Introduction
Exosomes are vesicles of 30–100 nm in length, that
were first identified in the 1980s and are secreted by a wide range
of mammalian cell types, including cancer cells (1). Studies have demonstrated that
tumor-derived exosomes may produce a double-effect in tumor
pathology. One study by Wolfers et al (2) demonstrated that tumor antigens can be
transferred by exosomes from tumor cells to dendritic cells (DCs),
and thus function in antigen cross-presentation. Like DC-derived
exosomes, exosomes from tumor cells carry major histocompatibility
complex molecules along with tumor-specific antigens expressed in
the parental tumor cells, including melan-A/MART1, mesothelin
(3), silv (2) and carcinoembryonic antigen (4), which can be recognized by T cells.
Together, these studies suggest that tumor-derived exosomes may be
a novel candidate for tumor vaccine development. By contrast, a
large number of studies have indicated diverse immunosuppressive
effects of exosomes from tumor cells, including suppression of
effector T cell activity (5),
induction of apoptosis in activated T cells (6) and natural killer cells (7), modulation of differentiation in
myeloid cells (8), and functional
enhancement of regulatory T cells.
CD133 is a marker of cancer stem cells and its
expression is associated with poor prognosis and chemoresistance in
several types of solid tumors, including breast cancer (9). However, there is little evidence of
the effects of tumor-derived exosomes on the CD133+ stem
cell-like tumor cells. The current study aimed to determine the
effect of 4T1 breast cancer cell line-derived exosomes on the
proliferation and apoptosis of CD133+ 4T1 cells.
Materials and methods
Cell culture
The 4T1 mouse mammary carcinoma cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) plus 100 U/ml
penicillin and 100 μg/ml streptomycin. NMuMG mouse mammary gland
epithelial cells (American Type Culture Collection) were cultured
in DMEM supplemented with 10% FBS with 10 μg/ml insulin, 100 U/ml
penicillin and 100 μg/ml streptomycin. All culture reagents were
obtained from Invitrogen (Carlsbad, CA, USA).
For exosome isolation, 4T1 and NMuMG cells were
cultured in medium with exosome-free FBS (exosomes in FBS were
removed by ultracentrifugation at 100,000 × g overnight at
4°C).
Exosome purification
Exosomes from 4T1 cells (4T1-Exo) or control
exosomes from NMuMG cells (NMuMG-Exo) were isolated using a Total
Exosome Isolation kit (#4478359) purchased from Invitrogen.
Briefly, culture media were collected and centrifuged at 2,000 × g
for 30 min to remove cells and debris. The required volume of
cell-free culture media was transferred to a fresh tube, and 0.5X
volumes of the Total Exosome Isolation reagent were added prior to
incubation at 4°C overnight. Following incubation, samples were
centrifuged at 10,000 × g for 1 h at 4°C. The supernatant was
discarded and the pellets were resuspended in 1X phosphate-buffered
saline (PBS) and stored at −80°C until use.
Dynamic light scattering and ζ
potential
Size distribution and ζ potential of exosomes were
measured as described previously (10). In brief, exosomes were washed with
double distilled (dd)H2O at 100,000 × g for 1 h and
resuspended in ddH2O for analysis. Prior to measurement,
samples were transferred to a cuvette (BrandTech Scientific, Inc.,
Essex, CT, USA) for dynamic light scattering analysis, or subjected
to an electric field for ζ potential determination using the
Zetasizer Nano ZS (Malvern Instruments, Ltd., Malvern, UK).
Transmission electron microscopy
(TEM)
For TEM analysis, exosomes were fixed with 2%
paraformaldehyde in PBS; then they were dropped onto Formvar/Carbon
on 200 mesh thick grids (Agar Scientific, Monterotondo, Italy) and
left to dry at room temperature for 20 min. Following a brief wash,
the grids were fixed with 1% w/v glutaraldehyde in PBS, followed by
several washing steps in distilled water. Samples were contrasted
by 4% w/v Uranyl Acetate (Sigma-Aldrich, St Louis, MO, USA) and by
UA-Methylcellulose mix solution (Sigma-Aldrich). The grid was dried
at room temperature, viewed in a Tecnai 12 G2 Transmission Electron
Microscope (FEI, Eindhoven, The Netherlands) and then analyzed with
Olympus iTEM CE software (Olympus Soft Imaging Solutions GmbH,
Münster, Germany).
