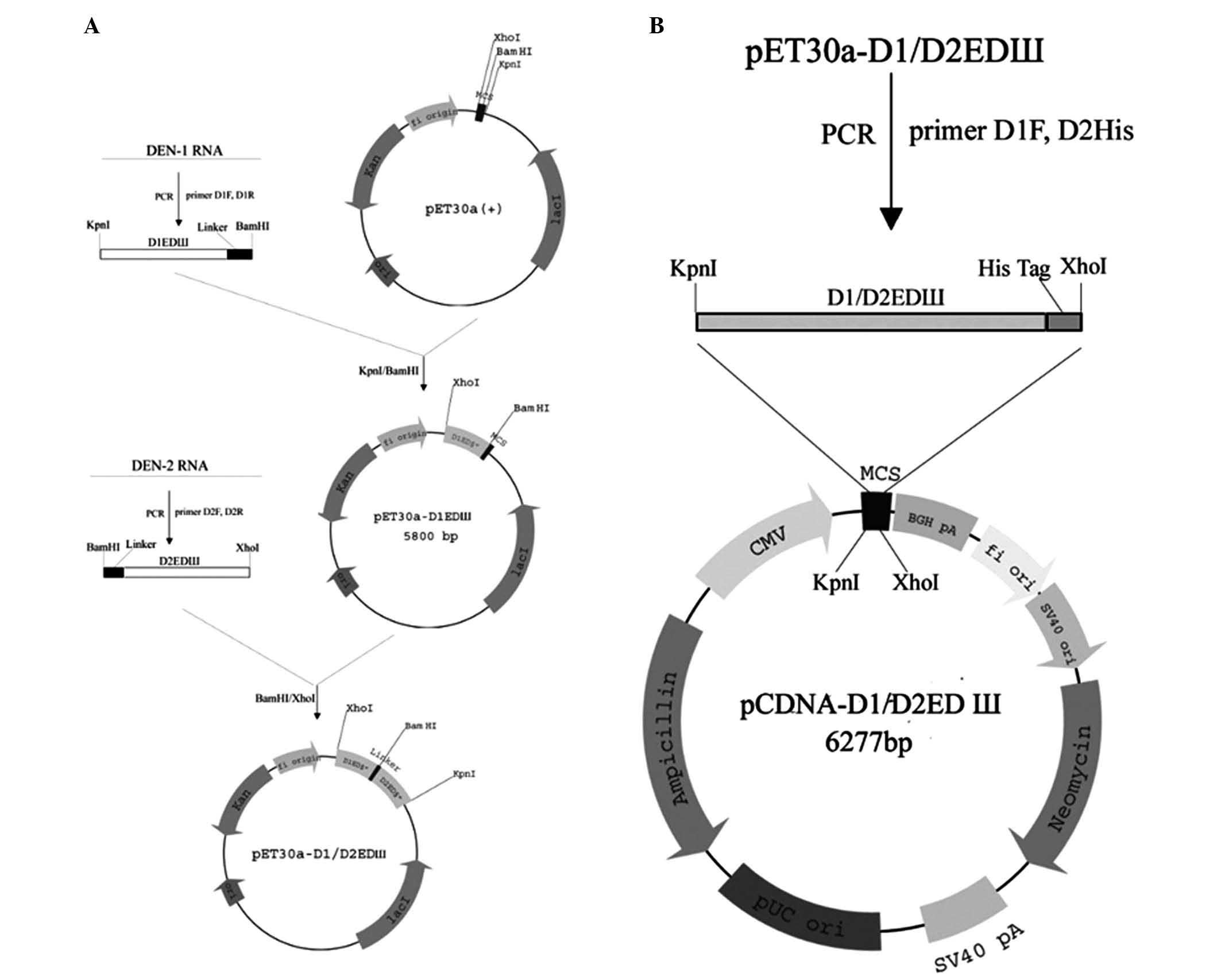
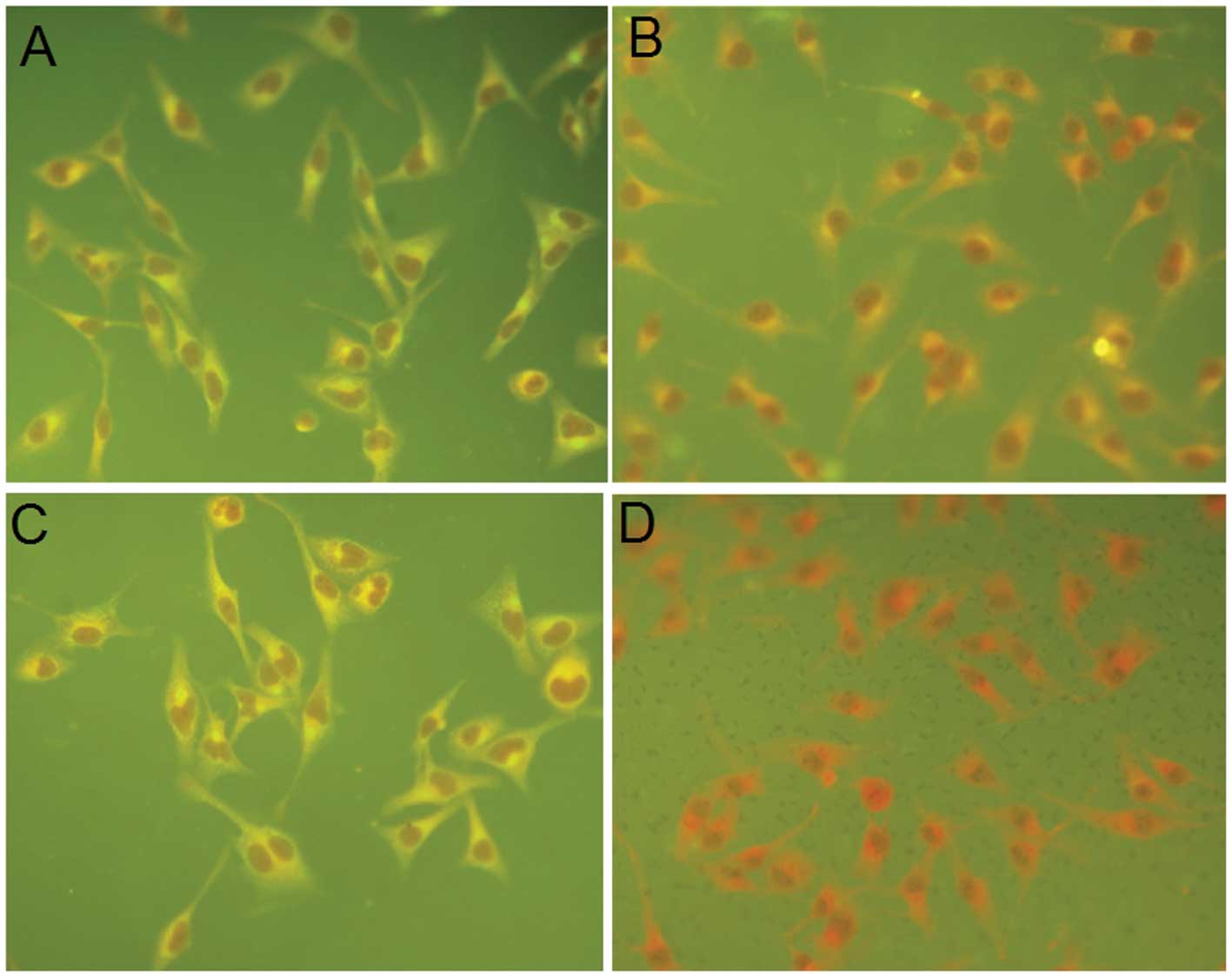
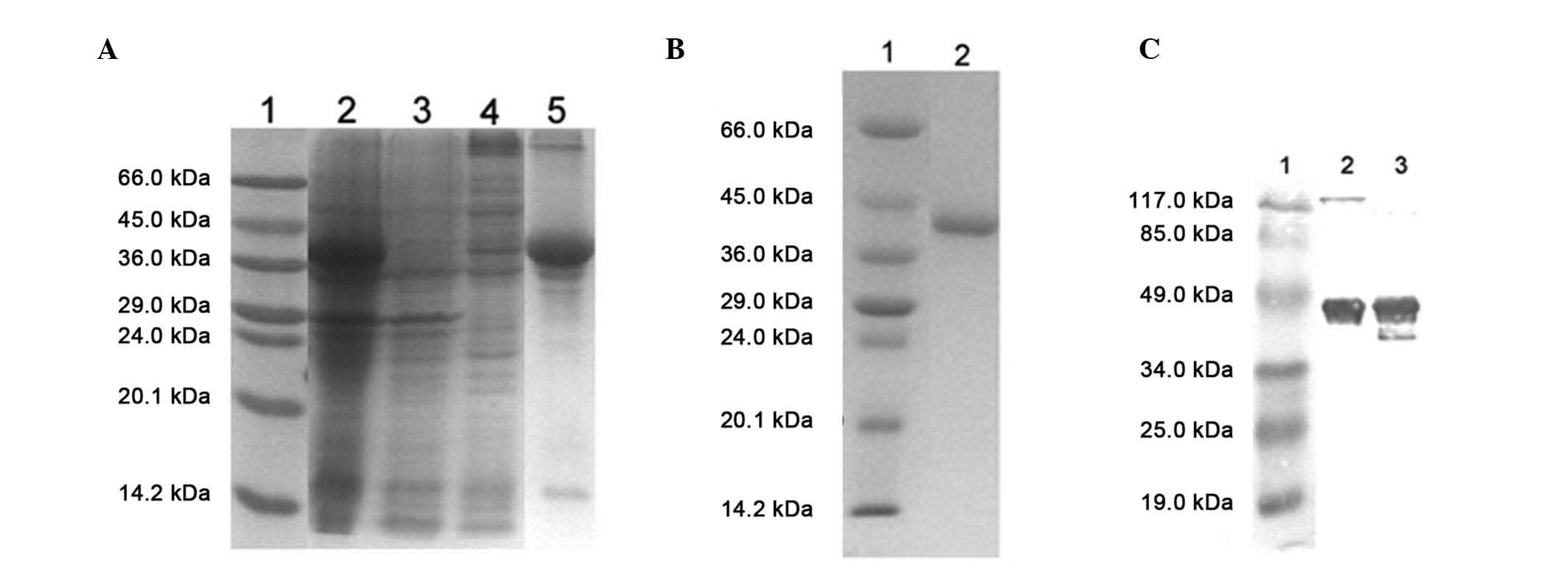
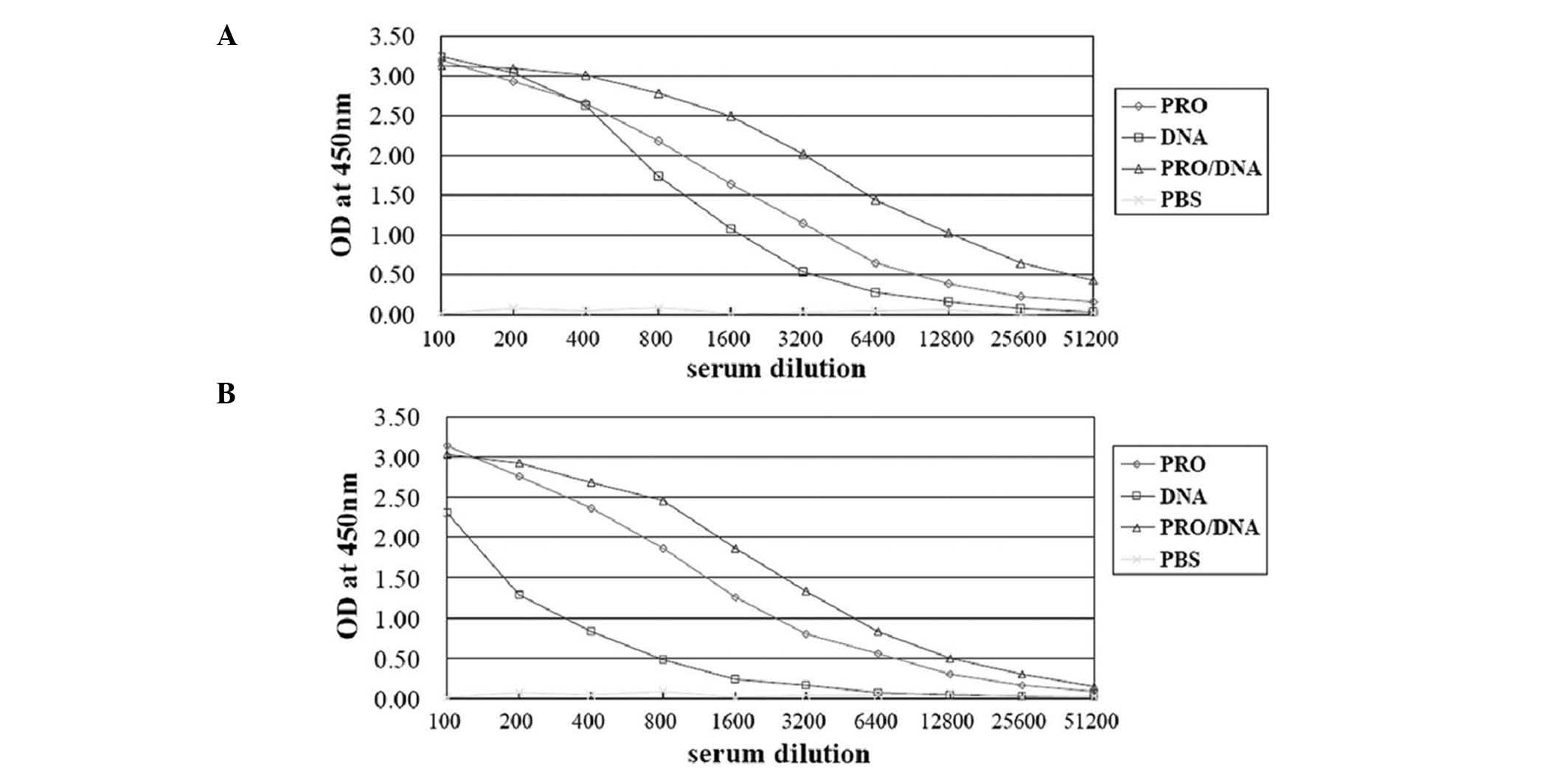
|
1
|
Agarwal R, Kapoor S, Nagar R, et al: A
clinical study of the patients with dengue hemorrhagic fever during
the epidemic of 1996 at Lucknow, India. Southeast Asian J Trop Med
Public Health. 30:735–740. 1999.
|
|
2
|
Rosen L: Comments on the epidemiology,
