Introduction
Neuroblastoma (NB) is a neuroendocrine cancer that
occurs most commonly in infants and young children (1). NB tumors are malignant tumors that
develop from the nerve cells of an embryo or fetus (2). Certain forms of NB are low risk,
while others are high risk and may require multiple treatments.
The Hippo signaling pathway manages organ size by
regulating cell proliferation and apoptosis (3,4).
Yes-associated protein (YAP) and TAZ are the primary downstream
effectors of the Hippo signaling pathway, and these two effectors
can be phosphorylated and inhibited by the Hippo pathway kinases
LATS1/2 (5,6). There are multiple studies on the
correlation between abnormal TAZ activation and human cancers,
including breast (7), ovarian
(8), colorectal (9), lung (10) and brain cancers (11).
Epithelial-mesenchymal transition (EMT) is a
complicated process that leads to the transdifferentiation of
epithelial cells into mesenchymal cells (12). EMT is induced by various molecular,
cellular, and microenvironmental signals, particularly those caused
by the transforming growth factor-β (TGF-β) signaling pathway and
its induction of key EMT regulators, including members of the
Twist, Zeb, and Snail families of transcription factors (13). Connective tissue growth factor
(CTGF) is a secreted matricellular protein that participates in a
series of events in cellular proliferation and angiogenesis.
Recently, CTGF has been implicated as an important regulator in
TGF-β-induced EMT (14).
The effect of TAZ on the metastatic progression of
neuroblastoma and the underlying mechanisms involved remain
elusive. The aim of the current study was to investigate the effect
of TAZ on the expression level of CTGF and consequently its role in
the EMT-like metastatic progression of neuroblastoma cells.
Materials and methods
Cell culture and transfection
The SK-N-SH and SK-N-BE(2) cell lines were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). SK-N-SH was cultured in Dulbecco’s modified Eagle’s medium
(DMEM; Invitrogen Life Technologies, Carlsbad, CA, USA) containing
10% fetal bovine serum (FBS; Invitrogen Life Technologies) and
SK-N-BE(2) was cultured in advanced DMEM/Nutrient Mixture F-12
(DMEM/F12; Invitrogen Life Technologies) containing 10% FBS. HEK
293 cells were maintained and cultured in DMEM supplemented with
10% FBS. All cell lines were incubated at 37°C in humidified air
containing 5% CO2.
Cells were transfected with human influenza
hemagglutinin (HA)-tagged TAZ or Flag-tagged Smad3 and Smad4 (all
OriGene Technologies, Rockville, MD, USA) using Lipofectamine™ 2000
reagent (Invitrogen Life Technologies), according to the
manufacturer’s instructions. siRNA oligonucleotides against human
TAZ (ON-TARGETplus SMARTpool) and non-targeting siRNAs control
oligonucleotides were obtained from GE Dharmacon (Lafayette, CO,
USA) and transfected using DharmaFECT1 siRNA transfection reagent
(GE Dharmacon).
Western blot analysis
Whole cell extracts were prepared using Laemmli
buffer (Invitrogen Life Technologies). Samples were run on a 10%
sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to
a nitrocellulose membrane (Invitrogen Life Technologies). Membranes
were blocked in 10% milk solution [Tris buffered saline with 0.2%
Tween 20 (TBST)] for 2 h at room temperature and incubated with the
indicated primary antibody overnight at 4°C. The membranes were
washed three times for 10 min in TBST at room temperature and
incubated for 2 h with the corresponding horseradish peroxidase
(HRP)-conjugated secondary antibody (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Proteins were detected using the
enhanced chemiluminiscence system (GE Healthcare Life Sciences,
Chalfont, UK) according to the manufacturer’s instructions. The
primary antibodies used for western blot analysis were mouse
monoclonal anti-TAZ (1:1,000; cat no. ab129153; Abcam, Cambridge,
UK), rabbit polyclonal anti-CTGF (1:1,000; cat no. sc-25440), mouse
monoclonal anti-GAPDH (1:1,000; cat no. sc-32233; Santa Cruz
Biotechnology, Inc.), mouse monoclonal anti-Vimentin (1:1,000; cat
no. 550513), mouse monoclonal anti-E-cadherin (1:1,000; cat no.
610182) and mouse monoclonal anti-β-catenin (1:1,000; cat no.