Western blot analysis
Exosomes were lysed with lysis buffer and boiled.
Proteins were quantified by a Lowry assay using Bio-Rad Protein
Assay kit I (Bio-Rad, Hercules, CA, USA). Lysates were subjected to
SDS/PAGE (10% gel) and the proteins were transferred on to a
polyvinylidine fluoride membrane (Life Technologies, Grand Island,
NY, USA). Subsequent to immunoblotting with antibodies, including
rat anti-mouse CD9, goat anti-mouse CD63 and goat anti-mouse CD81
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA), the reaction
product was revealed with an Amersham ECL Western Blotting system
(GE Healthcare Life Sciences, Chalfont, UK).
CD133+ cell sorting
To isolate the CD133+ and
CD133− fractions, 4T1 cells were resuspended in Hank’s
Balanced Salt Solution (HBSS; Gibco-BRL, Carlsbad, CA, USA)
containing 2% FBS and 10 mM HEPES (Fisher Scientific, MA, USA ).
The cell density was adjusted to 1×107/ml and the 4T1
cells were stained with fluorescein isothiocyanate-conjugated human
anti-mouse CD133 IgG antibody (#130-105-226; Miltenyi Biotec, Inc.,
Cambridge, MA, USA). Subsequent to staining, the 4T1 cells were
resuspended in HBSS containing 2% FBS and 1 mM HEPES, filtered
through a 40-μm mesh filter, and the CD133+ and
CD133− 4T1 fractions were sorted by a FACSAria cell
sorter II (BD Biosciences, San Jose, CA, USA).
Exosome uptake assays
CD133+ and CD133− 4T1 cells
were cultured in a 4-well chamber for 24 h, then the PKH26
(Sigma-Aldrich)-labeled NMuMG-Exo or 4T1-Exo were added into the
wells and incubated with the cells for 12 h at 37°C. The cells were
then washed three times with cold PBS following incubation, fixed
in 2% paraformaldehyde for 10 min at room temperature,
permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) for 2 min at
room temperature, washed three times with PBS and stained with DAPI
(Life Technologies). Internalization of exosomes was observed under
a A1R-A1 Confocal Microscope system (Nikon Corporation, Tokyo,
Japan).
Proliferation assay
An ATPlite Luminescence Assay system (#6016941;
PerkinElmer, Inc., Waltham, MA, USA) was used to identify levels of
proliferation in CD133+ and CD133− 4T1 cells
following incubation with the 4T1-Exo and NMuMG-Exo. Briefly,
CD133+ or CD133− 4T1 cells (5×104)
were cultured in 24 well plates for 24 h and then the 4T1-Exo and
NMuMG-Exo (10 μg) were respectively added and incubated for 24 or
48 h. Mammalian cell lysis buffer (50 μl) was added to each well
and the wells were shaken in a orbital shaker at 250 × g for 5 min.
Then, 50 μl substrate solution was added to the wells and the plate
was shaken at 700 rpm for another 5 min. The plates were dark
adapted for 10 min and the luminescence was measured.
Apoptosis assay
The 4T1 cells were treated with doxorubicin (1 μM)
to induce apoptosis, with or without 10 μg NMuMG-Exo or 4T1-Exo for
12 h, and then Annexin V/propidium iodide (PI) staining was
performed to quantify the apoptosis levels in 4T1 cells. In brief,
4T1 cells were harvested and washed twice with PBS, and then
resuspended in 1X binding buffer (BD Biosciences) at a
concentration of 1×106 cells/ml. Cells were incubated
with fluorescein isothiocyanate labeled anti-Annexin V antibody (5
μl, eBioscience, San Diego, CA, USA) for 15 min at room
temperature. After washing with binding buffer, the cells were
resuspended in 200 μl binding buffer. PI staining solution (5 μl,
eBioscience) was added and the cells were analyzed using a BD
Accuri C6 flow cytometer (BD Biosciences) within 1 h.
