Introduction
Lung cancer is a leading cause of global
cancer-associated mortality (1).
Non-small cell lung cancer (NSCLC) accounts for ~80% of all types
of lung cancer. Although recent advances have been made in the
development of diagnosis and treatment strategies, the prognosis of
patients with NSCLC remains poor (2).
Immunogene therapy using immunostimulatory molecules
to enhance anti-tumor immunity offers a promising therapeutic
option for lung cancer (3). The
CD40 ligand (CD40L), a type II membrane-bound protein belonging to
the tumor necrosis factor (TNF) superfamily, was used in cancer
gene therapy due to its ability to trigger Th1-type immune
responses that drive effector cells, including cytotoxic T
lymphocytes, natural killer cells and M1 macrophages, which
normalize the tumor microenvironment and directly suppress the
growth of certain CD40-positive tumors (4). Novel therapies based on CD40L,
including infusion of recombinant CD40L protein, anti-CD40
antibodies and CD40L gene therapy, potentially provide numerous
anti-cancer approaches in one therapy and offer an attractive
option for clinical trials (5–7).
Several studies indicated that the precise form of
the CD40 stimulus, inducing a membrane-bound and soluble version of
CD40L (sCD40L), influenced the therapeutic responses in carcinoma
cells. sCD40L is shed from membrane-bound forms via matrix
metalloproteinase (MMP) cleavage and provides local cytokine-like
amplification of CD40 signaling (8). A study indicated that sCD40L induced
pro-survival and pro-apoptotic signals in CD40-positive solid
carcinoma cells (9). In clinical
trials, elevated serum levels of sCD40L were found in autoimmune
diseases, metabolic and cardiovascular diseases and the
cytokine-like effects of sCD40L may contribute to the pathogenesis
of these disorders by acting on CD40(+) bystander cells (10,11).
Membrane-bound CD40L, however, through sustained activation of TNF
receptor-associated factor 3 (TRAF3)-dependent c-Jun N-terminal
kinase signals and suppression of TRAF6-dependent survival signals,
was able to directly induce cell death whilst creating few side
effects (12). Therefore, as a
potential anti-cancer therapeutic, membrane-bound CD40L presents a
more attractive option than its soluble form.
To circumvent the possible stimulating proliferative
activity and adverse inflammatory effects caused by sCD40L, a
membrane-stable mutant form of human CD40L (CD40L-M) was designed
and engineered. As a means of delivering CD40L-M for clinical
application, the transduction efficiency of various serotypes of
adeno-associated virus (AAV) vectors in an NSCLC cell line were
examined. The efficient recombinant self-complementary AAV5
(scAAV5) was subsequently selected as a therapeutic vehicle
(11). An scAAV5 encoding human
CD40L mutant (AAV5-CD40L-M), which results in consistent expression
of ligand at the cell membrane, was generated. The direct
anti-tumor effects of cleavage-resistant CD40L delivered by scAAV5
were examined in vitro and in vivo.
Materials and methods
Cell culture
The human NSCLC cell lines A549 and H1650 and the
human embryonic kidney cell line HEK293 (293T) were obtained from
the Cell Resource Center (Shanghai, China). The cells were
maintained in RPMI-1640 medium (Gibco, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco-BRL, Grand Island,
NY, USA).
Construction of scAAV5 vectors
Plasmids pdsAAV-CB-CD40L and pdsAAV-CB-CD40L-M,
which are self-complementary double-sequence AAV plasmids
containing human wild-type CD40L cDNA and CD40L-M cDNA (containing
six substitutions: Gln114 to Pro114, Lys115 to Arg115, Asp117 to
Glu117, Gln118 to Glu118, Asn119 to Asp119 and Pro120 to Ser120),
were constructed by Invitrogen Life Technologies (Carlsbad, CA,
USA) (13,14). AAV vectors were generated as
described by the Penn Vector Core (http://www.med.upenn.edu/gtp/vector_core/production.shtml).
Briefly, a plasmid encoding the transgene of interest, expressed
from a cytomegalovirus enhancer/β-actin (CB) promoter, flanked by
AAV2 inverted terminal repeats, was packaged by triple transfection
of HEK293 cells with plasmids encoding the AAV2 rep gene and the
AAV5 cap gene and an adenovirus helper plasmid (Fig. 1A). Vectors were purified by CsCl
ultracentrifugation and titers were determined by slot blot
analysis, as previouslt described (15).
Quantitative polymerase chain reaction
(qPCR)
PCR was performed using the SYBR Green RT-PCR kit
(Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer’s
instructions. The PCR primer pairs used were as follows: CD40L
(NM-000074, position 488–620, 132 bp fragment) forward, 5′-TGA GCA
ACA ACT TGG TAA CCC TGG-3′ and reverse, 5′-CTG GCT ATA AAT GGA GCT
TGA CTC G-3′; β-actin (NM-001101, position 939–1124, 205 bp
fragment) forward, 5′-TGA CGT GGA CAT CCG CAA AG-3′ and reverse,
5′-CTG GAA GGT GGA CAG CGA GG-3′ (Invitrogen Life
Technologies).
