Introduction
Esophageal cancer is the eighth most common type of
cancer, the sixth most common cause of mortality from cancer
worldwide and is more common in males (1). The incidence of esophageal cancer in
the high-risk northern Chinese population exceeds 100/10,0000 and
it has become a significant problem in Asian populations due to its
markedly poor prognosis (2,3).
Although certain studies have demonstrated that the incidence of
esophageal cancer is decreasing in Western countries (4), other studies have revealed that
esophageal cancer has become one of the fastest-growing types of
cancer in the Western world (5).
Therefore, the prevalence of esophageal cancer and its poor
survival rate following current therapy indicates a requirement to
identify novel drugs for its treatment. The use of botanical agents
or their derivatives, including isoflavone and curcumin, for the
treatment of cancer has been demonstrated to be effective (6,7).
Fructus forsythia, one of the most recognized Chinese
medicinal herbs, has been widely used as an anti-inflammatory,
diuretic, antidote and anti-cancer agent (8). Furthermore, a number of studies have
revealed that an extract of Fructus forsythia fruits induces
apoptosis in cancer cells, including liver, gastric and colon
cancer (9), and enhances the
sensitivity of cancer cells to chemotherapy (10). The root, fruit and leaf of
Fructus forsythia have different medical uses. However, the
plant part of Fructus forsythia with the most marked
anti-tumor activity has remained to be elucidated.
In the present study, the anti-tumor activity of the
root, leaf and fruit extract of Fructus forsythia was
compared. Furthermore, the underlying mechanism of the anti-cancer
effect of the ethanolic extract of Fructus forsythia root
(FSEER) on esophageal cancer cell lines was investigated in
vitro and in vivo. The ability of FSEER to inhibit the
growth of esophageal cancer cells and to induce apoptosis via
affecting levels of B-cell lymphoma (Bcl)-2 family proteins was
examined. Furthermore, the effect of FSEER on Janus kinase
(JNK)/signal transducer and activator of transcription (STAT) and
extracellular-signal-regulated kinase (ERK) signaling pathways was
investigated in vitro. In addition, the anti-tumor activity
of FSEER was evaluated in vivo using a TE-13 esophageal
cancer cell xenograft murine model.
Materials and methods
Reagents and antibodies
Fetal calf serum (FCS) and RPMI 1640 were purchased
from Gibco-BRL (Invitrogen Life Technologies, Carlsbad, CA, USA).
MTT, dimethyl sulfoxide (DMSO), RNase A and Annexin V/propidium
iodide (PI) apoptosis kits were from Sigma (St. Louis, MO, USA).
Monoclonal antibodies to B-cell lymphoma 2 (Bcl-2; mouse
anti-rabbit; 1:1,000), Bcl-2-associated X protein (Bax, mouse
anti-rabbit; 1:1,000) and GAPDH (rabbit anti-mouse; 1:10,000) and
polyclonal antibodies to poly(ADP ribose) polymerase (PARP; sheep
anti-rabbit, 1:1,000), caspase-3 (mouse anti-rabbit, 1:500),
caspase-8 (sheep anti-rabbit, 1:500), caspase-9 (mouse anti-rabbit,
1:500) and cytochrome c (Cyt-c; sheep anti-rabbit,
1:1,000) were supplied by Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Polyclonal mouse anti-rabbit antibodies targeting
phosphorylated (p)-ERK (1:1,000), p-Janus kinase (JAK; 1:1,000) and
p-signal transducers and activators of transcription (STAT)3
(1:1,000) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Tumor cell lines and culture
The esophageal cancer cell lines TE-1, TE-13 and
Eca-109 were obtained from the Cellular Biology Institute of the
Shanghai Academy of Sciences (Shanghai, China) and the Yes-2 cell
line was contributed by Professor Tagawa Masatoshi (Chiba Cancer
Center Research Institute, Chiba, Japan). The cells were maintained
in RPMI-1640 medium containing 10% FCS, 100 U/ml penicillin and 100
μg/ml phytomycin at 37°C in an incubator with a humidified
atmosphere of the 5% CO2.
Preparation of Forsythia suspensa
extracts
The plant material was purchased from Le Ren Tang
Pharmacy in Shijiazhuang (Hebei, China) and authenticated by
Professor Fengzhi Ren (Department of Natural Medicine Development,
New Drug Research and Development Center of North China
Pharmaceutical Group Corporation, Shijiazhuang, China). Following
drying by baking and grinding into a fine powder, the root, leaf
and fruits of Forsythia suspensa were separated and (2 kg of
each) was soaked in 95% ethanol (10 liters; Sigma) under reflux for
2×2 h. The extracts were then combined and concentrated under
reduced pressure at 40°C. The ethanolic extracts of the root, leaf
and fruit of Forsythia suspensa were termed FSEER, FSEEL and
FSEEF, respectively. The concentrated extracts were then separated
from the solid by filtration and concentrated using a rotary
evaporator to obtain dry extracts. These were then dissolved in 100
μl ethanol and resolved with 900 μl phosphate-buffered saline (PBS)
at 10 mg/ml for storage.
Cell viability assay
The viability of treated cancer cells was determined
using an MTT assay. Briefly, the cells (1×104) were
seeded into 96-well plates and cultured for 24 h, followed by
treatment with different concentrations of the extracts for a range
of durations for the different experiments. A volume of 10 μl 10
mg/ml MTT was added to each well and incubated for 3 h at 37°C,
following which the supernatant was removed and 150 μl DMSO was
added for 15–20 min. The absorbance was recorded using a microplate
reader (Titertek Multiskan; Flow Laboratories, North Ryde,
Australia) at a wavelength of 492 nm. All experiments were
performed in triplicate. The effect of FSREE on tumor cell
viability was detected by determining the IC50 value for
each cell line. The effect of each extract on the proliferation of
esophageal cancer cells was calculated as the percentage of cell
growth inhibition using the optical density (OD) with the following
formula: Inhibitory rate = ([OD control group - OD experiment
group] / OD control group) ×100%.
Flow cytometric analysis
To investigate apoptosis, 1×106 cells
were treated with FSREE (0.25, 0.5 and 1.0 mg/ml) for 48 h and 0.5
μg/ml FSREE for 0, 24, 48 and 72 h. The cells were collected and
PBS was added to a final volume of 500 μl. The cells were then
incubated with Annexin V-fluorescein isothiocyanate (FITC) and PI
double stain according to the manufacturer’s instructions and
analyzed using flow cytometry with a fluorescence activated cell
sorting (FACS) flow cytometer (FACSIII; Becton-Dickinson,
Sunnyvale, CA, USA). Data are expressed as the mean ± standard
error of the mean of three independent experiments. For analysis of
the mitochondrial membrane potential (MMP), 1×106 cells
were treated with FSREE (0.25, 0.5 and 1 mg/ml) for 48 h and then
measured by labeling the cells with 1 μm rhodamine JC-1 (Molecular
Probes Life Technologies, Carlsbad, CA, USA) at 37°C for 15 min and
performing flow cytometric analysis.