Statistical analysis
One-way analysis of variance was used to determine
statistical significance using Graphpad Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 and
P<0.01 were considered to indicate a statistically significant
difference.
Results
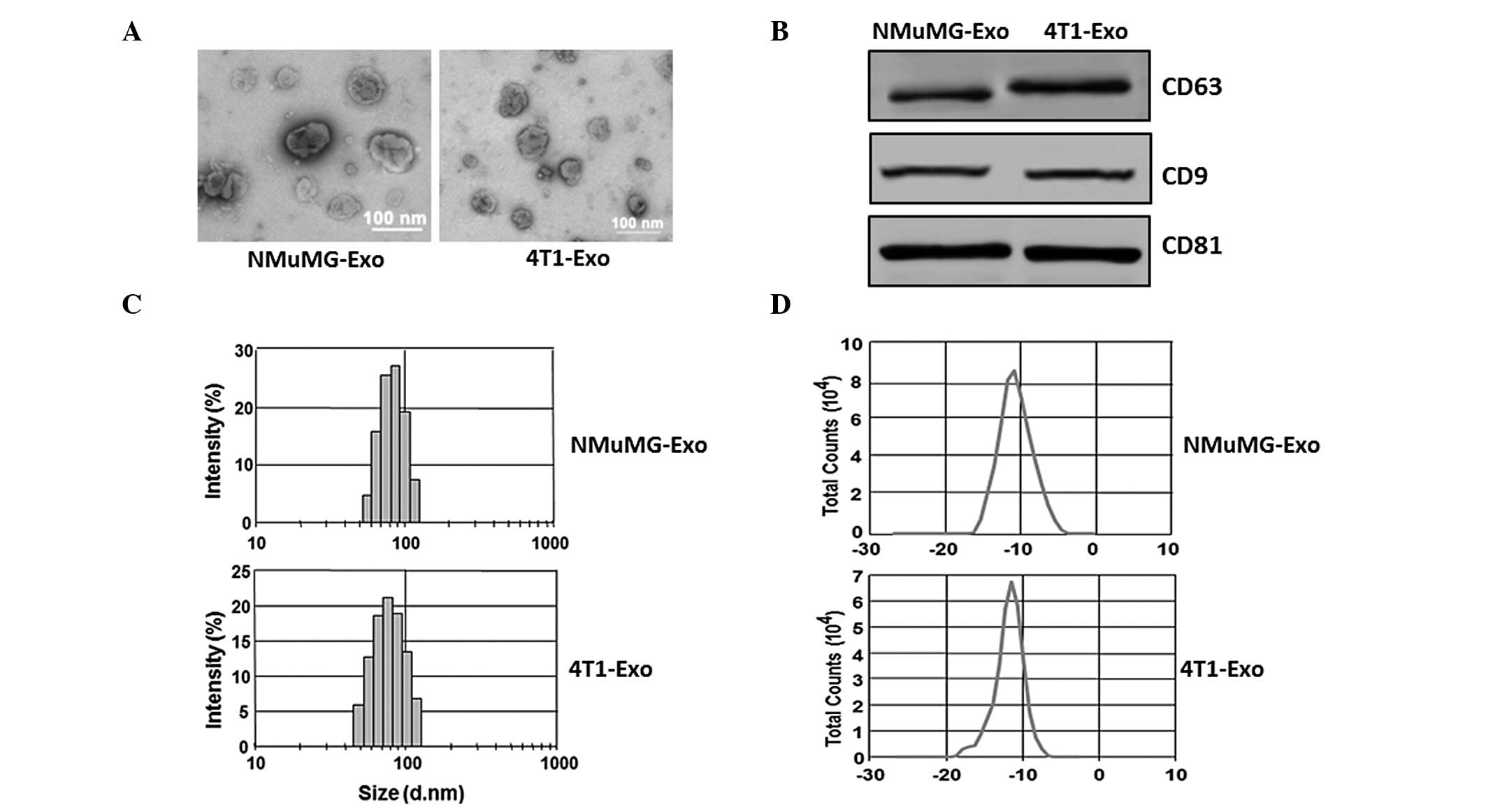
Characterization of exosomes
The exosomes secreted by the NMuMG and 4T1 cells
were isolated from serum-free culture supernatants using the
exosome isolation kit as mentioned above. TEM was performed in
order to observe the morphology of exosomes, and the size of the
particles were determined from the images to be ~100 nm (Fig. 1A). Western blot analysis was also
performed to detect the exosomal marker proteins. The exosomal
marker molecules CD63, CD81 and CD9 were detected in the exosome