Flow cytometric analysis
CD40L and CD40 expression was determined by flow
cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA)
using an allophycocyanin (APC)-conjugated CD154 antibody (Ab)
diluted at 1:25 or phycoerythrin (PE)-conjugated CD40 Ab diluted at
1:50. Appropriate APC- or PE-conjugated isotype control was used to
measure background staining. Annexin V-fluorescein isothiocyanate
(FITC) and/or propidium iodide (PI) staining (TACS Annexin V-FITC
kit; Trevigen, Gaithersburg, MD, USA) was used to detect apoptotic
cells. Caspase-3 inhibitor (Ac-DEVD-CHO; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was employed in a competition
experiment.
Cell viability assay
Cell viability was determined by MTT assay using a
Cell Proliferation Kit I (Roche Diagnostics GmbH, Mannheim,
Germany). Cancer cells were seeded into 96-well plates. After
incubation, the medium was replaced with 50 μl MTT reagent (2
mg/ml) followed by further incubation in an incubator for 2 h.
Next, the medium was removed and dimethylsulfoxide (150 μl) was
added to each well. The absorbance at 560 nm was measured with a
spectrophotometer (PerkinElmer, Waltham, MA, USA). A competition
experiment with anti-human CD40L monoclonal antibody (100 ng/l;
BioLegend, Inc., San Diego, CA, USA) was performed.
Western blot analysis
Briefly, proteins were separated by 6–15% SDS-PAGE
and transferred to polyvinylidene difluoride membranes (Hybond-P;
GE Healthcare, Little Chalfont, UK). The primary antibodies used
were: Rabbit anti-caspase-3 polyclonal antibody (pAb; 1:100;
sc-7148) and rabbit anti-β-actin pAb (1:1,000; sc-130656; Santa
Cruz Biotechnology, Inc.). The secondary antibodies were
horseradish peroxidase-conjugated antibodies against rabbit
immunoglobulin G (GE Healthcare, Waukesha, WI, USA). Immunoreactive
bands in the western blots were visualized using enhanced
chemiluminescence substrates (ECL Plus; GE Healthcare).
Caspase-3 activity assay
The activity of caspase-3 was evaluated using the
Caspase-3 Activity Assay kit (C115; Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer’s
instructions. The caspase-3 activity assay is based on
spectrophotometric detection of the chromophore
p-nitronanilide (pNA), following its cleavage from the
labeled substrate. The release of pNA was quantified by determining
the absorbance using a Sunrise™ microplate reader (Tecan Group,
Ltd., Männedorf, Switzerland) at 405 nm.
ELISA
The concentration of sCD40L in supernatants and
serum following treatment were measured using a human sCD40L ELISA
kit (ELH-CD40L-001; RayBiotech, Inc., Norcross, GA, USA) according
to the manufacturer’s instructions.
Mouse model studies
Xenografts were prepared by implanting tumor
fragments derived from A549 cells [5×106 cells suspended
in 100 μl phosphate-buffered saline (PBS)] subcutaneously into the
back of six-week-old male BALB/c nude mice. The mice were obtained
from the animal center at Nanjing Medical University (Nanjing,
China). All mice were maintained on a diet of standard rodent chow
and water supplied ad libitum, with a regular light-dark
cycle and at 23°C. When the tumor volume reached ~100
mm3, the mice were randomly divided into four groups
(six mice/group). Intratumoral injection with 0.5 ml scAAV5-GFP,
scAAV5-CD40L and scAAV5-CD40L-M vectors, respectively, [at 1×1,011
viral genomes (vgs)/mouse] or 0.5 ml PBS was performed.. Tumor
growth was subsequently monitored every three days for 30 days by
measuring tumor size with a caliper. The tumor volume was
calculated using the following formula: Tumor volume = length ×
width2 × 0.5. All animal experiments were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The protocol was
approved by the Committee on the Ethics of Animal Experiments of
Nanjing Medical University (permit number, 307005).
Immunohistochemistry
Immunohistochemistry using the rabbit anti-CD40L
antibody (ab65854; Abcam, Cambridge, UK) was employed to
investigate paraffin wax-embedded tissue sections using a routine
avidin-biotin-immunoperoxidase technique (Histostain-Plus kit,
Zymed, San Franzisco, USA). For quantitative analysis of apoptosis,
sections were assayed by the terminal deoxynucleotidyl
transferase-mediated dUTP-FITC nick end-labeling (TUNEL) method
using a One Step TUNEL Apoptosis Assay kit (C1086; Beyotime). The
number of positive cells was counted in ten random fields using
microscopy (magnification, ×100; LSM 700; Carl Zeiss, Oberkochen,
Germany).
Statistical analysis
The two-tailed Student’s t-test application of
Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA) was
used for all continuous outcome variables, including tumor size and
weight. A repeated Student’s t-test was used to compare the treated
groups with the control group. Values are presented as the mean ±
standard deviation and P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Expression of scAAV5-CD40L-M
The expression cassette containing the CD40L or
CD40L-M cDNA was inserted into the cloning site of pdsAAV-CB-GFP to
create the expression plasmid pdsAAV-CB-CD40L or pdsAAV-CB-CD40L-M,
respectively. The correct plasmids were identified by sequencing.