610153; BD Biosciences, Franklin Lakes, NJ, USA).
mRNA isolation and quantitative reverse
transcription polymerase chain reaction (RT-qPCR)
mRNA was isolated using TRIzol®
(Invitrogen Life Technologies) and cDNA was prepared using
Transcriptor First Strand cDNA Synthesis kit (Roche, Mannheim,
Germany) according to the manufacturer’s instructions. The mRNA was
reverse-transcribed, and 1/20 volume of the reverse-transcribed
product was used for the subsequent qPCR. The primers used for TAZ
gene were as follows: forward, 5′-CTTGGATGTAGCCATGACCTT-3′ and
reverse, 5′-TCAATCAAAACCAGGCAATG-3′.
Transwell® migration and
invasion assays
Transwell® invasion experiments were
performed with 24-well plates with Matrigel™-coated chambers (8 μm
pore size) from BD Biosciences. Briefly, cells were allowed to grow
to subconfluency (~80%) and serum starved for 24 h. Following
detachment with trypsin, cells were washed with PBS, resuspended in
serum-free medium and 2×104 cells were added to the
upper chamber. Complete medium was added to the bottom wells of the
chambers. After 24 h, the cells that had not migrated were removed
from the upper face of the filters using cotton swabs, and the
cells that had migrated were fixed and stained with crystal violet
solution. Cell migration assays were performed similarly, however,
without Matrigel™ coating.
Luciferase assay
HEK 293 cells were cotransfected with the indicated
plasmids. A TK-Renilla expression plasmid (Promega
Corporation, Madison, WI, USA). was used as an internal control.
Luciferase activity was measured after 24 h using a Dual Luciferase
Assay kit according the manufacturer’s instructions (Promega
Corporation). Statistical analysis was performed using GraphPad
Prism version 4.0 (La Jolla, CA, USA).
Chromatin immunoprecipitation (ChIP)
Cells were cross-linked using formaldehyde, the