preparations derived from the NMuMG and 4T1 cell lines (Fig. 1B). The size distribution (Fig. 1C) and surface ζ potential (Fig. 1D) were also determined with the
ZetaSizer Nano ZS. Results indicated that the average sizes of the
exosomes were 80.5 nm (NMuMG-Exo) and 89.6 nm (4T1-Exo) and the
average ζ potentials were −12.9 mV (NMuMG-Exo) and −15.8 mV
(4T1-Exo).
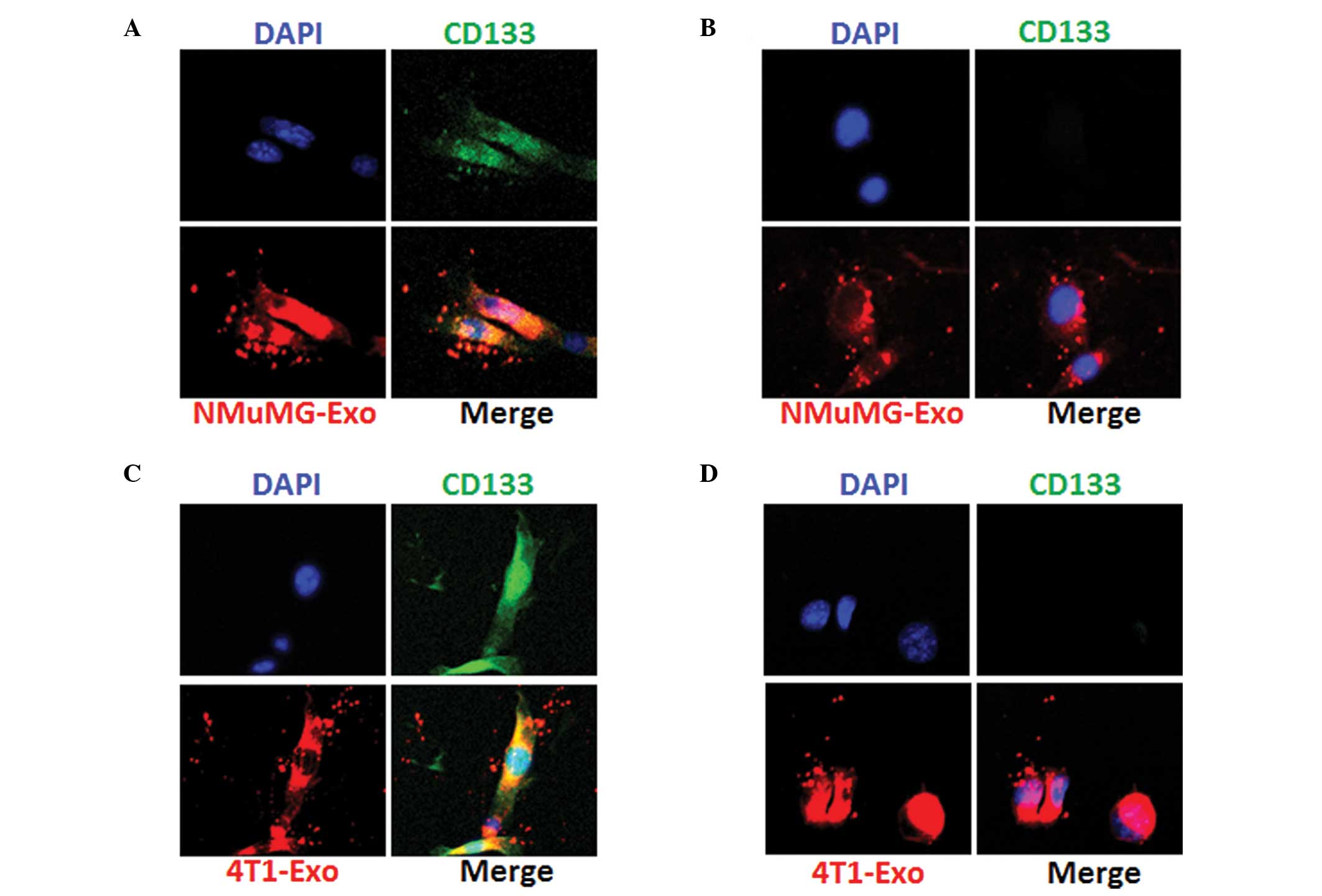
Exosomes from NMuMG or 4T1 cells are
internalized by the CD133+ and CD133− 4T1
cells
NMuMG and 4T1 cell-derived exosomes were purified
and characterized by size distribution, surface ζ potential and
morphology using the TEM microscope. The uptake of exosomes by
CD133+ and CD133− 4T1 cells was then
examined. Exosomes were labeled with PKH26 as described in the
methods. Next, 5 mg exosomes were incubated with the 4T1 cells.
After 12 h, the uptake of exosomes was imaged and the results