Isolation of cellular and cytoplasmic
extracts
The cellular and cytoplasmic extract proteins were
obtained as previously described (11). Briefly, cells were harvested by
trypsinizing and the whole cell protein was acquired by lysing the
cells on ice for 20 min in 700 μl lysis buffer with protease
inhibitors and mini protease inhibitor cocktail (Roche Diagnostics,
Indianapolis, IN, USA). The lysate was then centrifuged at 12,000 ×
g for 20 min and the supernatant was collected, and stored at
−80°C. To prepare the cytoplasmic proteins, the cell pellets were
suspended in 500 μl lysis buffer (see whole cell instructions)
without Tween-20 detergent, samples were sonicated (1 sec × 30) on
ice and then centrifuged at 10,000 × g for 20 min. The supernatant
(cytoplasmic fraction) was collected in accordance with the method
described in our previous report (11).
Western blot analysis
Protein levels were evaluated using bicinchoninic
acid assays (Pierce Biotechnology, Rockford, IL, USA) and 12%
SDS-PAGE and were electrotransferred onto a polyvinylidene
difluoride membrane (Millipore, Billerica, MA, USA). The membranes
were inhibited using 5% bovine serum albumin (Sigma) for 2 h at
room temperature and were incubated overnight at 4°C with the
primary antibodies diluted at 1:1,000. The bound primary antibody
was detected using the appropriate fluorochrome-labeled secondary
anti-rabbit or mouse immunoglobulin G (IRDye 800; LI-COR
Biosciences, Lincoln, NE, USA) for 1.5 h at room temperature.
Following washing three times with Tris-buffered saline (Sigma) and
Tween 20 (Sigma) for 10 min each, the membrane was imaged using an
Odyssey infrared imaging system (LI-COR Biosciences). The levels of
protein were calculated as the ratio of the intensity of protein to
that of GAPDH. The experiments were performed in triplicate wells
and repeated three times.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the treated cells using
TRIzol reagent (Sigma) according to the manufacturer’s
instructions. RT-qPCR was conducted as previously described
(11), with modifications, using
RT-qPCR kits from Promega Corp. (Madison, WI, USA). In brief, cDNA
was prepared using RNA samples (2 μg) to which 1 μg oligo(dT), 0.5
mM deoxynucleotide triphosphate and 200 units of the Revert
Aid™ H-Minus M-MuLV Reverse Transcriptase enzyme were
added (MBI Fermentas, Hanover, MD, USA). RT-qPCR analysis was
performed using primers synthesized by Sangon Biotech Co., Ltd
(Shanghai, China), as shown in Table
I, and 1 μl RT product was incubated with 1 unit Taq DNA
polymerase in a 20-μl reaction mixture (Promega Corp.). The
amplified fragments were detected on a 1.5% (w/v) agarose gel and
analyzed using an IS1000 image analysis system (Alpha Innotech, San
Leandro, CA, USA).
 | Table IPrimer sequences for the reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences for the reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer
sequence | Annealing
temperature (°C) | Length (base
pairs) |
|---|
| Noxa | Forward:
5′-GCTGGAAGTCGAGTGTGCTAC-3′
Reverse: 5′-CACATTCCTCTCAATTACAATGC-3′ | 55 | 211 |
| Bad | Forward:
5′-GTTCCAGATCCCAGAGTTTGAG-3
Reverse: 5′-GGCGAGGAAGTCCCTTCTTA-3′ | 59 | 392′ |
| Bax | Forward:
5′-CGGCGAATTGGAGATGAACTG-3′
Reverse: 5′-AGCAAAGTAGAAGAGGGCAACC-3′ | 62 | 178 |
| Bcl-XL | Forward:
5′-ACTGGACTGTGGCATTGAG-3′
Reverse: 5′-GATTGTCGGCATACTGTTTC-3′ | 55 | 312 |
| Bcl-2 | Forward:
5′-CGACTTCGCCGAGATGTCCAGCCAG-3′
Reverse: 5′-ACTTGTGGCCCAGATAGGCACCCAG-3′ | 55 | 252 |
| Mcl-1 | Forward:
5′-TCGAGTGATGATCCATGTTTTC-3
Reverse: 5′-GATATGCCAAACCAGCTCCTAC-3′ | 58 | 302′ |
| GAPDH | Forward:
5′-CGGATTTGGTCGTATTGGG-3′
Reverse: 5′-TGCTGGAAGATGGTGATGGGATT-3′ | 60 | 279 |
Effect of FSEER on tumor growth in
vivo
Twelve Balb/c nude mice (5–6 weeks old, 18–20 g)
were purchased from the Laboratory Animal Center of Hebei Medical
University (Shijiazhuang, China). The procedures using animals were
approved by the Animal Care and Use Committee. The Balb/c nude mice
were injected subcutaneously into the right axillary fossa with
TE-13 cells (2×106/0.1 ml). When the tumor growth
reached a volume of ~0.1 cm3, the mice were randomly
assigned into two groups (n=6/group). The treatment group were
intraperitoneally administered FSEER (50 mg/ml) and the control
group was administered an equal volume of PBS once every two days
for 14 days. The tumor volumes were estimated using the following
formula: 0.5 × length × width2, for which the length and
perpendicular width were measured using calipers. Subsequently, the
lung and liver tissues were stained for histological analysis using
hematoxylin and eosin (H&E; Invitrogen Life Technologies) under
an Olympus IX-70 microscope (Olympus Corp., Melville, NY, USA) to
analyze the toxicity of FSEER.
Statistical analysis
All data analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). The statistical
significance between values was determined by one-way analysis of
variance, and the Student’s t-test was used to compare two
independent samples. Fisher’s probability was used to analyze the
difference in protein expression between groups. P<0.05 was
considered to indicate a statistically significant difference. All
data are expressed as the mean ± standard deviation. Results shown
in the figures were obtained from at least three independent
experiments with a similar pattern.
