Introduction
Chronic myeloid leukemia (CML), is a clonal
myeloproliferative disorder of the hematopoietic stem cells, which
is defined by the overproduction of myeloid cells, leading to
marked splenomegaly and leukocytosis. If ill-controlled, CML
advances to the accelerated and blastic phases. The annual
incidence of CML in 2010 was reported to be one to two cases per
100,000 adults, accounting for 15% of newly diagnosed cases of
leukemia in adults (1). Exposure
to nuclear radiation increases the risk of CML, the peak incidence
occurs 5–12 years post-exposure and the risk is dose-dependent
(1,2). Due to an increase in exposure of the
general population to radiation, either medically (cancer patients
undergoing radiotherapy and radiologists) or incidentally (exposure
as a result of a nuclear accident, for example in Fukushima), it is
predicted that the prevalence of CML will increase gradually over
time. Hence, the development of more effective therapeutic options
for leukemia patients is indispensable.
In the human genome, protein-coding exons account
for only 1.5% of the DNA (3) and
the vast non protein-coding portions of the genome are of critical
functional importance. One class of small non-coding RNAs,
microRNAs, is of particular interest (4–6).
miRNAs have been previously reported to be involved in a number of
human diseases, including cancer, and it has been demonstrated that
epigenetic and genetic defects in miRNAs and their processing
machinery are common hallmarks of disease (3,7).
Certain miRNAs (for example miR-15, miR-21 and miR-29) are
considered to be therapeutic targets in the treatment of
cardiovascular diseases (8), and
it is almost certain that an increasing number of miRNAs will be
investigated for their functions in disease diagnosis, development
and therapeutics. In the present study, the involvement of miRNAs
in chronic myeloid leukemia was investigated using various
Bcr-Abl-expressing cell lines.
Materials and methods
Cell culture and erythroid
differentiation
K562 and KCL-22 human chronic myelogenous leukemia
cell lines were obtained from the American Type Culture Collection
(Manassas, VA, USA). The cell lines were cultivated in RPMI-1640
medium with 10% (v/v; Invitrogen Life Technologies, Carlsbad, CA,
USA) fetal calf serum (FCS; Invitrogen Life Technologies) and 1%
(v/v) penicillin/streptomycin (Gibco-BRL, Carlsbad, CA, USA). To
induce erythroid differentiation, cells were treated with 2 mM
sodium butyrate.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen Life technologies) and cDNA was
synthesized using the PrimeScript RT reagent kit (Takara
Biotechnology Co. Ltd., Dalian, China). qPCR was performed using
SYBR® Green master mix, Universal RT kit for the
detection of microRNA (Exiqon, Vedbaek, Denmark) and the relative
primers were all obtained from Exiqon. qPCR was carried out with
primers for miR-107, miR-17, miR-34c and Cacna2d1 (Table I) with SYBR in the 7900 HT
real-time PCR System (Qiagen, Valencia, CA, USA) using the
following protocol: 95°C for 5 min, followed by 40 cycles of 95°C
for 10 sec and 60°C for 30 sec. All experiments were performed in
triplicate. U6 RNA was used as an endogenous control.
 | Table IPrimers for miR-107, miR-17, miR-34c,
Cacna2d1. Primers for Cacna2d1-3′-UTR in the luciferase reporter
assay. |
Table I
Primers for miR-107, miR-17, miR-34c,
Cacna2d1. Primers for Cacna2d1-3′-UTR in the luciferase reporter
assay.