nuclei were isolated and sonicated and the DNA-protein complexes
were immunoprecipitated with an anti-HA antibody. The
immunoprecipitated DNA was de-cross-linked, digested with
proteinase K and purified for PCR amplification. The ChIP-enriched
DNA was subjected to PCR using the following CTGF primers: sense,
5′-GGAGTGGTGCGAAGAGGATA-3′, and antisense,
5′-GCCAATGAGCTGAATGGAGT-3′.
Statistical analysis
The Student’s t-test was used for comparisons
between two groups. Comparisons between three or more groups were
analyzed with a one-way analysis of variance followed by the
Duncan’s test using SPSS version 15.0 (SPSS Inc., Chicago, IL,
USA).
Results
Expression of TAZ migration and invasion
properties of SK-N-BE(2) cells correlate with increasing expression
levels of TAZ
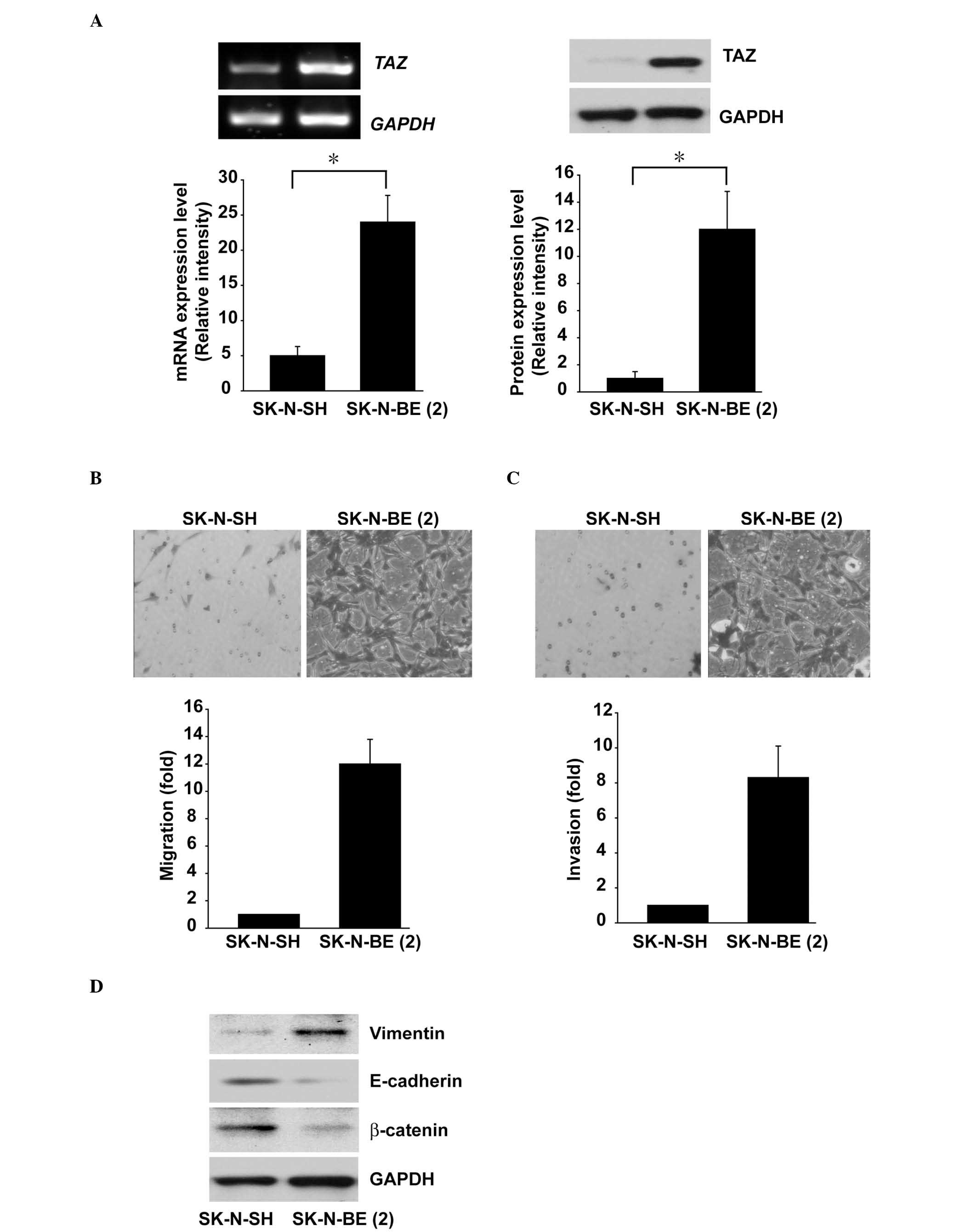
The SK-N-BE(2) cell line is a tumorigenic,
aggressive and MYCN gene amplified neuroblastoma cell line. The TAZ
mRNA and protein expression levels in SK-N-BE(2) cells were
significantly higher than those in SK-N-SH cells, which are less
aggressive and MYCN gene non-amplified (Fig. 1A). The migration and invasion
abilities of SK-N-BE(2) cells, analyzed via Transwell®
migration and Matrigel™ invasion assays, were higher than those in
the SK-N-SH cells (Fig. 1B and C).
These results indicated that TAZ may have a role in neuroblastoma
cell migration and invasion. Subsequently, the EMT protein
expression levels in the two cell lines were evaluated using
western blot analysis. The results revealed that the mesenchymal
marker Vimentin was upregulated in the SK-N-BE(2) cells compared
with the levels in the SK-N-SH cells; furthermore, the expression
levels of the epithelial markers E-cadherin and β-catenin were
downregulated in the SK-N-BE(2) cells compared with those of the
SK-N-SH cells (Fig. 1D).