Results
Inhibition of cell proliferation by
extracts of Forsythia suspensa
The TE-13 cells were treated with different extracts
of Forsythia suspensa for 48 h to evaluate their anti-cancer
activity. The IC50 values of FSEES, FSEER and FSEEL are
shown in Table II. On TE-13
cells, the IC50 values of FSEES, FSEER and FSEEL were
4.25, 0.58 and 78 mg/ml, respectively (Table II). This demonstrated that the
extract of the root, rather than that of the fruit or leaf, of
Forsythia suspensa had a more marked inhibitory effect on
the esophageal carcinoma cells. Therefore, FSEER was selected to
further investigate its inhibitory activity on esophageal cancer
cells and the underlying mechanism. The inhibitory rates of 0.5
mg/ml FSEER against the esophageal cancer cell lines TE-13,
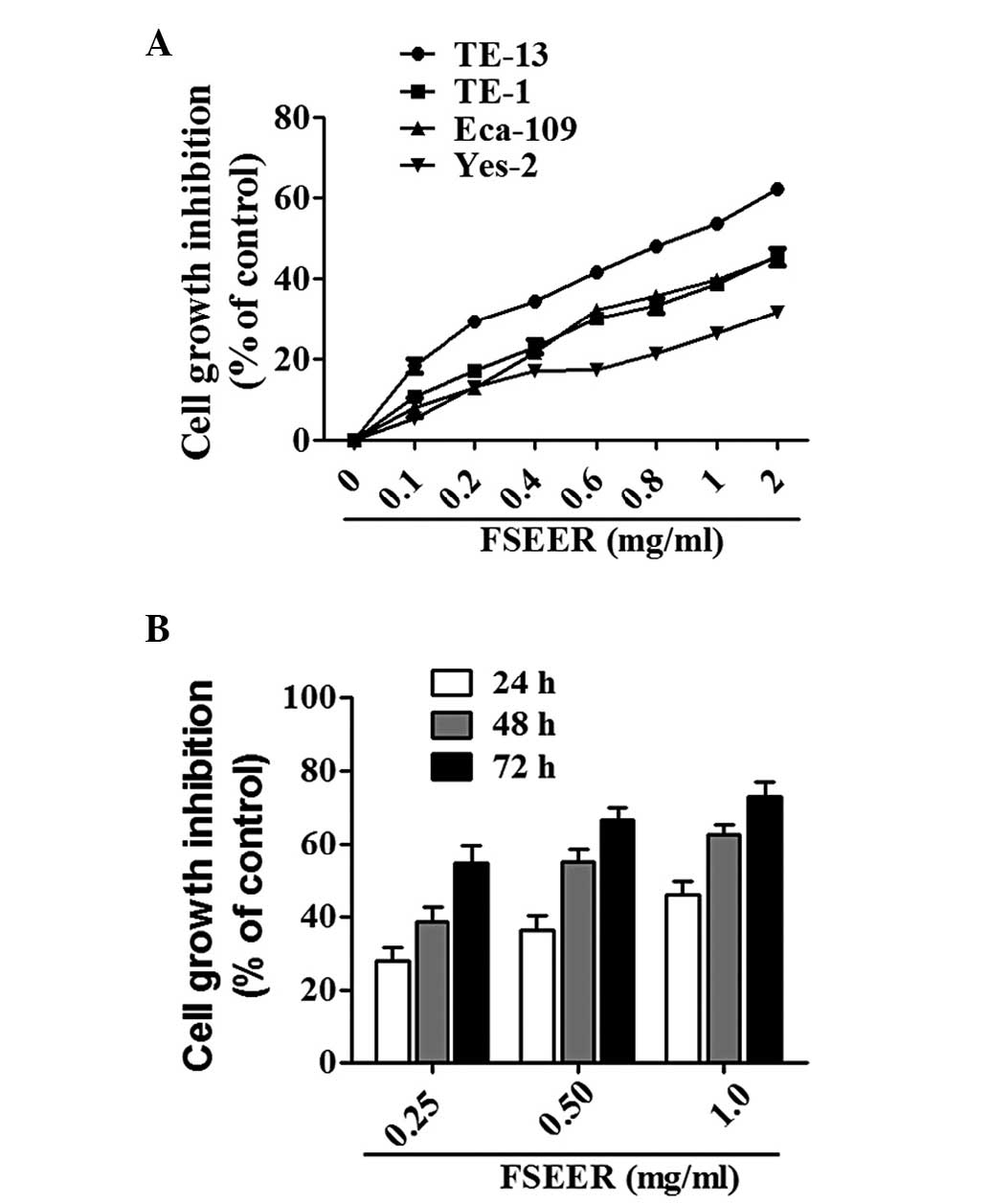
ECA-109, TE-1 and Yes-2 were 64.8, 51.6, 49.0 and 48.0%,
respectively (Fig. 1A). Therefore,
TE-13 cells were used in the subsequent experiments. Following
treatment with 0.251 mg/ml FSEER for 24, 48 and 72 h, the growth of
TE-13 cells was inhibited in a dose- and time-dependent manner
(Fig. 1B).
 | Table IIInhibitory effect of ethanolic
extract of Forsythia suspensa on esophageal cancer
cells. |
Table II
Inhibitory effect of ethanolic
extract of Forsythia suspensa on esophageal cancer
cells.
| IC50
(mgml) | TE-13 | TE-1 | Eca-109 | Yes-2 |
|---|
| FSEEL | 78.01 | 89.26 | 88.03 | 72.91 |
| FSEES | 4.25 | 8.92 | 10.90 | 7.82 |
| FSEER | 0.58 | 1.25 | 2.30 | 1.46 |
FSEER induces cell apoptosis in
vitro
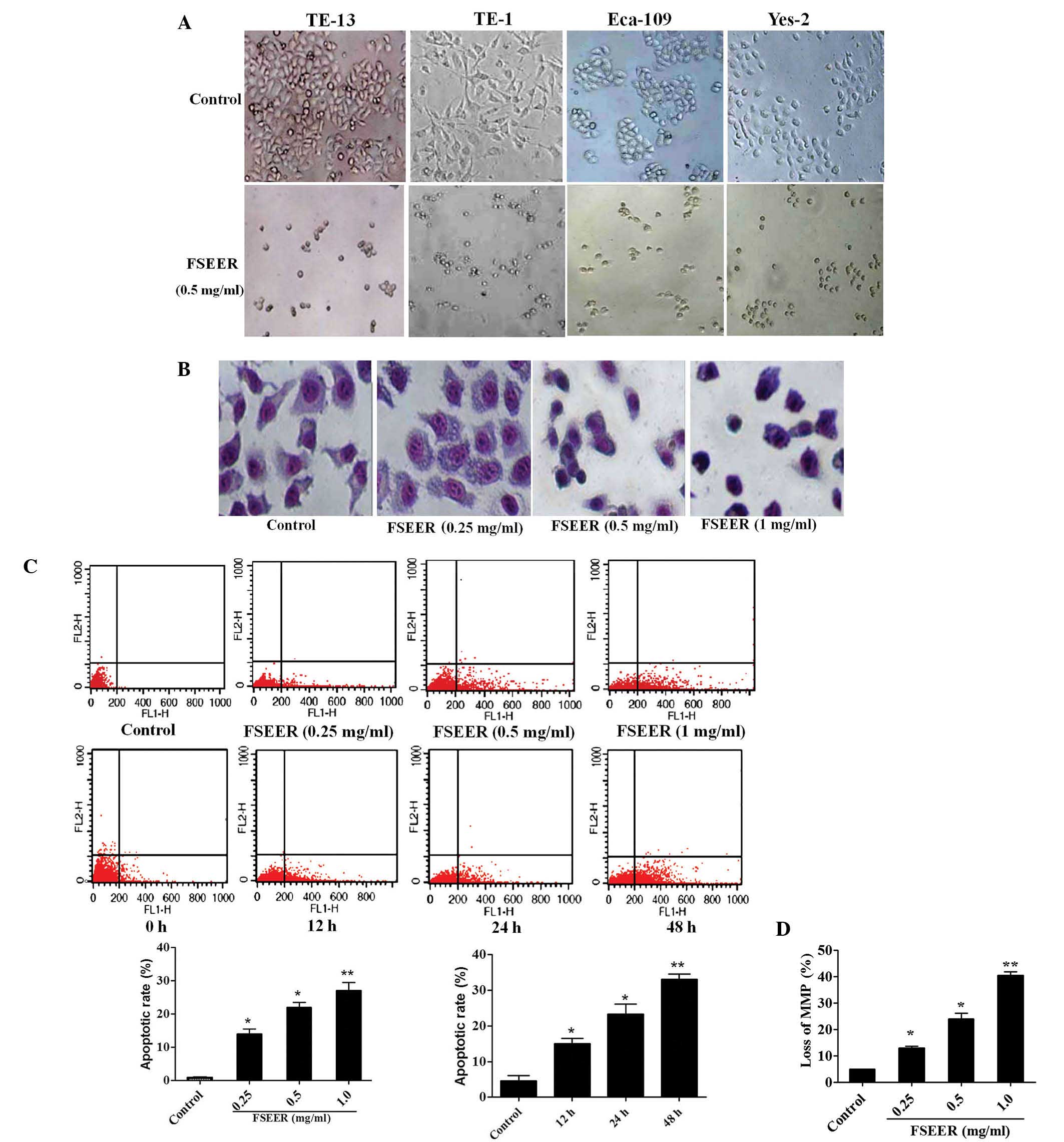
Giemsa staining and flow cytometry were performed to
investigate whether FSEER induced TE-13 cell apoptosis. As shown in
Fig. 2A and B, morphological
changes observed using microscopy and Giemsa staining revealed that
tumor cells exhibited decreased growth, loss of volume, cytoplasm
concentration, karyokinesis and deformation to a round appearance
following treatment with FSEER (0.5 mg/ml) for 48 h. However, the
cells in the control group were observed to maintain a regular
appearance, intensive growth and a polygonal shape. Flow cytometry
was performed to estimate the rate of apoptosis by quantitative
assessment of Annexin V/PI stained TE-13 cells. As shown in
Fig. 2C, FSEER treatment increased
the number of Annexin V-FITC-positive and PI-negative cells in a