pathogenesis and control of dengue. Med Trop (Mars). 59:495–498.
1999.
|
|
3
|
Morens DM and Halstead SB: Measurement of
antibody-dependent infection enhancement of four dengue virus
serotypes by monoclonal and polyclonal antibodies. J Gen Virol.
71:2909–2914. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson R, Wang S, Osiowy C and Issekutz
AC: Activation of endothelial cells via antibody-enhanced dengue
virus infection of peripheral blood monocytes. J Virol.
71:4226–4232. 1997.PubMed/NCBI
|
|
5
|
Bhamarapravati N and Sutee Y: Live
attenuated tetravalent dengue vaccine. Vaccine. 18(Suppl 2): 44–47.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eckles KH, Dubios DR, Putnak R, et al:
Modification of dengue virus strains by passage in primary dog
kidney cells: preparation of candidate vaccines and immunization of
monkeys. Am J Trop Med Hyg. 69(Suppl): 12–16. 2003.
|
|
7
|
Putnak R, Barvir DA, Burrous JM, et al:
Development of a purified, inactivated, dengue-2 virus vaccine
prototype in Vero cells: immunogenicity and protection in mice and
rhesus monkeys. J Infect Dis. 174:1176–1184. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guirakhoo F, Pugachev K, Zhang Z, et al:
Safety and efficacy of chimeric yellow fever-dengue virus
tetravalent vaccine formulations in nonhuman primates. J Virol.