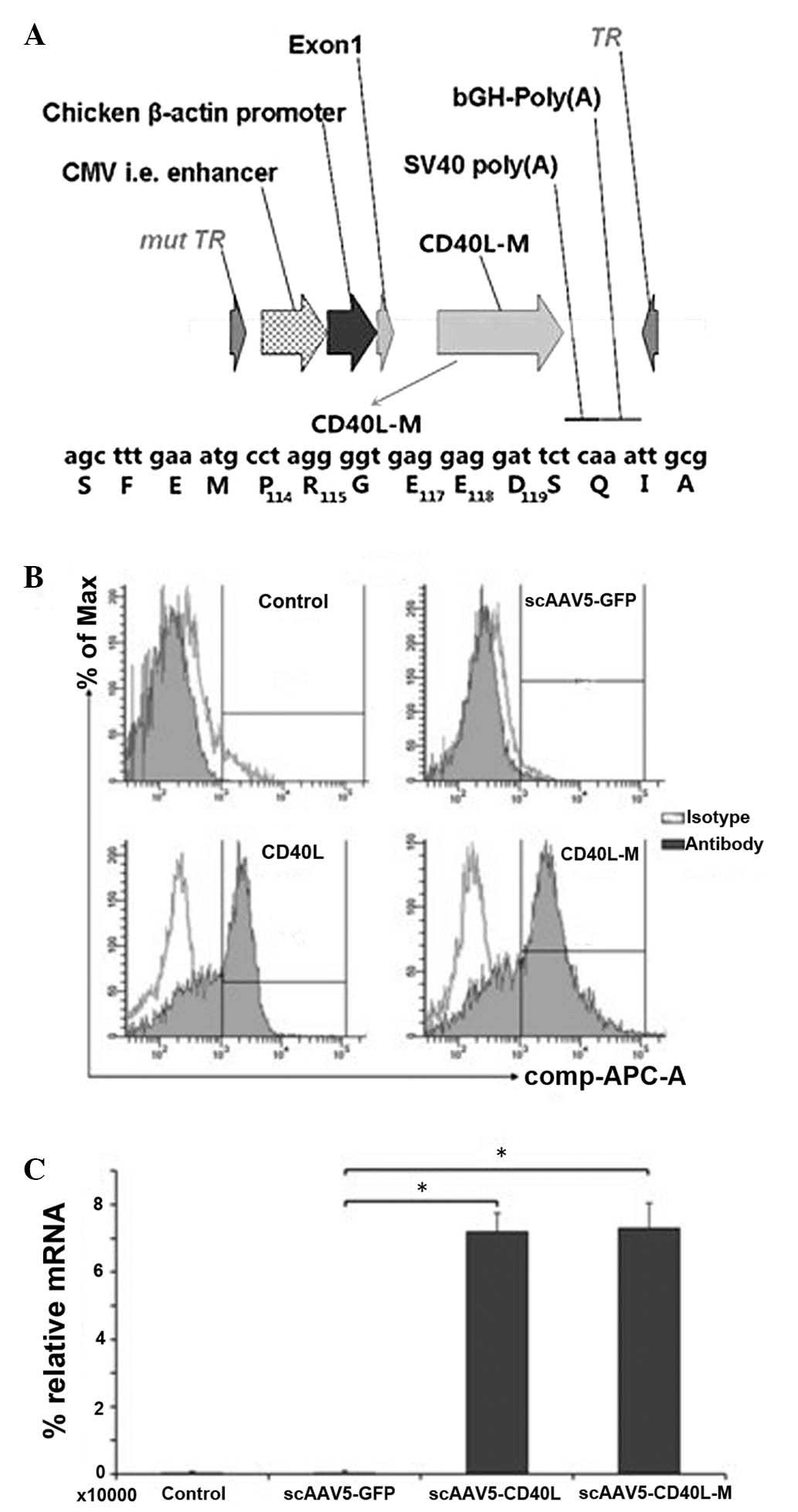
Recombinant scAAV5-CD40L and scAAV5-CD40L-M vectors (Fig. 1A) were generated as aforementioned.
These viruses were used to infect the A549, H1650 and 293T cell
lines. Expression of CD40 was detected in the A549 cell line
(65.63±6.02%), but not in the H1650 or 293T cell lines. Infection
with the equivalent multiplicity of infection (MOI, 104
vgs/cell) of CD40L-M resulted in membranous ligand expression
determined by flow cytometry (Fig.
1B). Expression of CD40L and CD40L-M was confirmed by qPCR
analysis (Fig. 1C).