dose- and time-dependent manner compared with that in the control
group. In order to determine whether FSEER-induced apoptosis of
TE-13 cells was mediated through mitochondrial dysfunction, the MMP
was measured using the mitochondrial-sensitive dye JC-1. As shown
in Fig. 2D, the number of cells
exhibiting depolarized mitochondrial membranes was significantly
increased in the FSEER (0.25, 0.5, 1.0 mg/ml)-treated cells
compared with that in the control group.
Involvement of the mitochondrial
signaling pathway in FSREE-induced apoptosis
Caspase-3 can be activated by a mitochondrial
apoptotic pathway involving caspase-9, termed the intrinsic
pathway, or by a death receptor pathway involving caspase-8, the
extrinsic pathway, contributing to cell apoptosis (12,13).
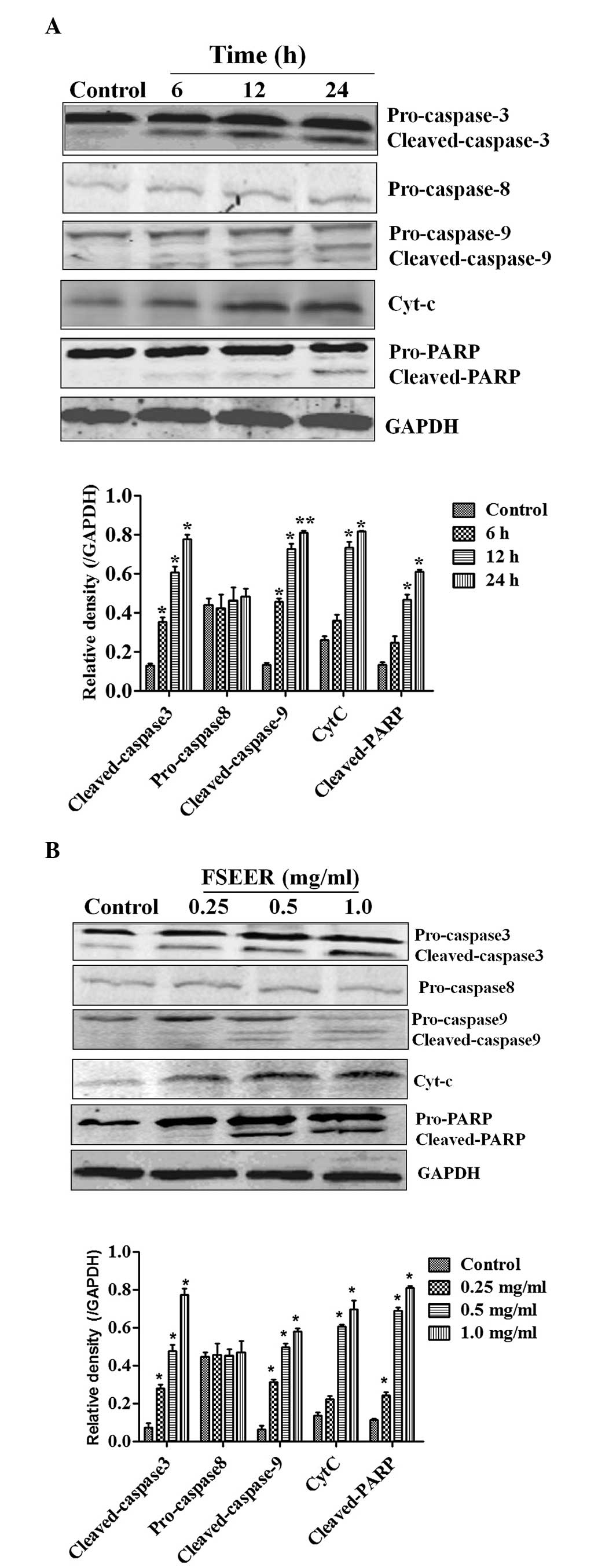
The results of the present study revealed that treatment of TE-13
cells with FSEER for 48 h resulted in cleavage of caspase-3, as
evidenced by the appearance of 19-kDa intermediates (Fig. 3A). Furthermore, treatment of the
TE-13 cells with FSEER also resulted in significantly increased
cleavage of caspase-9 without changes in procaspase-8 levels
(Fig. 3B). These results suggested
that FRSEE triggered apoptosis through the intrinsic pathway, but
not the extrinsic pathway. Activation of caspases during apoptosis
results in the cleavage of critical cellular substrates, including
PARP (14). Therefore, PARP has
become an essential marker of caspase-3 activity in intrinsic
apoptotic pathways (15). As shown
in Fig. 3A, the levels of cleaved
PARP fragment, which is the active form, were significantly
increased following exposure to FSREE for 48 h, further confirming
the activity of caspase-3 in the TE-13 cells. In addition, a key
step in the intrinsic apoptotic pathway is the damage of
mitochondria and the release of Cyt-c to activate apoptotic
protease activating factor 1, which in turn activates the caspase
cascade (16). Following treatment
of TE-13 cells with FSEER, Cyt-c levels increased in the
cytoplasmic fraction in a dose- and timedependent manner (Fig. 3). This result indicated that FSEER
induced the release of Cyt-c from the mitochondria to the
cytoplasm in TE-13 cells and further suggested that the
mitochondrial pathway was involved in FSREE-induced apoptosis.
