24:112–120. 2011.(In Czech).
|
|
28
|
Sharma DN, Goyal SG, Muzumder S, et al:
Radiation therapy in paediatric gliomas: our institutional
experience. Neurol Neurochir Pol. 44:28–34. 2010.PubMed/NCBI
|
|
29
|
Palomero T and Ferrando A: Therapeutic
targeting of NOTCH1 signaling in T-cell acute lymphoblastic
leukemia. Clin Lymphoma Myeloma. 9:S205–S210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zardawi SJ, O’Toole SA, Sutherland RL and
Musgrove EA: Dysregulation of Hedgehog, Wnt and Notch signalling
pathways in breast cancer. Histol Histopathol. 24:385–398.
2009.PubMed/NCBI
|
|
31
|
Sjolund J, Johansson M, Manna S, et al:
Suppression of renal cell carcinoma growth by inhibition of Notch
signaling in vitro and in vivo. J Clin Invest. 118:217–228. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai J, Ma D, Zang S, et al: Cross-talk
between Notch and EGFR signaling in human breast cancer cells.
Cancer Invest. 27:533–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yen WC, Fischer MM, Hynes M, et al:
Anti-DLL4 has broad spectrum activity in pancreatic cancer
dependent on targeting DLL4-Notch signaling in both tumor and
vasculature cells. Clin Cancer Res. 18:5374–5386. 2012. View Article : Google Scholar : PubMed/NCBI
|