scAAV5-CD40L-M inhibits the proliferation
of CD40-positive cell lines
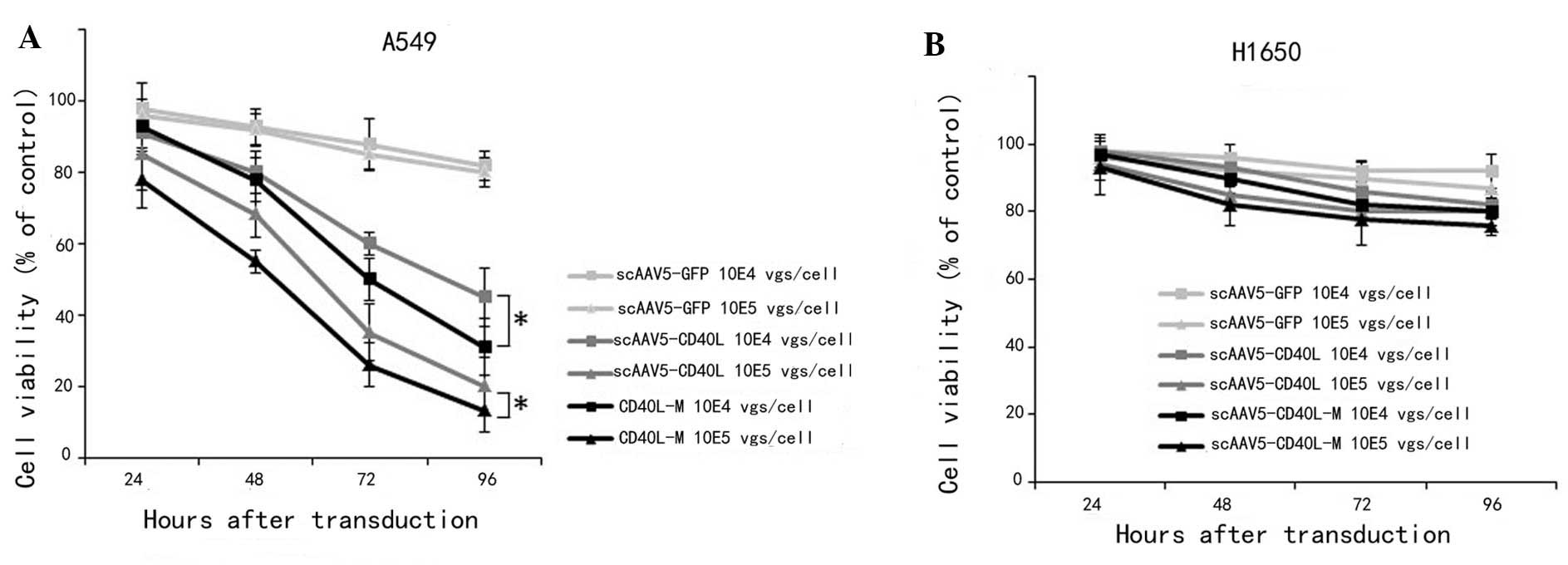
The direct growth-inhibitory activities of
scAAV5-CD40L and scAAV5-CD40L-M were assessed by MTT assay using
CD40-positive A549 and CD40-negative H1650 lung cancer cell lines.
Significantly decreased A549 viability was observed (Fig. 2A) following scAAV5-CD40L or
scAAV5-CD40L-M transduction at 96 h (MOI of 104
vgs/cell: scAAV5-GFP, 85.02±7.34%; scAAV5-CD40L, 49.53±6.05%;
scAAV5-CD40L-M, 23.56±3.67%; MOI of 105 vgs/cell:
scAAV5-GFP, 83.76±8.54%; scAAV5-CD40L, 35.37±4.78%; scAAV5-CD40L-M,
18.92±2.03%; versus scAAV5-green fluorescent protein (GFP);
P<0.01). A significant difference between the viability of
scAAV5-CD40L/A549 cells and that of scAAV5-CD40L-M/A549 cells was
also distinguishable (P<0.05). This inhibitory effect was
attenuated when cells were pre-incubated with an anti-CD40L
monoclonal antibody. By contrast, no difference was observed in the
CD40-negative H1650 cells following transduction (Fig. 2B).
scAAV5-CD40L-M induces CD40-positive A549
cell apoptosis
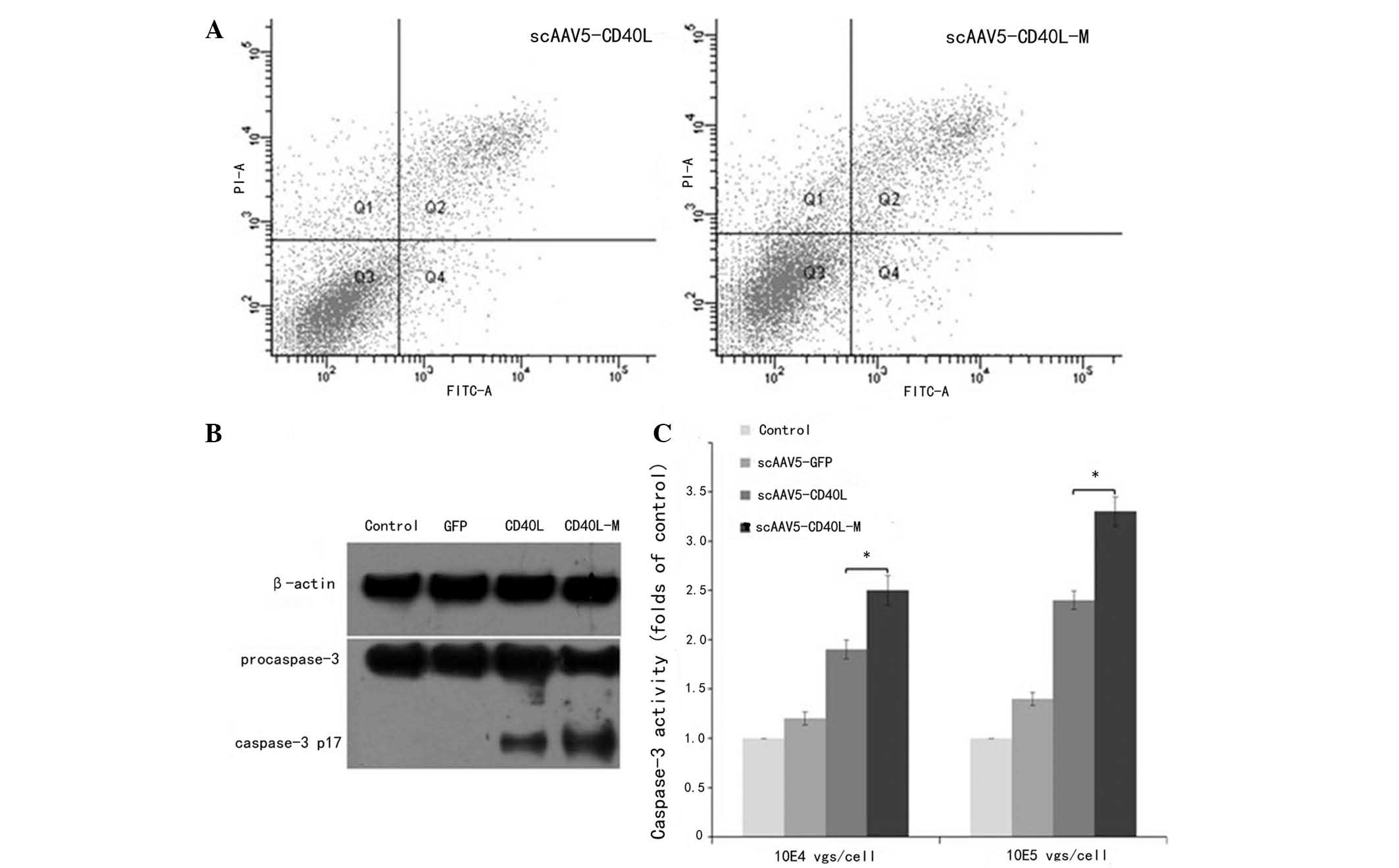
Relative to CD40-negative H1650 cells, scAAV5-CD40L
and scAAV5-CD40L-M (104 vgs/cell) were found to induce
CD40-positive A549 cell apoptosis as indicated by Annexin V-FITC/PI
double staining with apoptotic rates of 17.83±1.32 and 12.56±1.05%,