Members of the Bcl-2 family are involved
in FSREE-induced apoptosis of TE-13 cells
Mitochondrial integrity is regulated by the Bcl-2
family, which is constituted of pro-apoptotic members, including
Bcl-2, Bcl-xL and myeloid cell leukemia 1 (Mcl-1), and
anti-apoptotic members, including Bax, Bcl-2-associated death
promoter (Bad) and phorbol-12-myristate-13-acetate-induced protein
1 (Noxa) (17,18). Thus, the expression of these Bcl-2
family members was detected in TE-13 cells following treatment with
various concentrations of FSREE for different periods of time. As
shown in Fig. 4A and B, a decrease
in the expression of Bcl-2, Bcl-xL and Mcl-1 was observed,
accompanied by an increase in the expression of Bax, Bad and Noxa
mRNA in the TE-13 cells following treatment with FSEER (0.25–1
mg/ml) for 24 h (Fig. 4A). In
addition, the change in the expression levels of the above proteins
was consistent with the mRNA expression in response to treatment
with FSEER (0.25–1 mg/ml) for 48 h (Fig. 4B). These results further
demonstrated that the mitochondrial apoptotic pathway was activated
by the Bcl-2 family in FSERR-induced apoptosis in esophageal cancer
TE-13 cells.
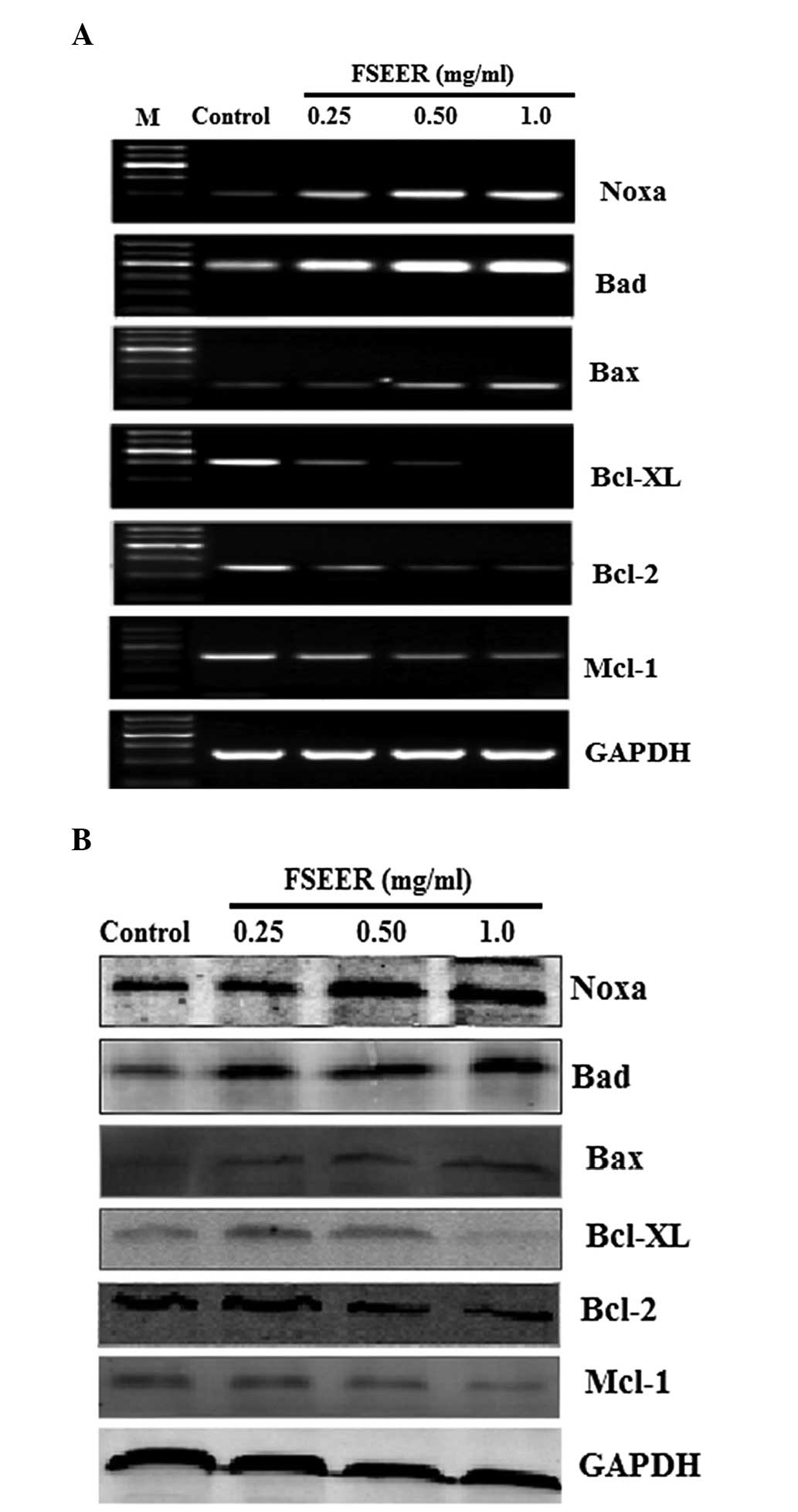 | Figure 4Reverse transcription quantitative
polymerase chain reaction and western blot analysis of the protein
expression of the Bcl2 family. TE-13 cells were treated with FSEER
(0.25, 0.5 and 1.0 mg/ml) for 48 h and the (A) mRNA and (B) protein
expression levels of Bcl-2, Bcl-xL, Mcl-1, Bax, Bad and Noxa were
examined. FSEER, Forsythia suspensa ethanolic extract of the
root; Bcl-2, B-cell lymphoma 2; Bcl-Xl, Bcl-extra large; Mcl-1,
myeloid cell leukemia 1; Bax, Bcl-2-associated X protein; Bad;
Bcl-2-associated death promoter; Noxa,
phorbol-12-myristate-13-acetate-induced protein 1. |
Effect of FSEER on the JAK/STAT3 and ERK
signaling pathways
The JAK/STAT3 and ERK signaling pathways are
important pathways in cell growth and apoptosis and the inactivity
of these pathways may regulate the Bcl2 family resulting in growth
arrest and apoptosis in certain tumor cells (19–21).