| Forward | Reverse |
|---|
| miR-107 |
5′-AAAGAATTCCTGTTTCACTCGCCAAGC-3′ |
5′-AAAGGATCCAGCGAGTGAGGAGGGAGA-3′ |
| miR-17 |
5′-GCCGCAAAGTGCTTACAGTG-3′ |
5′-TGCAGGGTCCGAGGTAT-3′ |
| miR-34c |
5′-GCGCTAGGCAGTGTAGTTAG-3′ |
5′-GTGCAGGGTCCGAGGT-3′ |
| Cacna2d1 |
5′-GCTCATTCTGGTGGACGTGAG-3′ |
5′-TCACGGCATCTTTCAACACCT-3′ |
| (for luciferase) |
5′-TCTCCCATCGCCGTGGGCCAAAACATGAGCCCTCAGT-3′ |
5′-CTTCTAGATCACTTCGCCGTCTGCCTAACCC-3′ |
| miR-107 mimics |
5′-AGCAGCAUUGUACAGGGCUAUCA-3′ |
5′-AUAGCCCUGUACAAUGCUGCUUU-3′ |
| miR-107
inhibitors |
5′-UGAUAGCCCUGUACAAUGCUGCU-3′ |
5′-TCATTGGCATGTACCATGCAGCT-3′ |
Luciferase reporter assay
The target gene predicted by scanning the database,
TargetScan (www.targetscan.org), was Cacna2d1. A
wild-type 3’-UTR segment of murine Cacna2d1 mRNA (1099 bp)
containing the putative binding site for miR-107 was PCR-amplified
and inserted into the BstXI/XbaI site downstream of the stop codon
in the pGL3 firefly luciferase reporter, which was based upon the
pGL3-control (Promega, Madison, WI, USA) and created by Professor
Shi-mei Zhuang (Sun Yat-sen University, Guangzhou, China). The
primers used are presented in Table
I.
The firefly luciferase construct was cotransfected
with a control Renilla luciferase vector into cells in the
presence of either miR-107 mimics or miRNA inhibitors. A dual
luciferase assay (Promega) was performed 48 h following
transfection. The experiments were performed independently and in
triplicate.
Oligonucleotide transfection
miR-107 inhibitors and mimics were synthesized by
Genepharma (Shanghai, China). Oligonucleotide transfection was
performed with the Lipofectamine 2000 reagent (Invitrogen Life
Technologies). miR-107 mimics were used at a concentration of 50
nM, while miR-107 inhibitors were transfected at a concentration of
100 nM.
Western blot analysis
Whole cell lysates were prepared using a
radioimmunoprecipitation assay buffer (Sigma-Aldrich, St. Louis,
MO, USA). Equal amounts of proteins (10 mg/lane) were separated by
10% SDS-PAGE and transferred to polyvinylidene fluoride membranes.
Rabbit anti-mouse Cacna2d1 polyclonal antibody was purchased from
Santa Cruz Biotechnology (sc-133436; Santa Cruz, CA, USA).
Secondary goat anti-rabbit horseradish peroxidase-conjugated
antibody was obtained from Cell Signaling Technology (Beverly, MA,
USA). Signals were detected using an ECL chemiluminescence kit (EMD
Millipore, Billerica, MA, USA).
MTT assay
K562 and KCL-22 cells at the exponential growth
phase were harvested and adjusted to a final concentration of
5×104 cells per ml. The cells that were pretreated with
miR-107 or miR-control using Lipofectamine 2000 were set as
experiment groups, while untreated cells were used as the control
group, and cells that were cultured with gabapentin adjusted to
10−6 mol/l served as the positive control group. Cells
(5×103) were seeded into 96-well plates, with each of
the four groups assigned three columns of wells, and the plates
were incubated at 37°C in 5% CO2 for 5 days. Cell
Counting Kit-8 solution (10 μl; Beyotime Institute of
Biotechnology, Jiangsu, China) was added to each well and incubated
for 2 h at 37°C, with 5% CO2. The absorbance was
detected at a wavelength of 450 nm to measure the growth of the
cells.
Hemoglobin histochemical stain
The cells were treated as previously described for
the MTT assay and seeded into 24-well plates. Hemoglobin
histochemical staining was performed to measure the erythroid
differentiation level of the K562 and KCL-22 cells. Briefly, the
cells were harvested on day 4, 1×106 cells were
incubated with 1 ml benzidine (2 mg/ml) and 10 μl 30%
H2O2 and subsequently seeded on to a 96-well
plate with a total volume of 200 μl in each well. The absorbance
was detected at a wavelength of 570 nm.
Flow cytometric analysis
Cells (1×106 from each group) were
harvested and 1 μl OKT9 anti-human CD71-FITC antibody (eBioscience,
San Diego, USA) was added. Following incubation at 4°C for 30 min,
the cells were washed with phosphate buffered-saline (PBS) and
resuspended in 100 μl PBS for further analysis with a Calibur flow
cytometer equipped with Cellquest software (BD Biosciences,
Franklin Lakes, NJ, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed using a two-way
analysis of variance and a two-tailed paired Student’s t-test where
appropriate. P≤0.05 was considered to indicate a statistically
significant difference. All experiments were performed at least
three times.