indicated that the NMuMG (Fig. 2A and
2B) and 4T1 cell-derived exosomes (Fig. 2C and 2D) were taken up by the
CD133+ and CD133− 4T1 cells. These results
suggest that exosomes from NMuMG or 4T1 cells may produce effects
on CD133 positive and negative cells.
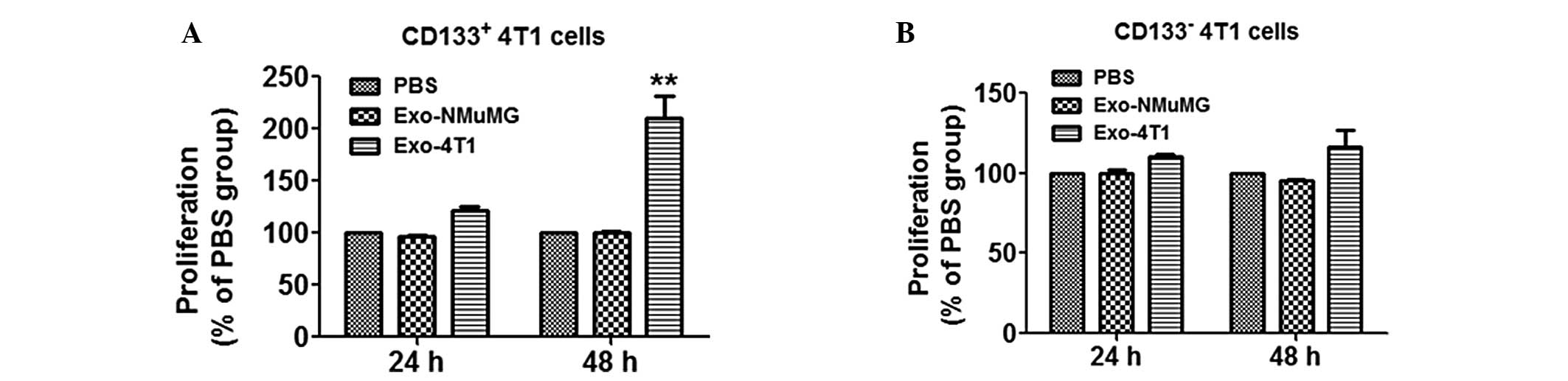
Exosomes from 4T1 cells promote
proliferation of CD133+ 4T1 cells
The microscope observations indicated that exosomes
from NMuMG and 4T1 cells have the potential to be taken up by
CD133+ and CD133− cells, thus the effects of
exosomes on proliferation of CD133+ and
CD133− cells were subsequently investigated by an
ATPlite assay. Notably, exosomes from 4T1 cells significantly
promoted the proliferation of CD133+ cells after 48 h
(Fig. 3A; **P<0.01
compared with control) but not CD133− cells (Fig. 3B).