Several studies have suggested that anti-apoptotic genes are
regulated by interleukin 6 and STAT3, including Bcl-2, Bcl-xL and
Mcl-1 (22). While these genes are
induced by STAT3, the most important anti-apoptotic gene is
considered to be Mcl-1 and Bcl-xL (23). The results of the present study
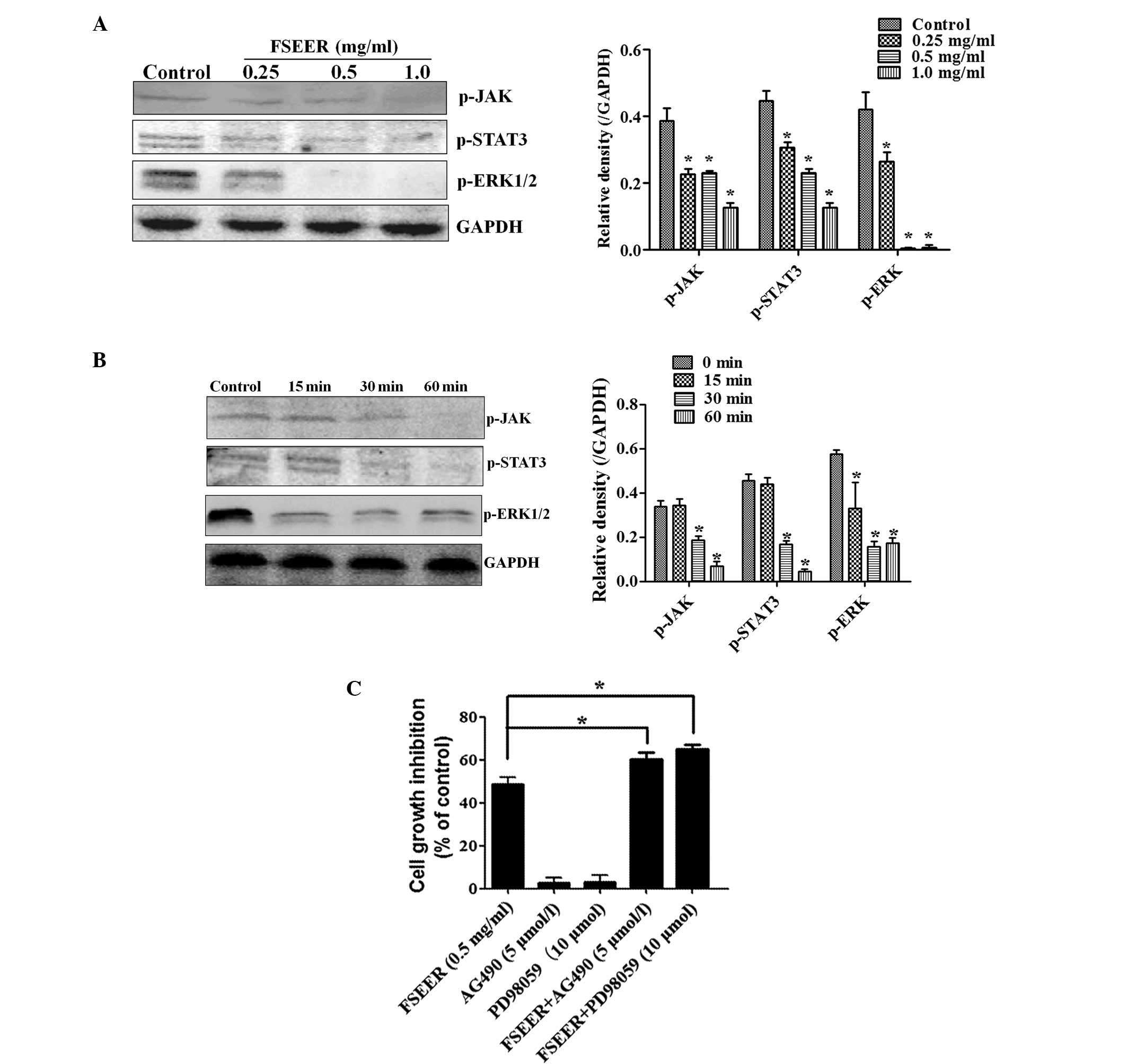
revealed that FSEER markedly reduced the expression of p-JAK/STAT3
and p-ERK in a concentration and time-dependent manner (Fig. 5A and B), indicating that FSEER
inhibited the activation of the JAK/STAT3 and ERK signaling
pathways in TE-13 cells. In order to verify the involvement of
these pathways in FSEER-induced apoptosis, the effect of FSEER on
the proliferation of TE-13 cells was observed in the presence of an
inhibitor of the signaling pathway. AG490 is a member of the
typhostin family of tyrosine kinase inhibitors, which inhibit the
JAK/STAT3 signaling pathway in several types of cancer cell,
including esophageal carcinoma cells (24,25).
Beales and Ogunwobi (26)
demonstrated that the P4244 MAP kinase inhibitor PD98059 enhanced
the activity of leptin-mediated esophageal adenocarcinoma cell
apoptosis. The present study revealed that, although AG490 (5
μmol/l) and PD98059 (10 μmol/l) alone were not able to inhibit the
proliferation of TE-13 cells, they significantly enhanced the
inhibitory effect of FSEER (0.5 mg/ml) on the proliferation of
TE-13 cells by ~25 and 35%, respectively (Fig. 5C). Taken together, the findings of
the present study demonstrated that the induction of apoptosis of
TE-13 cells by FSEER was achieved through downregulation of the
JAK/STAT3 and ERK signaling pathways.
Anti-tumor efficacy of FSEER in vivo
The established TE-13 cells implanted into nude mice
were used as a model to observe the effect of FSEER on the tumor
burden in vivo. The treatment regimens were performed as
described previously. As shown in Fig.
6A, compared with the control group, the FSEER-treated group
demonstrated significant inhibition of tumor growth. Following
treatment with FSEER for 20 days, the mean tumor volume of the
treated group was 0.79+0.17 cm3 and the mean weight was
0.35+0.08 mg. These were significantly lower compared with those of
the control group, which were 2.56+0.18 cm3 and
1.35+0.11 mg, respectively (Fig.
6B) Following inoculation of the TE-13 cells, a clear increase
in tumor volume was observed from day 7 in the vehicle group until
the animals were sacrificed. However, tumor volume in mice treated
with FSEER (50 mg/ml) from the day of inoculation started to
increase from day 10 and tumor volume increased slowly (Fig. 6C). Furthermore, no clear
pathological changes were observed in the liver and lung in the
H&E-stained sections of FSEER-treated mice (Fig. 6D), indicating that FSEER had no
detectable toxicity in mice. In conclusion, these results indicated
that FSEER exerted anti-tumor effect in vitro and in
vivo.
Discussion
Forsythia suspensa is used as an anti-pyretic
and analgesic and is one of the essential components of Chinese
Traditional Medicines used in cancer treatment. Although the leaf,
root and fruit of Forsythia suspensa exhibit various
pharmacological effects, their anti-cancer effectiveness remains to
be elucidated. In the present study, the anti-proliferative effects
of ethanolic extracts of leaf, root and fruit of Forsythia
suspensa on esophageal carcinoma cells were examined. The
results demonstrated that the extract of the root rather than that
of the leaf or fruit produced the most marked arrest of cell
growth. The present study was the first, to the best of our
knowledge, to demonstrate which part of Forsythia suspensa
is the most potent inducer of apoptosis in esophageal carcinoma
cells. Of note, the leaf of Forsythia suspensa is commonly
used for the preparation of tea in China (27) and the fruit is used for the
preparation of certain oils, although these are not used for
medicinal purposes (28). Previous
studies have demonstrated that ethanolic extracts of Forsythia
suspensa fruit have significant inhibitory effects against
murine hepatocellular carcinoma cells (H22), human hematology cells
(SMMC-7721), intestinal cancer cells (LOVo) and gastric carcinoma
cells (BGC-823) (8,9). The present study revealed that FSEER
inhibited the proliferation of esophageal carcinoma TE-13 cells by
inducing apoptosis.