Results
miR-107 promotes erythroid
differentiation of chronic myeloid leukemia cells
Through microRNA screening, it has previously been
demonstrated that miR-107 is downregulated in chronic myeloid
leukemia cells (manuscript in preparation). The current study used
qPCR to detect the expression of Bcr-Abl, a marker for the chronic
myeloid leukemia and miR-107. The results revealed a reciprocal
association between miR-107 and Bcr-Abl (Fig. 1A). The aim of this study was to
investigate the role of miR-107 in the biological behavior of
leukemia cells using the K562 and KCL-22 chronic myeloid leukemia
cell lines. The endogenous miR-107 expression of the cells was
altered via transfection with miR-107 using Lipofectamine 2000.
K562 and KCL-22 cells overexpressing miR-107 were cultured for 4
days. When compared with that of the miR-107-control or
gabapentin-treated cells, the proliferation rate of the miR-107
cells displayed no significant differences in the K562 or KCL-22
cell lines (Fig. 1B and C).
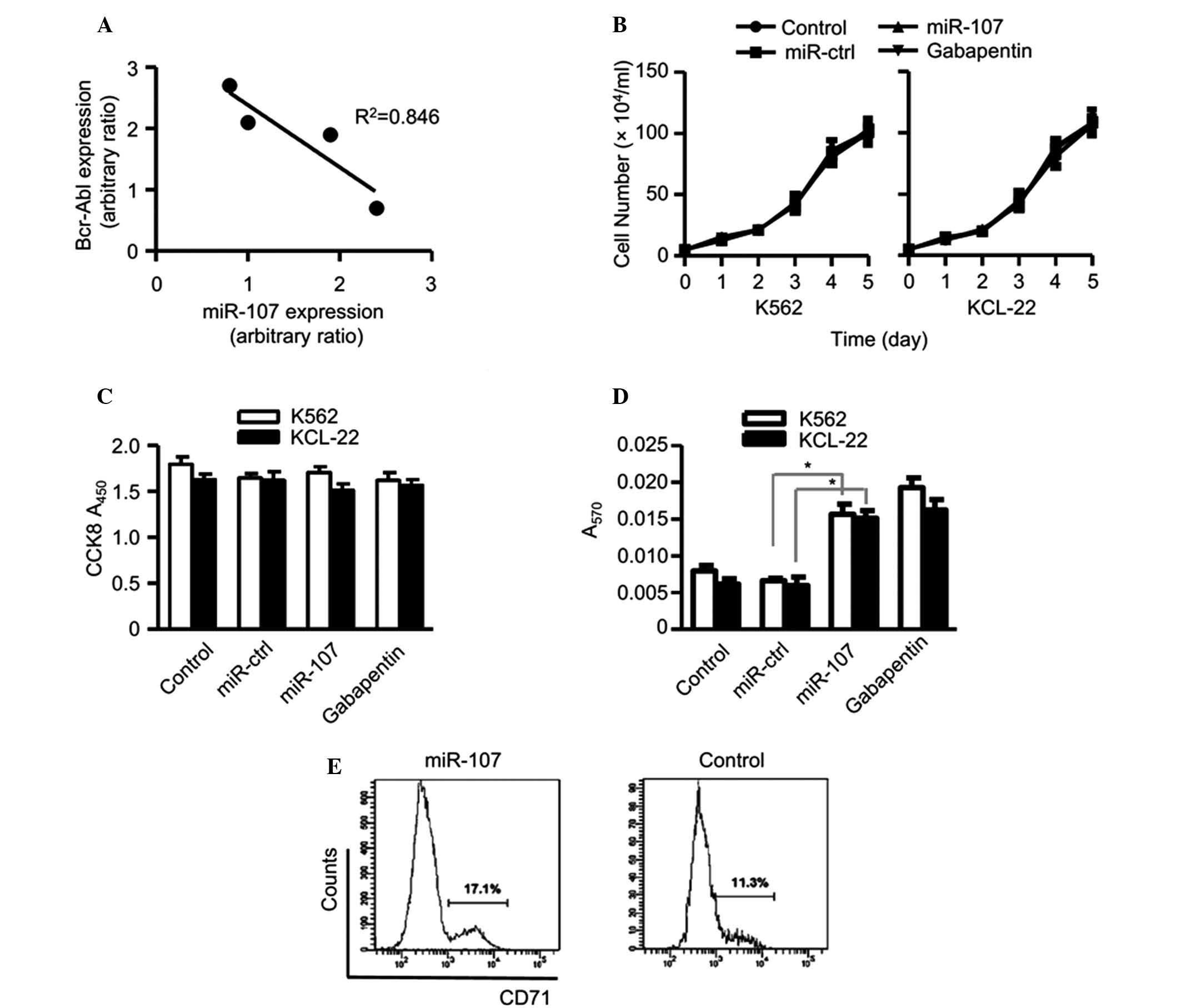 | Figure 1Overexpression of microRNA (miR)-107
may promote the erythroid differentiation of chronic myeloid
leukemia cells. Cell lines were harvested at the exponential growth
phase, adjusted to a final concentration of 5×104/ml and
divided into four groups: Control, no treatment; miR-control,
transfected with unrelated miRNAs; miR-107, transfected with 10 nM
miR-107 and gabapentin-positive control, treated with 10 nM
gabapentin. (A) The relative expression of Bcr-Abl and miR-107 were
set as the Y-axis and the X-axis, respectively. (B) The cells were
cultured in six-well plates for five days. Each day, the number of
cells was counted. There was no difference in the growth rate
between the four groups. (C) The cells were cultured in 96-well
plates for four days. On day four, Cell Counting kit-8 solution was
added to the medium and A450 was measured. There was no difference