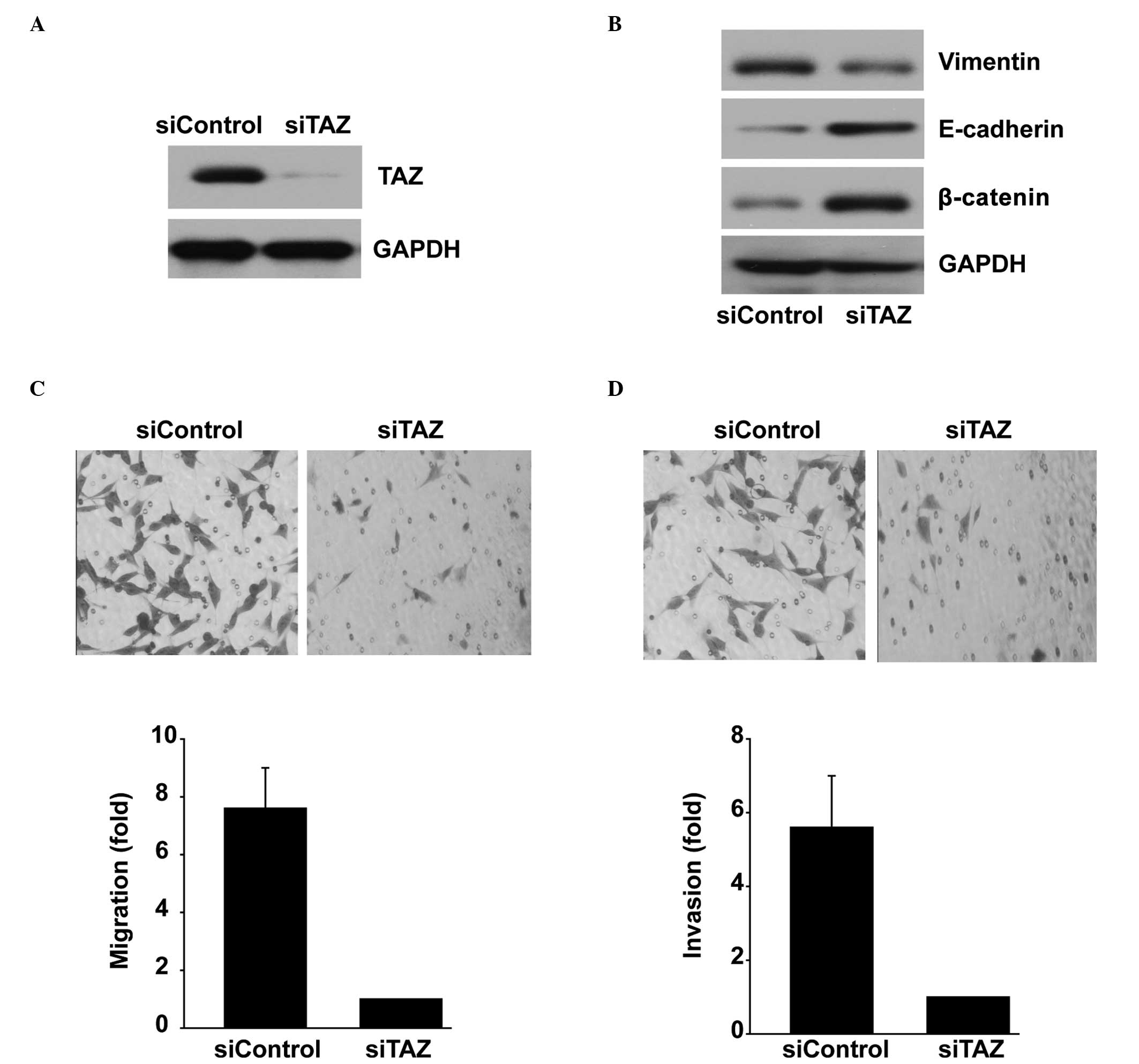
Repression of TAZ expression in
SK-N-BE(2) cells results in a decreased aggressiveness of the cell
line
TAZ was knocked down by siRNA in the aggressive
SK-N-BE(2) neuroblastoma cell line, which expresses high levels of
TAZ. Knockdown of TAZ in SK-N-BE(2) cells resulted in a marked
reduction at the TAZ protein expression level (Fig. 2A). Specific TAZ RNA interference
(RNAi) suppressed the protein expression levels of the mesenchymal
marker Vimentin. In contrast, the expression levels of the
epithelial markers E-cadherin and β-catenin were increased
(Fig. 2B). Compared with the mock
control, the reduced expression of TAZ via RNAi reduced the
migration and invasion abilities of SK-N-BE(2) cells (Fig. 2C and D).