indicating a significant difference between the two groups
(P<0.05; Fig. 3A).
To investigate whether the apoptotic effect observed
was associated with caspase-3 activation, western blot analysis was
used to examine the expression levels of cleaved caspase-3 in A549
cells. The expression levels of cleaved caspase-3 protein in
scAAV5-CD40L-M-infected cells were higher than those in
scAAV5-CD40L-infected cells (104 vgs/cell; Fig. 3B). Caspase-3 activity was further
analyzed by enzymatic assay. The level of caspase-3 activation was
markedly higher in cells transduced with scAAV5-CD40L-M for 48 h
than that of cells exposed to scAAV5-CD40L or AAV5-GFP (Fig. 3C). Apoptosis was not induced when
the cells were pre-incubated with caspase-3 inhibitor (data not
shown). These results indicated that CD40L-associated cell death
may be mediated by a caspase-dependent signaling pathway.
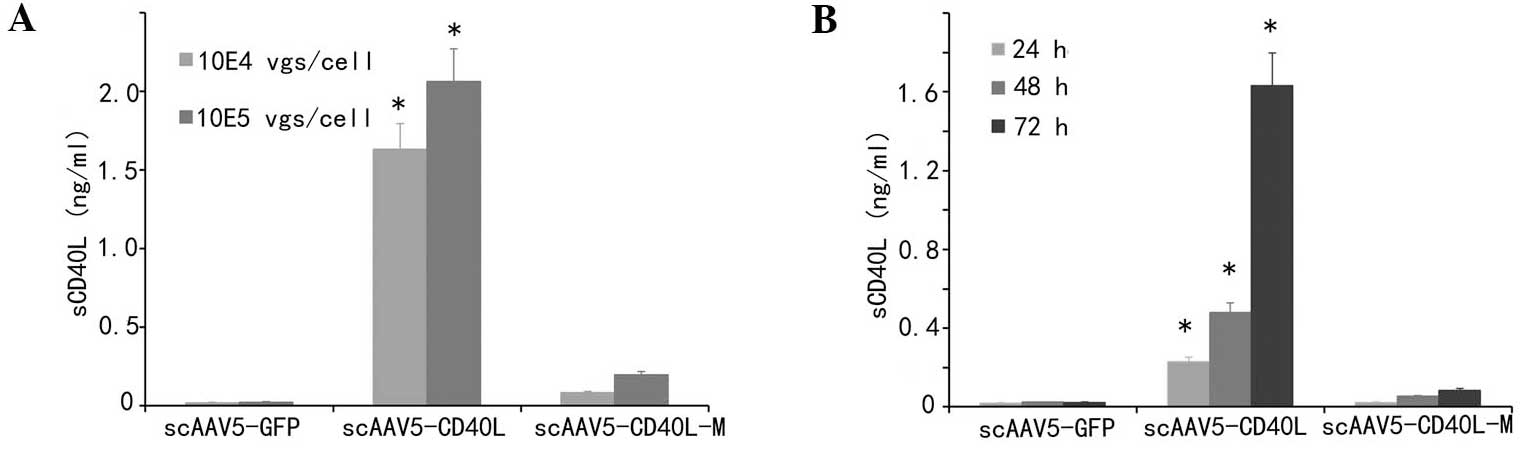
sCD40L secretion is significantly
decreased in scAAV5-CD40L-M-transduced cells
Secretion of sCD40L following transduction was
detected in supernatants to examine whether CD40L-M was resistant
to cleavage on the cell surface. As indicated in Fig. 4A and B, the amount of sCD40L from
the culture supernatants of scAAV5-CD40L-transduced cells was
significantly increased following transduction compared with that
of the corresponding mock and CD40L-M groups (P<0.01).