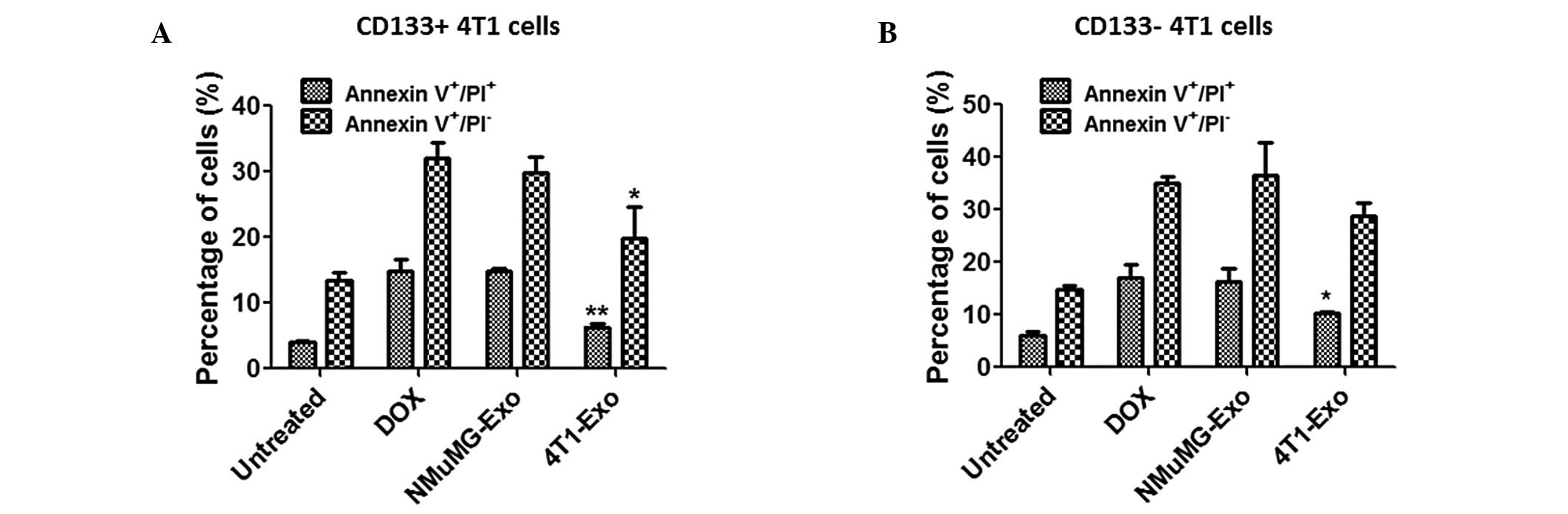
Exosomes from 4T1 cells inhibit apoptosis
of CD133+ and CD133− 4T1 cells
As presented in Fig.
3, it was demonstrated that exosomes from 4T1 cells
significantly promoted the proliferation of CD133+ cells
but had no effect on the proliferation of the CD133−
cells. Therefore, the effect of exosomes on apoptosis of 4T1 cells
was investigated. Following treatment with doxorubicin, Annexin
V/PI staining was performed to quantify the apoptosis levels of 4T1
cells. The percentages of cells in each group were determined and
presented as the mean ± standard error. As shown in Fig. 4A, apoptosis of CD133+
4T1 cells was markedly suppressed by 4T1 cell-derived exosomes
following treatment with doxorubicin (early apoptosis,
*P<0.05; late apoptosis, **P<0.01). In
contrast to the proliferation data, the apoptosis of
CD133− 4T1 cells was also inhibited by 4T1 cell-derived
exosomes to a certain extent (Fig.