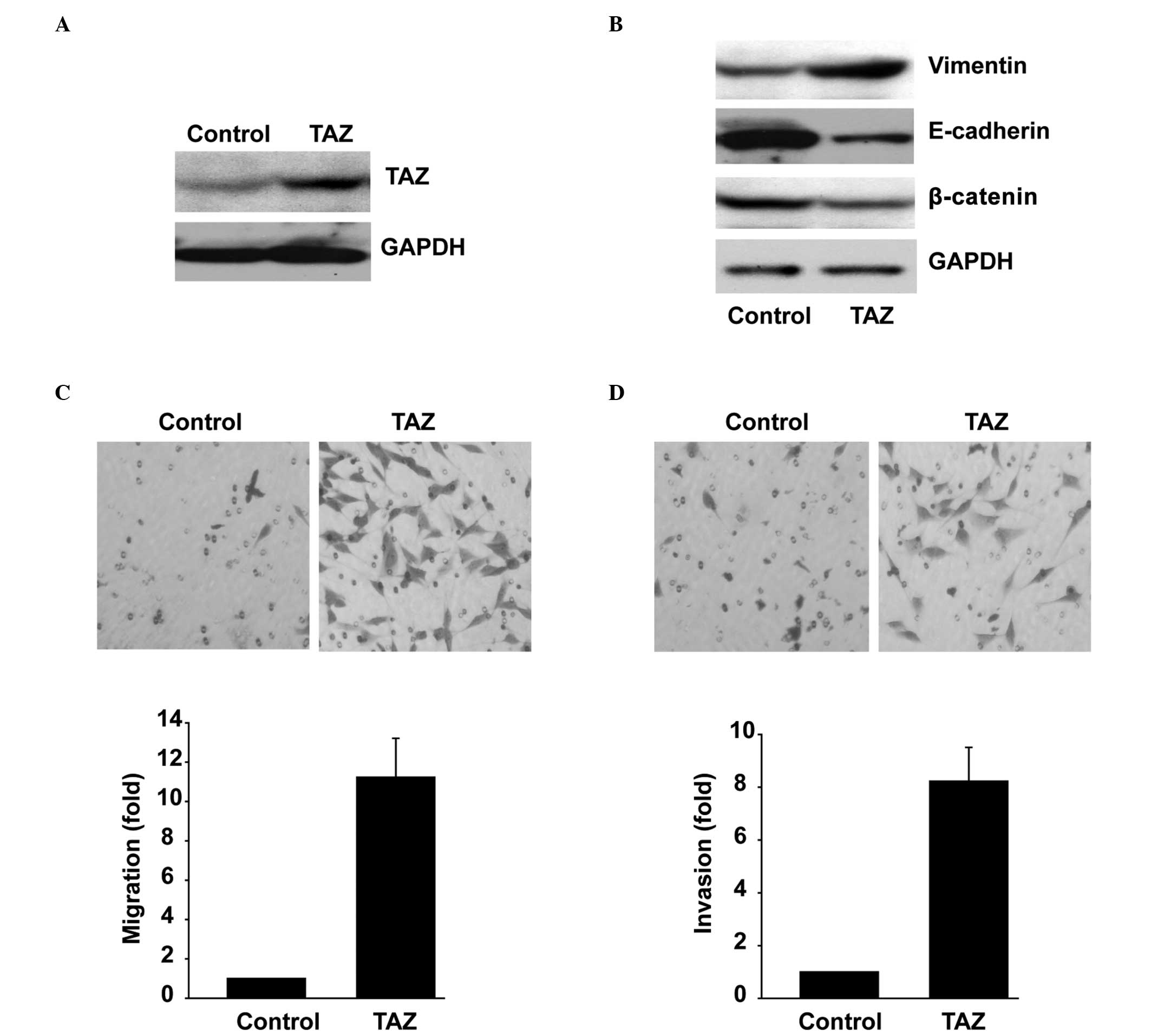
Overexpression of TAZ in SK-N-SH cells
results in EMT and increased invasiveness
SK-N-SH cells overexpressing TAZ expressed increased
levels of the mesenchymal marker Vimentin, and reduced levels of
epithelial markers, including E-cadherin and β-catenin, compared
with those in the mock control (Fig 3A
and B). To determine if the TAZ-induced EMT-like phenotype
could be translated into the aggressive ability of the SK-N-SH
cells, the migration and invasion of SK-N-SH cells was
investigated. SK-N-SH cells overexpressing TAZ showed an increase
in motility in the migration and invasion assays, compared with
that of the mock control cells (Fig.
3C and D).