in the cellular proliferation between the four groups. (D) The
cells were seeded in 24-well plates in conditional medium with
sodium butyrate. Hemoglobin histochemical staining was performed to
measure the erythroid differentiation level of K562 and KCL-22
cells. The absorbance was detected at 570 nm. In the miR-107 and
gabapentin groups, the differentiation rate was higher than that in
the control group. (E) The CD71 level was analyzed by flow
cytometry. In the miR-107 group, the level was higher than that in
control group. *P<0.05. |
Subsequently the differentiation status of the cells
was analyzed. Since it has previously been reported that K562 cells
have the ability to undergo erythroid differentiation, the current
study investigated the effect of miR-107 on this process. The cells
were treated with sodium butyrate to induce erythroid
differentiation. The results showed that the levels of hemoglobin
and CD71 were considerably higher in miR-107 overexpressed cells
than those in the controls (Fig. 1D
and E). These results demonstrate that miR-107 promotes
erythroid differentiation of chronic myeloid leukemia cells, while
having no effect on cellular proliferation.
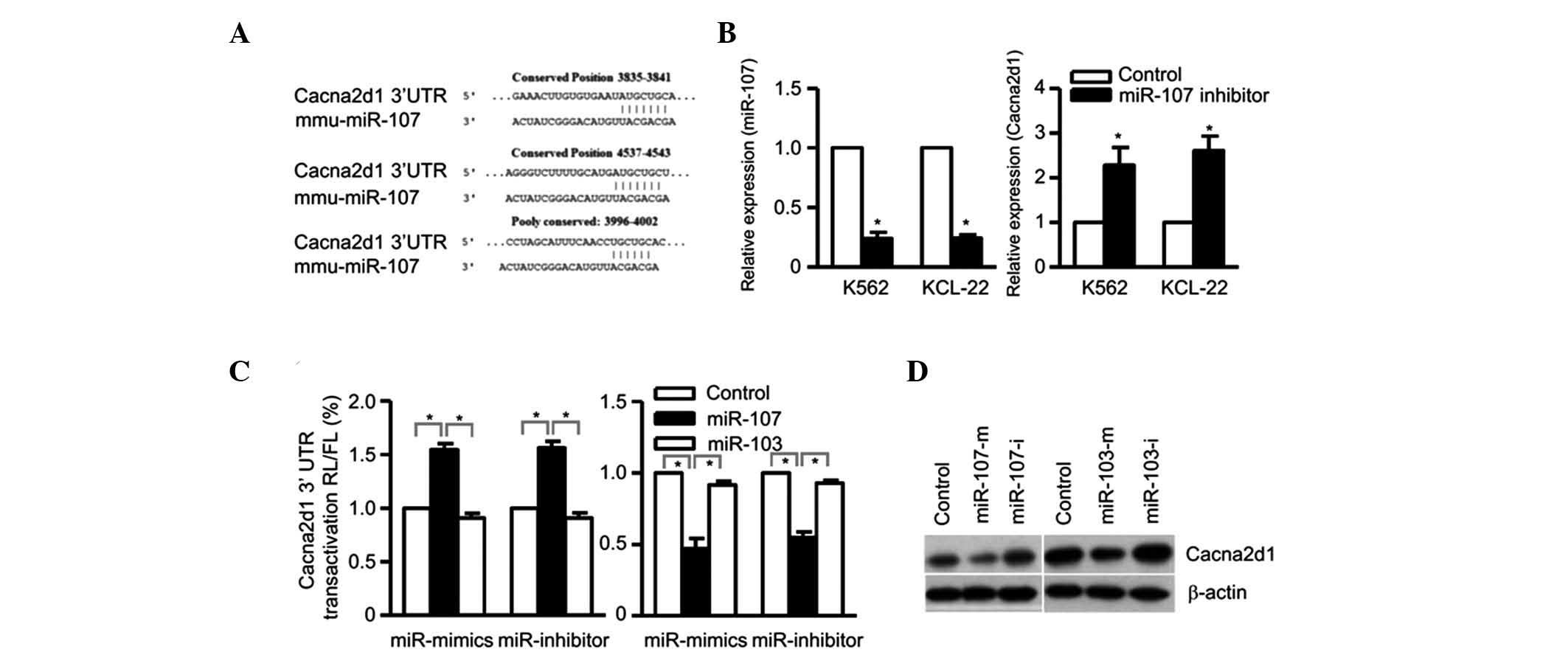
Cacna2d1 is a target of miR-107
In order to further elucidate the underlying
mechanism of mir-107 in K562 and KCL-22 erythroid differentiation,
the target of miR-107 had to be determined. By searching the
potential targets of miR-107 with TargetScan, Pictar (http://pictar.mdc-berlin.de), miRanda (www.microrna.org) and Microcosm (http://www.ebi.ac.uk/enright-srv/microcosm) software,
a list was compiled of all the predicted genes for functional
clustering analysis classified by the DAVID 6.7 (http://david.abcc.ncifcrf.gov), Kegg (www.genome.jp/kegg) and Panther (www.pantherdb.org) databases. Cacna2d1 contains a
putative target sequence, with two conserved and one poorly