4B; *P<0.05).
Discussion
Cell-to-cell communication is required for
appropriate coordination among the different cell types in one
organism. Cells may communicate with each other through soluble
factors, through adhesion molecule-mediated cell-to-cell
interactions, including cytonemes that connect neighboring cells,
enabling ligand-receptor-mediated transfer of surface-associated
molecules, or through tunneling nanotubules that establish conduits
between cells, allowing the transfer of surface molecules and
cytoplasmic components.
Tumor-derived exosomes exert antitumorigenic and
protumorigenic effects by targeting different types of cells.
Various studies have focused on the protumorigenic function
(11) of tumor-derived exosomes,
including their immunosuppressive properties (12), facilitation of tumor invasion and
metastasis (13), and the
promotion of tumor survival by transportation of RNA and protein
(14). Diverse immunosuppressive
effects of tumor-derived exosomes have been identified. Certain
tumor cell lines are able to produce exosomes expressing death
ligands, such as FasL and TRAIL, which can trigger the apoptotic
death of activated T cells (15,16).
Additionally, Epstein-Barr virus-infected nasopharyngeal carcinoma
has been demonstrated to release exosomes containing high levels of
galectin-9, which induces apoptosis of mature Th1 lymphocytes
subsequent to binding with the membrane receptor Tim-3 (17). Exosomes from murine-derived GL26
cells promote glioblastoma tumor growth by reducing the number and
function of CD8+ T cells (18). Another study demonstrated that
human melanoma- and colorectal carcinoma cell line-derived exosomes
are also able to regulate monocyte differentiation into DCs toward
the generation of myeloid-derived suppressor cells, and exert a
TGF-β1-mediated suppressive function on T cells in vitro
(19). In addition to the
suppression of the antitumor immunity, tumor-derived exosomes also
contribute to the establishment of a premetastatic niche,
generating a suitable microenvironment in metastatic sites by
regulation of stromal cells, stimulating angiogenesis and
remodeling the extracellular matrix (20). Protein and RNA, particularly miRNAs
transported by exosomes to target cells, are another effective
approach for cell-to-cell communication (21). There are few studies regarding the
effects of tumor-derived exosomes on tumor stem cells. However, Cho
et al (22) reported that
exosomes from ovarian cancer cells induce adipose tissue-derived
mesenchymal stem cells to acquire the physical and functional
characteristics of tumor-supporting myofibroblasts, and that
exosomes from breast cancer cells can convert adipose
tissue-derived mesenchymal stem cells into myofibroblasts via a
SMAD-mediated pathway. Myofibroblasts are a key source of
matrix-remodeling proteins within the tumor microenvironment and
thus participate in tumor angiogenesis.
To the best of our knowledge, the present study was
the first to assess the function of 4T1 mouse breast cancer
cell-derived exosomes on CD133+ 4T1 cells in
vitro. Uptake results indicated that exosomes from 4T1 cells
were taken up by the CD133+ and CD133− 4T1
cells. The in vitro proliferation assay demonstrated that
the exosomes from 4T1 cells significantly enhanced proliferation of
CD133+ but not CD133− 4T1 cells. Also, the
level of apoptosis in CD133+ 4T1 cells following
treatment with the apoptosis-inducing drug, doxorubicin, was
significantly suppressed by 4T1-derived exosomes whilst
CD133− 4T1 cells were not. This phenotype suggests that
tumor-derived exosomes may function as protumorigenic factors by
promoting the proliferation and suppression of apoptosis of
CD133+ tumor stem cells. The current study provides
further understanding of the communication between tumor-derived
exosomes and target cells.