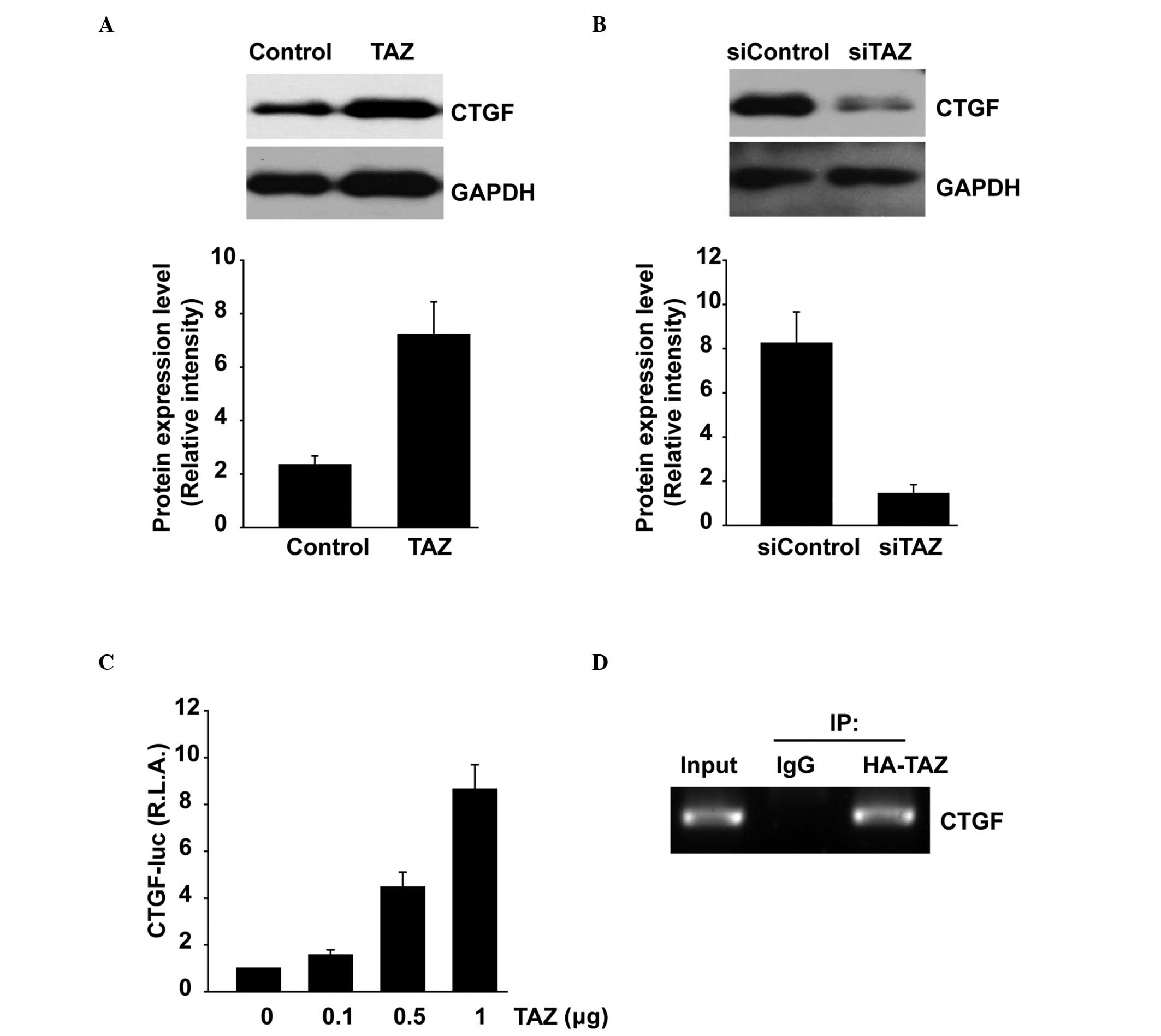
Overexpression of TAZ upregulates CTGF
expression but knockdown of TAZ downregulates it
To explore the mechanism by which TAZ induces EMT
and increased invasiveness, the regulatory effect of TAZ on the
expression of CTGF was investigated. As expected, ectopic TAZ
expression clearly induced CTGF at the protein level (Fig. 4A). In contrast, TAZ suppression via
RNAi reduced the CTGF expression level compared to that in the
controls (Fig. 4B). Subsequently,
it was assessed whether TAZ promotes the activity of the CTGF gene
promoter. The CTGF luciferase reporter constructs (CTGF-Luc) were
transiently transfected into HEK 293 cells with the TAZ expression
plasmids. The luciferase reporter activity increased in a
concentration-dependent manner in cells with ectopic TAZ expression
compared with that in the mock control (Fig. 4C), suggesting that ectopic TAZ
expression promotes the activity of CTGF. To determine if TAZ
interacted with the endogenous CTGF promoter, ChIP assays were
performed. CGTF promoter was detected in PCR-amplified DNA
fragments immunoprecipitated with an anti-HA antibody but not in
DNA fragments precipitated with an IgG control antibody, indicating
that TAZ bound to the CTGF promoter (Fig. 4D).