78:4761–4775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van der Most RG, Murali-Krishna K, Ahmed R
and Strauss JH: Chimeric yellow fever/dengue virus as a candidate
dengue vaccine: Quantitation of the dengue virus-specific CD8
T-cell response. J Virol. 74:8094–8101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guirakhoo F, Arroyol J, Pugachev KV, et
al: Construction, safety, and immunogenecity in nonhuman primates
of a chimeric yellow fever dengue virus tetravalent vaccine. J
Virol. 75:7290–7304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simmons M, Murphy GS, Kochel T, et al:
Characterization of antibody responses to combinations of a
dengue-2 DNA and dengue-2 recombinant subunit vaccine. Am J Trop
Med Hyg. 65:420–426. 2001.PubMed/NCBI
|
|
12
|
Mota J, Acosta M, Argotte R, et al:
Induction of protective antibodies against dengue virus by
tetravalent DNA immunization of mice with domain III of the
envelope protein. Vaccine. 23:3469–3476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu SF, Liao CL, Lin YL, et al: Evaluation
of protective efficacy and immune mechanisms of using a
non-structural protein NS1 in DNA vaccine against dengue 2 virus in
mice. Vaccine. 21:3919–3929. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Men R, Wyatt L, Tokimatsu I, et al:
Immunization of rhesus monkeys with a recombinant of modified
vaccina virus Ankara expressing a truncated envelope glycoprotein
of dengue type 2 virus induced resistance to dengue type 2 virus
challenge. Vaccine. 18:3113–3122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simmons M, Nelson WM, Wu SJ and Hayes CG:
Evaluation of the protective efficacy of a recombinant dengue
envelope B domain fusion protein against dengue 2 virus infection
in mice. Am J Trop Med Hyg. 58:655–662. 1998.PubMed/NCBI
|
|
16
|
Simmons M, Murphy GS and Hayes CG: Short
report: Antibody response of mice immunized with a tetravalent
dengue recombinant protein subunit vaccine. Am J Trop Med Hyg.
65:159–161. 2001.PubMed/NCBI
|
|
17
|
Hermida L, Rodriguez R, Lazo L, et al: A
dengue-2 envelope fragment inserted within the structure of the
P64k meningococcal protein carrier enables a functional immune
response against the virus in mice. J Virol Methods. 115:41–49.
2004. View Article : Google Scholar
|
|
18
|
Kelly EP, Greene JJ, King AD and Innis BL:
Purified dengue 2 virus envelope glycoprotein aggregates produced
by baculovirus are immunogenic in mice. Vaccine. 18:2549–2559.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Maguire T and Marks RM:
Demonstration of binding of dengue envelope protein to target
cells. J Virol. 70:8765–8772. 1996.PubMed/NCBI
|
|
20
|
Bray M and Lai CJ: Dengue virus
premembrane and membrane proteins elicit a protective immune
response. Virology. 185:505–508. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rey FA, HeinA FX, Mandl C, et al: The
envelope glycoprotein from tick-borne encephalitis virus at 2 A
resolution. Nature. 375:291–298. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roehrig JT, Volpe KE, Squires J, et al:
Contribution of disulfide bridging to epitope expression of the
dengue type 2 virus envelope glycoprotein. J Virol. 78:2648–2652.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhardwaj S, Holbrook M, Shope RE, et al:
Biophysical characterization and vector-specific antagonist
activity of domain III of the tick-borne flavivirus envelope
protein. J Virol. 75:4002–4007. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crill WD and Roehrig RT: Monoclonal
antibodies that bind to domain III of dengue virus E glycoprotein
are the most efficient blockers of virus E glycoprotein are most
efficient blockers of virus adsorption to Vero cells. J Virol.
75:7769–7773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Megret F, Hugnot JP, Falconar MK, et al:
Use of recombinant fusion proteins and monoclonal antibodies to
define linear and discontinuous antigenic sites on the dengue
envelope glycoprotein. Virology. 187:480–491. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roehrig JT, Johnson AJ, Hunt AR, et al:
Antibodies to dengue 2 virus E-glycoprotein synthetic peptides
identity antigenic conformation. Virology. 177:668–675. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hung JJ, Hsieh MT, Young MJ, et al: An
external loop region of domain III of dengue virus type 2 envelope
protein is involved in serotype-specific binding to mosquito but
not mammalian cells. J Virol. 78:378–388. 2004. View Article : Google Scholar :
|
|
28
|
Simmons M, Nelson WM, Wu SJ and Hayes CG:
Evaluation of the protective efficacy of a recombinant dengue
envelope B domain fusion protein against dengue 2 virus infection
in mice. Am J Trop Med Hyg. 58:655–662. 1998.PubMed/NCBI
|
|
29
|
Morens DM, Halstead SB, Repik PM, et al:
Simplified plaque reduction assay for dengue viruses by
semi-micromethods in BHK21 cells: comparison of the BHK suspension
test standard plaque reduction neutralization. J Clin Microbio.