conserved positions, and was identified as the possible target of
miR-107 (Fig. 2A). To validate
this in silico prediction, qPCR was used to determine
whether the expression of Cacna2d1 could be repressed by miR-107.
The results showed that with the miR-107 inhibitors in K562 and
KCL-22 cells, the mRNA expression level of Cacna2d1 was
significantly downregulated compared with that in the negative
control (P<0.05) (Fig. 2B).
To determine whether Cacna2d1 is a direct downstream
target of miR-107, a fragment of the 3′-UTR of Cacna2d1 (1099 bp)
containing the potential miR-107 binding site was cloned into a
vector with the firefly luciferase reporter gene. Luciferase
activity in cells treated with miR-107 was reduced by ~20% compared
with the control. Subsequently, K562 or KCL-22 cells were
transfected with either miR-107 mimics or inhibitors. The results
showed that miR-107 mimics downregulate the Cacna2d1 expression
levels while miR-107 inhibitors upregulate the Cacna2d1 expression
levels at the mRNA level, compared with those observed in the
controls. It has been reported that miR-103 and miR-107 share
numerous functions and are always co-expressed (10), hence, in the current study the
expression of miR-103 was also analyzed, and the results indicated
that miR-103 does not exhibit the same effects on Cacna2d1,
implying this is a specific function of miR-107 (Fig. 2C). This ensures the specificity of
the binding between miR-107 and the 3′-UTR of Cacna2d1. The
Cacna2d1 expression levels were also monitored in K562 cells
subjected to both overexpression and knockdown of miRNAs, and the
results demonstrated that miR-107 had strong effects on Cacna2d1
expression, while miR-103 had marginal effects (Fig. 2D).
Taken together, these results indicated that the
downregulation of miR-107 may cause the increased expression levels
of Cacna2d1, which is the target gene of miR-107.