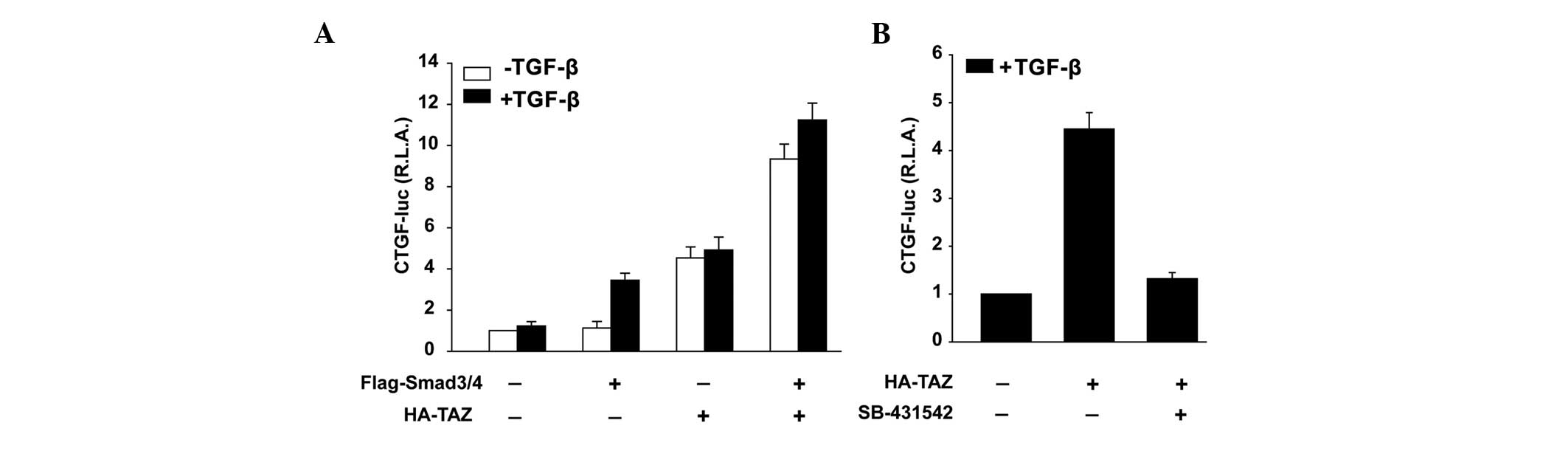
TAZ induces CTGF expression via
modulating the activation of TGF-β/Smad3 signaling pathway
Smad3 and Smad4 are the two predominant Smads that
form complexes in response to TGF-β and are primarily responsible
for the EMT phenotype. Cotransfection of Smad3 and Smad4 in SK-N-SH
cells overexpressing TAZ led to a strong induction of CTGF promoter
activity compared with that of the untransfected cells (Fig. 5A). In contrast, treatment with
SB-431542, a specific and selective inhibitor of TGF-β signaling,
inhibited the CTGF promoter activity induced by TAZ (Fig. 5B).
Discussion
TAZ, a 14-3-3 binding protein, can interact with a
number of transcription factors (15–17).
In the present study, the results demonstrated that TAZ
transcriptionally activates CTGF, thereby promoting EMT and
invasive progression. Furthermore, this effect is mediated by the
TGF-β/Smad3 signaling pathway, as blocking the pathway inhibits
TAZ-induced CTGF promoter activity. The expression of TAZ in
neuroblastoma cell lines was investigated at the mRNA level by
RT-qPCR and at the protein level by western blotting. Compared with
SK-N-SH cells, the migration and invasion of SK-N-BE(2) cells was
markedly higher which was accompanied by higher expression of
TAZ.
EMT is necessary for promoting cancer metastasis
(18–20). To demonstrate the role of TAZ in
EMT, the expression of TAZ was silenced using siRNA in SK-N-BE(2)
cells with highly invasive potential and TAZ high expression.
Furthermore, TAZ was overexpressed in SK-N-SH cells with low
invasion and TAZ low expression. It was determined that variation
in the TAZ expression level correlates with the expression levels
of several putative EMT marker. In addition, assessment of the
migratory and invasive potential of SK-N-BE(2) cells following
transfection with TAZ-siRNA indicated that the rate of cell
invasion was markedly reduced compared to those in mock control
group, suggesting that TAZ contributes to the migration and
invasive potential of neuroblastoma cells.