Antitumor activity of scAAV5-CD40L-M
against lung cancer xenografts
To focus on the direct tumor growth-inhibitory
features of AAV5-CD40L-M, in vivo analyses were performed
via lung cancer heterotransplants in athymic nude mice.
scAAV5-CD40L and scAAV5-CD40L-M each effectively decreased the
tumor growth rate (Fig. 5A). The
mean tumor weights were 0.43±0.03, 0.31±0.02 and 0.29±0.03 g in the
scAAV5-GFP, scAAV5-CD40L and scAAV5-CD40L-M groups, respectively
(Fig. 5B).
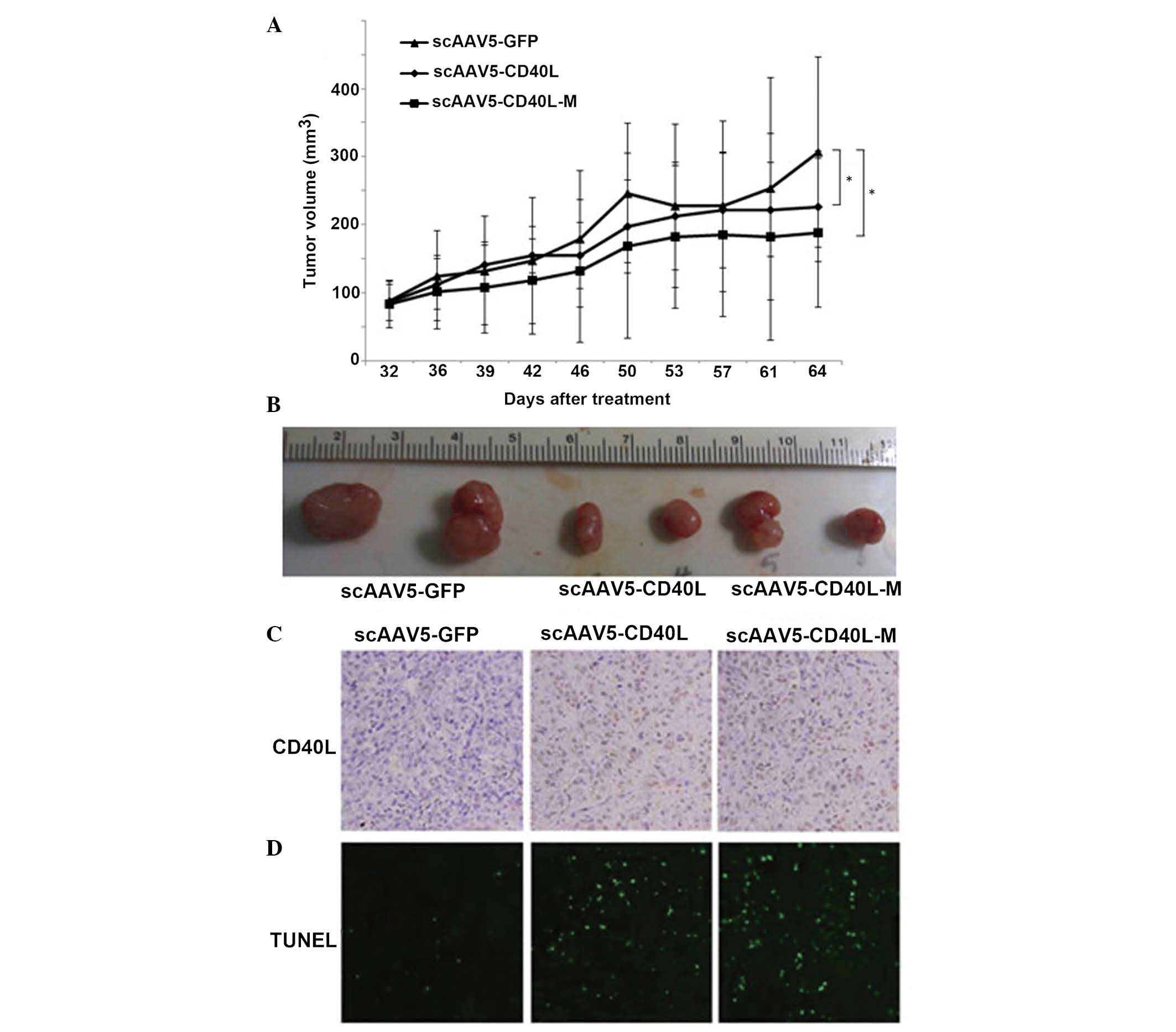 | Figure 5scAAV5-CD40L-M inhibits tumor growth
in vivo. Athymic nude mice were xenotransplanted with A549
cells. When 100 mm3-tumors were formed, intratumoral
injection with scAAV5 vectors (at 1×1011 vgs/mouse) or
0.5 ml phosphate-buffered saline was performed every five days. (A)
Tumor growth curves. Treatment of scAAV5-CD40L-M enhanced the
inhibition of tumor growth. *P<0.05 vs. scAAV5-GFP.