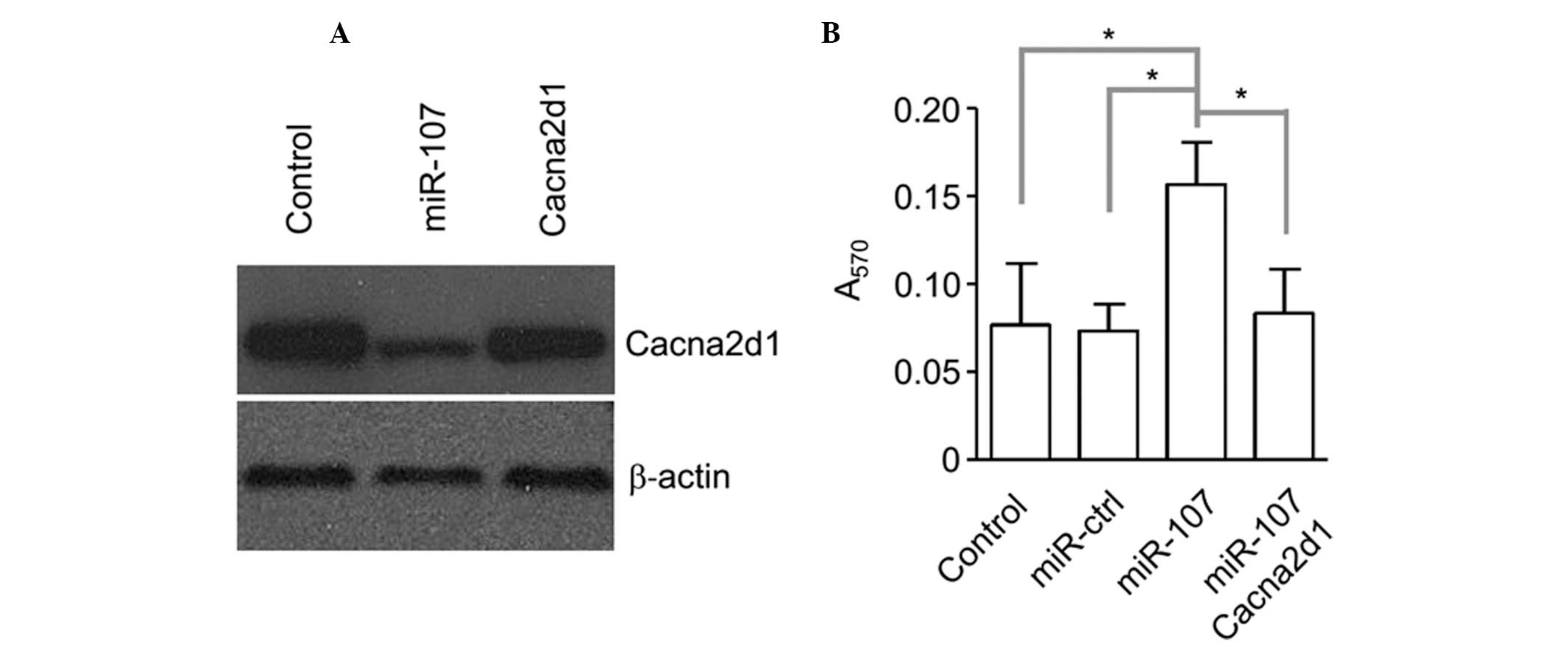
miR-107 promotes erythroid
differentiation of chronic myeloid leukemia cells via
downregulation of Cacna2d1
As shown in Fig.
2D, miR-107 overexpxression contributes to the accelerated
downregulation of Cacna2d1. To further confirm the function of
miR-107 in K562 cells, a rescue experiment was performed via the
transfection of a plasmid expressing Cacna2d1 and the detection of
erythroid differentiation. The rescue reversed the downregulation
of miR-107, as observed using an immunoblotting assay (Fig. 3A). Meanwhile, the K562 cells with
rescued Cacna2d1 expression demonstrated better erythroid
differentiation (Fig. 3B).
Therefore, this study clearly showed that miR-107 promotes K562
erythroid differentiation via the downregulation of Cacna2d1.
Discussion
miRNAs have been reported to have important roles in
the development and progression of a number of diseases through the
repression of downstream target genes (11,12).
Since this initial discovery, abnormal miRNA expression levels have
been detected in numerous types of hereditary diseases. However,
little is known about the dysregulation of miRNAs in laminopathies.
It has been reported that miR-107 has strong influences on health
and disease in human biology. It can regulate the expression of
genes involved in metabolism, angiogenesis, cell division and
stress response in numerous vertebrate species. miR-107 has also
been implicated in cardiovascular disease, a number of cancers and
certain neurodegenerative diseases in humans (13–15).
miR profiling experiments have revealed that miR-107 is expressed
in numerous mammalian tissues in moderate to high levels, including
heart, skeletal muscle, brain, lung, liver, kidney, spleen and
placenta tissues (4,10).