A time- and dose-dependent investigation was
conducted over 72 h, with assays performed at 24, 48 and 72 h,
using human TE-13 cells treated with 0.25–1.0 mg/ml FSEER.
Significant growth inhibition of the TE-13 cells was observed over
the entire period of the experiment compared with control cells.
Morphological and flow cytometric analyses of the FSEER-treated
cells demonstrated an increase in apoptotic cells, suggesting that
apoptosis is important in the growth inhibitory effects of
FSEER.
The activation of caspase in apoptosis occurs via
two distinct pathways. Caspase 3 activation is involved in two
apoptotic signaling cascades as a final apoptotic executioner. In
the present study, caspase-3 and caspase-9, but not caspase-8, were
activated by FSEER in the TE-13 cells, indicating that apoptosis
was induced by FSEER through the intrinsic apoptotic pathway. This
mechanism was similar to the role of certain chemotherapeutics on
cancer cells, including paclitaxel and camptothecin (29,30),
which suggested that certain compounds with anti-tumor activity
were present in FSEER. The quantity of mitochondrial Cyt-c
released into the cytoplasm is a signaling event in the intrinsic
apoptotic activation pathway (31). The release of Cyt-c from the
mitochondria into the cytoplasm supports the activation of the
intrinsic apoptotic pathway. Of note, in the present study,
downstream events of caspase-3, including PARP cleavage, were
detected 48 h after treatment, while Cyt-c was observed to
increase in the cytoplasm following FSEER treatment. In addition,
mitochondrial outer membrane permeabilization and disruption of the
MMP are independent triggers of the mitochondrial cell death
cascade, resulting in the release of Cyt-c from the
intermembrane space of the mitochondria into the cytoplasm
(32). The present study
demonstrated that treatment of TE-13 cells with FSREE caused rapid
depolarization of MMP, depicted by representative dot blots, which
demonstrated that FSEER disrupted the MMP. This accounted for the
Cyt-c release from the mitochondria into the cytoplasm and
confirmed that FSREE treatment induced TE-13 apoptosis via the
mitochondrial pathway.
In addition, the Bcl-2 family, which consists of
anti-apoptotic and proapoptotic proteins, are central regulators in
the mitochondrial apoptotic pathway, acting to either suppress or
promote the MMP changes required for release of Cyt-c
(33,34). The Bcl-2 family has been identified
as a major regulator in controlling the mitochondrial apoptotic
pathway (35). The present study
demonstrated that the major anti-apoptotic proteins Bcl-2, Mcl-1
and Bcl-xL were downregulated, whereas the proapoptotic proteins
Bad, Bax and Noxa were upregulated in the TE-13 cells following
treatment with FSEER. Therefore, the release of Cyt-c from
the mitochondria into the cytoplasm induced by FSEER resulted from
deregulation of Bcl2 family proteins.
Certain deregulated signaling pathways are involved
in the occurrence and development of cancer, including esophageal
carcinoma (36,37). As important signaling molecules,
JAK/STAT3 and ERK are deregulated in various types of cancer cell
and can phosphorylate a series of transcription factors, which
regulate gene expression and are important in cell proliferation,
differentiation and survival. Furthermore, the Bcl2 family is
regulated mainly by the JAK/STAT3 and ERK pathways in certain tumor
cells (38,39). Therefore, the present study
investigated whether FSEER regulated the balance of Bcl2 family
proteins via these two signaling pathways. The results revealed
that levels of p-JAK/STAT3 and pERK were significantly decreased by
FSEER in vitro, which contributed to the decreases in
survival rate and induction of apoptosis in TE-13 cells. Further
investigation is required to analyze whether other signaling
pathways are involved in FSEER.
In the present study, the effect of FSEER on the
growth of TE-13 cells in vivo was also assessed. The results
demonstrated that FSEER (50 mg/kg) decreased the cancer burden in
xenograft mice. In addition, no toxicity to lung and liver was
observed at the concentration of FSEER used, which suggested that
FSEER may be a potential, safe anti-cancer drug.
In conclusion, the present study demonstrated that
FSEER, as an anti-tumor agent, induced the apoptosis of esophageal
carcinoma cells. However, important questions regarding the
inhibitory effect of FSEER remain to be elucidated, including which
active components of FSEER trigger the apoptosis of cancer cells.
The compounds quercetin, phillyrin and pinoresinol, which are
present in the fruit of Forsythia suspensa, have been shown
to induce apoptosis in cancer cells (8,40).
However, the compounds in Forsythia suspensea root that
exert an anti-tumor effect remain to be elucidated. Identification
of these active components may assist in examining the
physiological mechanisms and functions of Forsythia suspensa
root.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 81173611) and Chinese Medical Research of
Hebei Province (no. 2011011. The authors would like to thank the
New Drug Research and Development Co., Ltd, the North China
Pharmaceutical Corporation, the China and Chinese Academy of
Medical Sciences and Peking Union Medical College, China for their
support.
References
|
1
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou J, Liao LD, Xie YM, Zeng FM, Ji X,
Chen B, Li YL, Zhu MX, Yang CX, Zhao Q, Chen T, Xu XE, Shen Jian,
Guo MZ, Li EM and Xu LY: DACT2 is a candidate tumor suppressor and
prognostic marker in esophageal squamous cell carcinoma. Cancer
Prev Res. 6:791–800. 2013. View Article : Google Scholar
|
|
3
|
Wang LD, Zhou Q, Feng CW, Liu B, Qi YJ,
Zhang YR, Gao SS, Fan ZM, Zhou Y, Yang CS, Wei JP and Zheng S:
Intervention and follow-up on human esophageal precancerous lesions
in Henan, northern China, a high-incidence area for esophageal
cancer. Gan To Kagaku Ryoho. 1:159–172. 2002.
|
|
4
|
Mawhinney MR and Glasgow RE: Current
treatment options for the management of esophageal cancer. Cancer
Manag Res. 4:367–377. 2012.PubMed/NCBI
|
|
5
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Horie S: Chemoprevention of prostate
cancer: soy isoflavones and curcumin. Korean J Urology. 53:665–672.
2012. View Article : Google Scholar
|
|
7
|
Sarkar FH, Li Y, Wang Z and Padhye S:
Lesson learned from nature for the development of novel anti-cancer
agents: implication of isoflavone, curcumin, and their synthetic
analogs. Curr Pharm Des. 16:1801–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu WJ, Qian XP, Tu YX, Shen ZT, Yu LX and
Liu BR: Anti-tumor effect of extract of Fructus forsythiae alcohol.
Nanjing Zhong Yi Yao Da Xue Xue Bao. 23:379–381. 2007.(In
Chinese).
|
|
9
|
Liu GX, Wang TT, Hu WJ, Qian XP, Yu LX and
Liu BR: Anticancer effect of ethanol extract of Fructus forsythiae
on primary cancer cells isolated from ascites and pleural fluids.
Shi Yong Lao Nian Bing Xue. 23:359–363. 2009.(In Chinese).
|
|
10
|
Wang CL, Yin HT and Liu BR: Effects of
antiproliferation and radiosensitivity on PC-3 cell of prostate
cancer induced by triterpenes component. Shandong Yi Xue Za Zhi.