Although TAZ is thought to contribute to EMT
(21), it remains unknown by which
molecular mechanism the transcription factor achieves its
deleterious effects. It has been established that the TGF-β
signaling pathway induces EMT during cancer progression. A recent
report revealed that CTGF is required for TGF-β-induced EMT
(14). The present study provides
a mechanistic explanation in the case of TAZ; i.e., through
inducing transcription as exemplified for the CTGF gene.
In conclusion, the present study is, to the best of
our knowledge, the first to show that TAZ induces EMT to elicit
invasion in neuroblastoma cells. The function of TAZ as an oncogene
may be associated with several important molecules involved in the
invasion of cancer cells. These results further suggest that TAZ
may serve as a potential target for the development of therapies
for neuroblastoma.
References
|
1
|
Barbieri E, De Preter K, Capasso M, et al:
A p53 drug response signature identifies prognostic genes in
high-risk neuroblastoma. PLoS One. 8:e798432013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bénard J, Raguénez G, Kauffmann A, et al:
MYCN-non-amplified metastatic neuroblastoma with good prognosis and
spontaneous regression: a molecular portrait of stage 4S. Mol
Oncol. 2:261–271. 2008. View Article : Google Scholar
|
|
3
|
Couzens AL, Knight JD, Kean MJ, et al:
Protein interaction network of the mammalian Hippo pathway reveals
mechanisms of kinase-phosphatase interactions. Sci Signal.
6:rs152013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwon Y, Vinayagam A, Sun X, et al: The
Hippo signaling pathway interactome. Science. 342:737–740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao J, Zhang Y, Wang Y, et al: Role of
extracellular matrix and YAP/TAZ in cell fate determination. Cell
Signal. 26:186–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dupont S, Morsut L, Aragona M, et al: Role
of YAP/TAZ in mechanotransduction. Nature. 474:179–183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan SW, Lim CJ, Guo K, et al: A role for
TAZ in migration, invasion, and tumorigenesis of breast cancer
cells. Cancer Res. 88:2592–2598. 2008. View Article : Google Scholar
|
|
8
|
Jeong GO, Shin SH, Seo EJ, et al: TAZ
mediates lysophosphatidic acid-induced migration and proliferation
of epithelial ovarian cancer cells. Cell Physiol Biochem.
32:253–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Shi S, Guo Z, et al:
Overexpression of YAP and TAZ is an independent predictor of
prognosis in colorectal cancer and related to the proliferation and
metastasis of colon cancer cells. PLoS One. 8:e655392013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie M, Zhang L, He CS, et al: Prognostic
significance of TAZ expression in resected non-small cell lung
cancer. J Thorac Oncol. 7:799–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhat KP, Salazar KL, Balasubramaniyan V,
et al: The transcriptional coactivator TAZ regulates mesenchymal
differentiation in malignant glioma. Genes Dev. 25:2594–2609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mikaelian I, Malek M, Gadet R, et al:
Genetic and pharmacologic inhibition of mTORC1 promotes EMT by a
TGF-β-independent mechanism. Cancer Res. 73:6621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sonnylal S, Xu S, Jones H, et al:
Connective tissue growth factor causes EMT-like cell fate changes
in vivo and in vitro. J Cell Sci. 126:2164–2175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong JH, Hwang ES, McManus MT, et al: TAZ,
a transcriptional modulator of mesenchymal stem cell
differentiation. Science. 309:1074–1078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murakami M, Nakagawa M, Olson EN and
Nakagawa O: A WW domain protein TAZ is a critical coactivator for
TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc
Natl Acad Sci USA. 102:18034–18039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park KS, Whitsett JA, Di Palma T, et al:
TAZ interacts with TTF-1 and regulates expression of surfactant
protein-C. J Biol Chem. 279:17384–17390. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype. Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao D, Dai C and Peng S: Mechanism of the
mesenchymal-epithelial transition and its relationship with
metastatic tumor formation. Mol Cancer Res. 9:1608–1620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lei QY, Zhang H, Zhao B, et al: TAZ
promotes cell proliferation and epithelial-mesenchymal transition
and is inhibited by the hippo pathway. Mol Cell Biol. 28:2426–2436.
2008. View Article : Google Scholar : PubMed/NCBI
|