The present study investigated the target gene that
is regulated by miR-107. Through the luciferase reporter assay
combined with target prediction software, including Targetscan,
Pictar, Miranda and Microcosm, a number of possible target genes
were identified (data not shown). Cacna2d1 was the most likely
candidate. The official name of this gene is calcium channel,
voltage-dependent, α-2/δ subunit 1. This gene encodes a member of
the α-2/δ subunit family, which is a protein in the
voltage-dependent calcium channel complex. Calcium channels mediate
the influx of calcium ions into the cell upon membrane
polarization, and they comprise a complex of α-1, α-2/δ, β and γ
subunits in a 1:1:1:1 ratio. Studies on a similar protein in
rabbits indicate that the protein described in this record is
cleaved into α-2 and δ subunits. Alternative transcriptional splice
variants of this gene have been observed, however, they have not
been thoroughly characterized (16,17).
In the current study, miR-107 was revealed to
promote the erythroid differentiation of K562 and KCL-22 chronic
myeloid leukemia cells via the downregulation of Cacna2d1, while
having no effect on the cell growth. Further investigation of the
regulation mechanism is required to provide information on the
association between Cacna2d1 and other diseases, which may offer a
novel insight into Cacna2d1.
In conclusion, the present study indicated that
miR-107 promotes the erythroid differentiation of K562 and KCL-22
cells via the downregulation of Cacna2d1, providing potential
therapeutic approaches for the treatment of chronic myeloid
leukemia.
Acknowledgements
This study was funded by grants from the National
Natural Science Foundation of China (nos. 30871440, 81170327), the
Guangdong Medical Scientific Research Project (no. A2014470), the
Dongguan Health Project (no. 201410515200159) and the Science and
Technology Innovation Fund of Guangdong Medical College (nos.
STIF201102, 1057113036, ZZDC006)
References
|
1
|
Jabbour E and Kantarjian H: Chronic
myeloid leukemia: 2012 update on diagnosis, monitoring, and
management. Am J Hematol. 87:1037–1045. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maru Y: Molecular biology of chronic
myeloid leukemia. Cancer Sci. 103:1601–1610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rottiers V and Näär AM: MicroRNAs in
metabolism and metabolic disorders. Nat Rev Mol Cell Biol.
13:239–250. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
O’Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar
|
|
6
|
Taby R and Issa JP: Cancer epigenetics. CA
Cancer J Clin. 60:376–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taft RJ, Pang KC, Mercer TR, Dinger M and
Mattick JS: Non-coding RNAs: regulators of disease. J Pathol.
220:126–139. 2010. View Article : Google Scholar
|
|
8
|
van Rooij E and Olson EN: MicroRNA
therapeutics for cardiovascular disease: opportunities and
obstacles. Nat Rev Drug Discov. 11:860–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su H, Yang JR, Xu T, et al: MicroRNA-101,
down-regulated in hepatocellular carcinoma, promotes apoptosis and
suppresses tumorigenicity. Cancer Res. 69:1135–1142. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Finnerty JR, Wang WX, Hébert SS, Wilfred
BR, Mao G and Nelson PT: The miR-15/107 group of microRNA genes:
evolutionary biology, cellular functions, and roles in human
diseases. J Mol Biol. 402:491–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foley NH and O’Neill LA: miR-107: a
toll-like receptor-regulated miRNA dysregulated in obesity and type
II diabetes. J Leukoc Biol. 92:521–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen HY, Lin YM, Chung HC, et al:
miR-103/107 promote metastasis of colorectal cancer by targeting
the metastasis suppressors DAPK and KLF4. Cancer Res. 72:3631–3641.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Datta J, Smith A, Lang JC, et al:
microRNA-107 functions as a candidate tumor-suppressor gene in head
and neck squamous cell carcinoma by downregulation of protein
kinase Cɛ. Oncogene. 31:4045–4053. 2012. View Article : Google Scholar :
|
|
16
|
Singer D, Biel M, Lotan I, Flockerzi V,
Hofmann F and Dascal N: The roles of the subunits in the function
of the calcium channel. Science. 253:1553–1557. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dolphin AC: Calcium channel auxiliary α2δ
and β subunits: trafficking and one step beyond. Nature reviews
Neuroscience. 13:542–555. 2012. View
Article : Google Scholar
|