Each point represents the mean ± standard deviation (n=6 tumors).
(B) Representative image of the various tumors. Mean weights of the
tumors were 0.43±0.03, 0.31±0.02 and 0.29±0.03 g for the
scAAV5-GFP, scAAV5-CD40L and scAAV5-CD40L-M groups, respectively.
(C) Representative images of tumor sections examined by
immunohistochemical staining for CD40L protein expression
(magnification, ×100). (D) Representative images of tumor sections
examined by TUNEL assay. TUNEL-positive cell nuclei (green) were
observed under a fluorescence microscope (magnification, ×100).
scAAV5, self-complementary adeno-associated virus 5; GFP, green
fluorescent protein; TUNEL, terminal deoxynucleotidyl
transferase-mediated dUTP-fluorescein isothiocyanate nick
end-labeling; CD40L-M, CD40 ligand mutant. |
CD40L expression was detected in the scAAV5-CD40L-
and scAAV5-CD40L-M-injected tumors but not in the
scAAV5-GFP-injected tumors (Fig.
5C). The effect of scAAV5-CD40L-M treatment on apoptosis was
assessed using an in situ TUNEL assay. The most significant
level of apoptosis was observed in the tumors of mice receiving
scAAV5-CD40L- or scAAV5-CD40L-M-treatment, compared with that of
scAAV5-GFP mice (Fig. 5D;
P<0.05, respectively).
The systemic toxicity of scAAV5-CD40L-M treatment
was examined. Histopathological studies indicated no apparent
lesions in the lung, liver, spleen or kidney. Levels of sCD40L in
serial serum samples were ~0. Serum alanine transaminase, aspartate
aminotransferase, serum urea nitrogen and creatinine were
determined. No significant difference was detected between groups
(Data not shown).
Discussion
CD40L transgene expression in human CD40-positive
solid carcinoma cells was found to produce a direct
growth-inhibitory effect through apoptotic induction and/or cell
cycle blockage (16–22). However, sCD40L cleaved from
membrane-bound CD40L may regulate CD40L activity by downregulating
surface CD40L and quenching CD40L with an sCD40L ‘decoy’ (23), which is unable to induce survival
signals in cancer cells (24) or
enter into the systemic circulatory system and cause inflammatory
diseases (10,11). Therefore, in the present study, to
reduce the adverse effects caused by sCD40L, a human CD40L mutant
that was resistant to proteolytic cleavage was generated as a
candidate gene for immunogenetic therapy of lung cancer.
Successful gene therapy is dependent upon the
efficiency and availability of gene delivery systems. Recombinant
AAVs have been widely used for gene delivery in animal models and
their use in human gene therapy is currently being evaluated in
clinical trials (http://www.abedia.com/wiley/). However, limitations in
vector tropism, including limited tissue specificity and
insufficient transduction efficiencies, preclude therapeutic
applications in certain tissues (25). To achieve greater efficacy of
AAV-gene therapy for lung carcinoma, the transduction efficiency of
various AAV vector serotypes in NSCLC cell lines were examined. The
results demonstrated that scAAV5 vectors, which bypass the
requirement of conventional viral second-strand DNA synthesis, are
the most robust to deliver the gene in A549 cells (13). Recently, a study verified that
N-linked sialic acid on the surface of lung cancer cells mediates
tropism of vectors based on AAV5 (26). Therefore, based on previous
studies, a recombinant scAAV5 expressing mutant CD40L was generated
in the present study to further explore the direct antitumor
effects of wild-type and cleavage-resistant CD40L in vitro
and in vivo.
The results of the present study indicated that
scAAV5-CD40L-M produced a more profound growth-inhibitory effect in
CD40-positive cells compared with that in wild-type scAAV5-CD40L.
Caspase-3 activation assay and Annexin V staining confirmed that
CD40L-M was a more potent inducer of apoptosis than the wild-type
CD40L, which was in agreement with previous studies by Elmetwali
et al (12)and Vardouli
et al (17). It is possible
that this augmented antitumor activity was a result of ‘suicide’
and ‘fratricide’ through constitutive stimulation of CD40 signals
by CD40L expression. This antitumor activity was further confirmed
in vivo by treatment of pre-existing A549 xenografts without
detectable side effects.
In conclusion, the results of the present study
demonstrated the direct antitumor effects of scAAV5 delivery of
membrane-stable mutant CD40L on human CD40-positive lung carcinomas
in vitro and in vivo with few side effects. The
balance between the direct cancer growth inhibition of
scAAV-CD40L-M with its immune-activating properties will be
examined in forthcoming studies, using a murine, immunocompetent
syngeneic cancer model.