51:25–27. 2011.(In Chinese).
|
|
11
|
Zhao LM, Shan BE, Du YY, Wang MX, Liu LH
and Ren FZ: Periplocin from Cortex periplocae inhibits cell growth
and down-regulates survivin and c-myc expression in colon cancer in
vitro and in vivo via β-catenin/TCF signaling. Oncol Rep.
24:375–83. 2010.PubMed/NCBI
|
|
12
|
Slee EA, Harte MT, Kluck RM, Wolf BB,
Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri
ES, Green DR and Martin SJ: Ordering the cytochrome c-initiated
caspase cascade: hierarchical activation of caspases-2,−3,−6,−7,−8,
and −10 in a caspase-9-dependent manner. J Biol Chem. 144:281–292.
1999.
|
|
13
|
Berg CP, Engels IH, Rothbart A, Lauber K,
Renz A, Schlosser SF, Schulze-Osthoff K and Wesseborg S: Human
mature red blood cells express caspase-3 and caspase-8, but are
devoid of mitochondrial regulators of apoptosis. Cell Death Differ.
8:1197–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soldani C and Scovassi AI: Poly
(ADP-ribose) polymerase-1 cleavage during apoptosis: an update.
Apoptosis. 7:321–328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sánchez-Hidalgo M, Lee M, Lastra CA,
Guerrero JM and Pachham G: Melatonin inhibits cell proliferation
and induces caspase activation and apoptosis in human malignant
lymphoid cell lines. J Pineal Res. 53:366–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marsden VS, O’Connor L, O’Reilly LA, Silke
J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ,
Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM and Strasser A:
Apoptosis initiated by Bcl-2-regulated caspase activation
independently of the cytochrome c/Apaf-1/caspase-9 apoptosis.
Nature. 419:634–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gross A, McDonnell JM and Korsmeyer SJ:
Bcl-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danial NN: Bcl-2 family proteins: critical
checkpoints of apoptotic cell death. Clin Cancer Res. 13:7254–7263.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,2/STAT3
signaling induces apoptosis, cell cycle arrest, and reduces tumor
cell invasion in colorectal cancer cells. Neoplasia. 10:287–297.
2008.PubMed/NCBI
|
|
20
|
Kuo ML, Chuang SE, Lin MT and Yang SY: The
involvement of PI3-KAkt-dependent up-regulation of Mcl-1 in the
prevention of apoptosis of Hep3B cells by interleukin-6. Oncogene.
20:677–685. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu HS, Huang PI, Chang YL, Tzao C, Chen
YW, Shih HC, Hung SC, Chen YC, Tseng LM and Chiou SH: Cucurbitacin
I inhibits tumorigenic ability and enhances radiochemosensitivity
in non-small cell lung cancer-derived CD133-positive cells. Cancer.
117:2970–2985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spets H, Strömberg T, Georgii-Hemming P,
Siljason J, Nilsson K and Jernberg-Wiklund H: Expression of the
bcl-2 family of pro- and anti-apoptotic genes in multiple myeloma
and normal plasma cells: regulation during interleukin-6
(IL-6)-induced growth and survival. Eur J Haematol. 69:76–89. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song L, Li Y, Sun Y and Shen B: Mcl-1
mediates cytokine deprivation induced apoptosis of human myeloma
cell line XG-7. Chinese Med J (Engl). 115:1241–1243. 2002.
|
|
24
|
Rahaman SO, Harbor PC, Chernova O, Barnett
GH, Vogelbaum MA and Haque SJ: Inhibiton of constitutively active
STAT3 suppresses proliferation and induces apoptosis in
glioblastoma multiforme cells. Oncogene. 21:8404–8413. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao J, Tian J, Lv Y, Shi F, Kong F, Shi H
and Zhao L: Leptin induces function activation of cyclooxygenase-2
through JAK2/STAT3, MAPK/ERK and PIK3/AKT pathways in human
endometrial cancer cells. Cancer Sci. 100:389–395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beales IL and Ogunwobi OO: Microsomal
prostaglandin E synthase-1 inhibition blocks proliferation and
enhances apoptosis in oesophageal adenocarcinoma cells without
affecting endothelial prostacyclin production. Int J Cancer.
126:2247–2255. 2010.
|
|
27
|
Kang HS, Lee JY and Kim CJ:
Anti-inflammatory activity of arctigenin from Forsythiae fructus. J
Ethnopharmacol. 116:305–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiao J, Gai QY, Wei FY, Luo M, Wang W, Fu
YJ and Zu YG: Biodiesel from Forsythia suspens e [(Thunb.) Vahl
(Oleaceae)] fruit oil. Bioresour Technol. 143:653–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haldar S, Chintapalli J and Croce CM:
Taxol induces bcl-2 phosphorylation and death of prostate cancer
cells. Cancer Res. 56:1253–1255. 1996.PubMed/NCBI
|
|
30
|
Han Z, Wei W, Dunaway S, Calabresi P,
Sedivy J, Hendrickson EA, Balan KV, Pantazis P and Wyche JH: Role
of p21 in apoptosis and senescence of human colon cancer cells
treated with camptothecin. J Biol Chem. 277:17154–17160. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Green R and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strathmann J, Klimo K, Sauer SW, Okun JG,
Prehn JH and Gerhäuser C: Xanthohumol-induced transient superoxide
anion radical formation triggers cancer cells into apoptosis via a
mitochondria-mediated mechanism. FASEB J. 24:2938–2950. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid: a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang X and Wang X: Cytochrome C-mediated
apoptosis. Annu Rev Biochem. 73:87–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nelson EA, Sharma SV, Settleman J and
Frank DA: A chemical biology approach to developing STAT
inhibitors: molecular strategies for accelerating clinical
translation. Oncotarget. 2:5182011.PubMed/NCBI
|
|
37
|
Wang Z, Zhu S, Shen M, Liu J, Wang M, Li
C, Wang Y, Deng A and Mei Q: STAT3 is involved in esophageal
carcinogenesis through regulation of Oct-1. Carcinogenesis.
34:678–688. 2013. View Article : Google Scholar
|
|
38
|
Sepúlveda P, Encabo A, Carbonell-Uberos F
and Miñana MD: Bcl2 expression is mainly regulated by JAK/STAT3
pathway in human CD34+ hematopoietic cells. Cell Death
Differ. 14:378–380. 2006. View Article : Google Scholar
|
|
39
|
Boucher MJ, Morisset J, Vachon PH, Reed
JC, Lainé J and Rivard N: MEK/ERK signaling pathway regulated the
expression of Bcl-2, Bcl-XL, and Mcl-1 and promotes survival of
human pancreatic cancer cells. J Biol Chem. 79:355–369. 2000.
|
|
40
|
Park CH, Chang JY, Hahm ER, Park S, Kim HK
and Yang CH: Quercetin, a potent inhibitor against β-catenin/Tcf
signaling in SW480 colon cancer cells. Biochem Biophys Res Commun.
328:227–234. 2005. View Article : Google Scholar : PubMed/NCBI
|