22:250–254. 1985.
|
|
30
|
Trirawatanapong T, Chandran B, Putnak R
and Padmanabhan R: Mapping of a region of dengue virus type-2
glycoprotein required for binding by a neutralizing monoclonal
antibody. Gene. 116:139–150. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun W, Edelman R, Kanesa-Thasan N, et al:
Vaccination of human volunteers with monovalent and tetravalent
live-attenuated dengue vaccine candidates. Am J Trop Med Hyg.
69(Suppl): 24–31. 2003.
|
|
32
|
Edelman R, Wasserman SS, Bodison SA, et
al: Phase I trial of 16 formulations of a tetravalent
live-attenuated dengue vaccine. Am J Trop Med Hyg. 69(Suppl):
48–60. 2003.
|
|
33
|
Kitchener S, Nissen M, Nasveld P, et al:
Immunogenicity and safety of two live-attenuated tetravalent dengue
virus vaccine formulations in healthy Australian adults. Vaccine.
24:1238–1241. 2006. View Article : Google Scholar
|
|
34
|
Sabchareon A, Lang J, Chanthavanich P, et
al: Safety and immunogenicity of a three dose regimen of two
tetravalent live-attenuated dengue vaccines in five- to
twelve-year-old Thai children. Pediatr Infect Dis J. 23:99–109.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swaminathan S and Khanna N: Viral vaccines
for dengue: the present and the future. WHO Dengue Bul. 27:181–191.
2003.
|
|
36
|
Kanesa-thasan N, Sun W, Kim-Ahn G, et al:
Safety and immunogenicity of attenuated dengue virus vaccines
(Aventis Pasteur) in human volunteers. Vaccine. 19:3179–3188. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jaiswal S, Khanna N and Swaminathan S:
High-level expression and one-step purification of recombinant
dengue virus type 2 envelope domain III protein in Escherichia
coli. Protein Expr Purif. 33:80–91. 2004. View Article : Google Scholar
|
|
38
|
Robinson CR and Sauer RT: Optimizing the
stability of single-chain proteins by linker length and composition
mutagenesis. Pro Natl Acad Sci USA. 95:5929–5934. 1998. View Article : Google Scholar
|
|
39
|
Putnak R, Feighny R, Burrous J, et al:
Dengue-1 virus envelope glycoprotien gene expressed in recombinant
baculovirus elicits virus-neutralizing antibody in mice and
protects them from virus challenge. Am J Trop Med Hyg. 45:159–167.
1991.PubMed/NCBI
|
|
40
|
Delenda C, Staropoli I, Frenkiel MP, et
al: Analysis of C-terminally truncated dengue 2 and dengue 3 virus
envelope glycoproteins: processing in insect cells and immunogenic
properties in mice. J Gen Virol. 75:1569–1578. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Khanam S, Etemad B, Khanna N and
Swaminathan S: Induction of neutralizing antibodies specific to
dengue virus serotypes 2 and 4 by a bivalent antigen composed of
linked envelope domains III of these two serotypes. Am J Trop Med
Hyg. 74:266–277. 2006.PubMed/NCBI
|
|
42
|
Chang GJ, Davis BS, Hunt AR, et al:
Flavivirus DNA vaccines: current status and potential. Ann N Y Acad
Sci. 951:272–285. 2001. View Article : Google Scholar
|
|
43
|
Sedegah M, Jones TR, Kaur M, et al:
Boosting with recombinant vaccinia increases immunogenicity and
protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA.
95:7648–7653. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barnett SW, Rajasekar S, Legg H, et al:
Vaccination with HIV-1 gp120 DNA induces immune responses that are
boosted by a recombinant gp 120 protein subunit. Vaccine.
15:869–873. 1997. View Article : Google Scholar : PubMed/NCBI
|