Acknowledgements
The present study was funded by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (Jiangsu, China) and supported by grants from the
International Science & Technology Cooperation Program of China
(no. 2014DFA31940), the National Natural Science Foundation of
China (Beijing, China; nos. 30971320, 81272602 and 81302014) and
the Universities Natural Science Research Project of Jiangsu
Province (Nanjing, China; no. 12KJB320004).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
Thomas A and Hassan R: Immunotherapies for
non-small-cell lung cancer and mesothelioma. Lancet Oncol.
13:e301–e310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reck M: What future opportunities may
immuno-oncology provide for improving the treatment of patients
with lung cancer? Ann Oncol. 23(Suppl 8): viii28–viii34. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loskog AS and Eliopoulos AG: The Janus
faces of CD40 in cancer. Semin Immunol. 21:301–307. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khong A, Nelson DJ, Nowak AK, et al: The
use of agonistic anti-CD40 therapy in treatments for cancer. Int
Rev Immunol. 31:246–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ullenhag G and Loskog AS: AdCD40L -
crossing the valley of death? Int Rev Immunol. 31:289–298. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vonderheide RH and Glennie MJ: Agonistic
CD40 antibodies and cancer therapy. Clin Cancer Res. 19:1035–1043.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pietravalle F, Lecoanet-Henchoz S, Blasey
H, et al: Human native soluble CD40L is a biologically active
trimer, processed inside microsomes. J Biol Chem. 271:5965–5967.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Georgopoulos NT, Steele LP, Thomson MJ, et
al: A novel mechanism of CD40-induced apoptosis of carcinoma cells
involving TRAF3 and JNK/AP-1 activation. Cell Death Differ.
13:1789–1801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goules A, Tzioufas AG, Manousakis MN, et
al: Elevated levels of soluble CD40 ligand (sCD40L) in serum of
patients with systemic autoimmune diseases. J Autoimmun.
26:165–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferroni P, Santilli F, Guadagni F, et al:
Contribution of platelet-derived CD40 ligand to inflammation,
thrombosis and neoangiogenesis. Curr Med Chem. 14:2170–2180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elmetwali T, Young LS and Palmer DH: CD40
ligand-induced carcinoma cell death: a balance between activation
of TNFR-associated factor (TRAF) 3-dependent death signals and
suppression of TRAF6-dependent survival signals. J Immunol.
184:1111–1120. 2010. View Article : Google Scholar
|
|
13
|
Wu JQ, Zhao WH, Li Y, et al:
Adeno-associated virus mediated gene transfer into lung cancer
cells promoting CD40 ligand-based immunotherapy. Virology.
368:309–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masuta Y, Kato K, Tomihara K, et al: Gene
transfer of noncleavable cell surface mutants of human CD154
induces the immune response and diminishes systemic inflammatory
reactions. J Immunother. 30:694–704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Zhao W, Zhong L, et al:
Self-complementary recombinant adeno-associated viral vectors:
packaging capacity and the role of rep proteins in vector purity.
Hum Gene Ther. 18:171–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gomes EM, Rodrigues MS, Phadke AP, et al:
Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154)
transgene construct in human breast cancer cells. Clin Cancer Res.
15:1317–1325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vardouli L, Lindqvist C, Vlahou K, et al:
Adenovirus delivery of human CD40 ligand gene confers direct
therapeutic effects on carcinomas. Cancer Gene Ther. 16:848–860.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dzojic H, Loskog A, Tötterman TH and
Essand M: Adenovirus-mediated CD40 ligand therapy induces tumor
cell apoptosis and systemic immunity in the TRAMP-C2 mouse prostate
cancer model. Prostate. 66:831–838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iida T, Shiba H, Misawa T, et al:
Adenovirus-mediated CD40L gene therapy induced both humoral and
cellular immunity against rat model of hepatocellular carcinoma.
Cancer Sci. 99:2097–2103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Serba S, Schmidt J, Wentzensen N, et al:
Transfection with CD40L induces tumour suppression by dendritic
cell activation in an orthotopic mouse model of pancreatic
adenocarcinoma. Gut. 57:344–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lindqvist C, Sandin LC, Fransson M and
Loskog A: Local AdCD40L gene therapy is effective for disseminated
murine experimental cancer by breaking T-cell tolerance and
inducing tumor cell growth inhibition. J Immunother. 32:785–792.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
von Euler H, Sadeghi A, Carlsson B, et al:
Efficient adenovector CD40 ligand immunotherapy of canine malignant
melanoma. J Immunother. 31:377–384. 2008.PubMed/NCBI
|
|
23
|
Matthies KM, Newman JL, Hodzic A and
Wingett DG: Differential regulation of soluble and membrane CD40L
proteins in T cells. Cell Immunol. 241:47–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davies CC, Mason J, Wakelam MJ, et al:
Inhibition of phosphatidylinositol 3-kinase- and ERK MAPK-regulated
protein synthesis reveals the pro-apoptotic properties of CD40
ligation in carcinoma cells. J Biol Chem. 279:1010–1019. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dickey DD, Excoffon KJ, Koerber JT, et al:
Enhanced sialic acid-dependent endocytosis explains the increased
efficiency of infection of airway epithelia by a novel
adeno-associated virus. J Virol. 85:9023–9030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nonnenmacher M and Weber T: Intracellular
transport of recombinant adeno-associated virus vectors. Gene Ther.
19:649–658. 2012. View Article : Google Scholar : PubMed/NCBI
|