References
|
1
|
Fan W, Tian XD, Huang E and Zhang JJ:
Exosomes from CIITA-transfected CT26 cells enhance anti-tumor
effects. Asian Pac J Cancer Prev. 14:987–991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfers J, Lozier A, Raposo G, et al:
Tumor-derived exosomes are a source of shared tumor rejection
antigens for CTL cross-priming. Nat Med. 7:297–303. 2001.PubMed/NCBI
|
|
3
|
Clayton A, Mitchell JP, Court J, et al:
Human tumor-derived exosomes selectively impair lymphocyte
responses to interleukin-2. Cancer Res. 67:7458–7466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartman ZC, Wei J, Glass OK, et al:
Increasing vaccine potency through exosome antigen targeting.
Vaccine. 29:9361–9367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clayton A, Al-Taei S, Webber J, et al:
Cancer exosomes express CD39 and CD73, which suppress T cells
through adenosine production. J Immunol. 187:676–683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai Z, Yang F, Yu L, et al: Activated T
cell exosomes promote tumor invasion via Fas signaling pathway. J
Immunol. 188:5954–5961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munich S, Sobo-Vujanovic A, Buchser WJ, et
al: Dendritic cell exosomes directly kill tumor cells and activate
natural killer cells via TNF superfamily ligands. Oncoimmunology.
1:1074–1083. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Webber J, Steadman R, Mason MD, et al:
Cancer exosomes trigger fibroblast to myofibroblast
differentiation. Cancer Res. 70:9621–9630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonito MD, Cantile M, Malzone G, et al:
The prognostic role of cancer stem cells in breast tumors. J Clin
Med Res. 5:325–326. 2013.PubMed/NCBI
|
|
10
|
Wang Q, Zhuang X, Mu J, et al: Delivery of
therapeutic agents by nanoparticles made of grapefruit-derived
lipids. Nat Commun. 4:18672013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang C and Robbins PD: The roles of
tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol.
2011:8428492011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang C and Robbins PD: Immunosuppressive
exosomes: a new approach for treating arthritis. Int J Rheumatol.
2012:5735282012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stoeck A, Keller S, Riedle S, et al: A
role for exosomes in the constitutive and stimulus-induced
ectodomain cleavage of L1 and CD44. Biochem J. 393:609–618. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zomer A, Vendrig T, Hopmans ES, et al:
Exosomes: Fit to deliver small RNA. Commun Integr Biol. 3:447–450.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andreola G, Rivoltini L, Castelli C, et
al: Induction of lymphocyte apoptosis by tumor cell secretion of
FasL-bearing microvesicles. J Exp Med. 195:1303–1316. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huber V, Fais S, Iero M, et al: Human
colorectal cancer cells induce T-cell death through release of
proapoptotic microvesicles: role in immune escape.
Gastroenterology. 128:1796–1804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keryer-Bibens C, Pioche-Durieu C,
Villemant C, et al: Exosomes released by EBV-infected
nasopharyngeal carcinoma cells convey the viral latent membrane
protein 1 and the immunomodulatory protein galectin 9. BMC Cancer.
6:2832006. View Article : Google Scholar
|
|
18
|
Liu ZM, Wang YB and Yuan XH: Exosomes from
murine-derived GL26 cells promote glioblastoma tumor growth by
reducing number and function of CD8+ T cells. Asian Pac
J Cancer Prev. 14:309–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai Z, Zhang W, Yang F, et al:
Immunosuppressive exosomes from TGF-β1 gene modified dendritic
cells attenuate Th17-mediated inflammatory autoimmune disease by
inducing regulatory T cells. Cell Res. 22:607–610. 2012.
|
|
20
|
Peinado H, Lavotshkin S and Lyden D: The
secreted factors responsible for pre-metastatic niche formation:
old sayings and new thoughts. Semin Cancer Biol. 21:139–146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Channavajjhala SK, Rossato M, Morandini F,
et al: Optimizing the purification and analysis of miRNAs from
urinary exosomes. Clin Chem Lab Med. 52:345–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho JA, Park H, Lim EH, et al: Exosomes
from ovarian cancer cells induce adipose tissue-derived mesenchymal
stem cells to acquire the physical and functional characteristics
of tumor-supporting myofibroblasts. Gynecol Oncol. 123:379–386.
2011. View Article : Google Scholar
|