Introduction
Osteosarcoma is the most common bone primary
malignant tumor and mostly occurs in late childhood and early
adulthood (1). The application of
adjuvant chemotherapy has increased the overall survival rate of
osteosarcoma patients to more than 70% (2). However in patients with metastasis or
recurrence, prognosis remains poor, with survival rates of only
20–30% (3,4). Without formal treatment, osteosarcoma
cells tend to metastasize to new body sites, most commonly to the
lung, within 6 months to 1 year, eventually leading to death
(5). The mechanisms underlying
progression and metastasis of this cancer type are only beginning
to be elucidated, and novel, effective therapeutic agents are
urgently needed.
Intratumoral hypoxia is a typical feature of solid
tumors. It is mainly due to the abnormal formation of vasculature
in the rapidly growing tumor mass. Tumor hypoxia has been
correlated to increased tumor invasion, angiogenesis and distant
metastasis (6–8). The adaptation of tumor cells to
hypoxia has given rise to tumor heterogeneity and the selection of
resistant clones, evolving into a more aggressive phenotype
(9). The transcription factor
hypoxia inducible factor-1α (HIF-1α) mediates cellular responses to
hypoxia. Overexpression of the HIF-1α protein has been reported in
numerous solid tumors and their metastases (9). Stabilization and activation of the
HIF-1α/HIF-1β transcription complex is closely associated with cell
proliferation, metastasis, angiogenesis, epithelial to mesenchymal
transition (EMT), increased resistance to chemotherapy and
radiotherapy, and tumor progression (10–15).
Hence, it is likely that HIF-1α may be used as an independent
prognostic marker and as a predictor of the mortality risk and
treatment failure in tumors (16–19).
Resveratrol (3,4′,5-trihydroxystilbene) is a natural
polyphenolic phytoalexin mainly found in plants, including grapes,
peanuts, root extracts of the weed Polygonum cuspidatum, and
various fruits (20). Resveratrol
has been shown to have multiple bioactivities, including
anti-oxidative, chemopreventive, antitumor, growth inhibitory,
anti-inflammatory, estrogen-like, and immunoregulatory activity
(21,22). Resveratrol can affect various
cellular events related to distinct steps of carcinogenesis, i.e.,
tumor initiation, promotion and progression (23,24).
Resveratrol has therapeutic potential due to its ability to
suppress tumor cell growth via inducing cell-cycle arrest and
apoptosis, and to suppress tumor angiogenesis via inhibiting the
vascular endothelial growth factor (VEGF) protein (25–34).
At the molecular level, these effects may be attributed to the
inhibition of the formation of free radicals, and the inhibition of
cyclooxygenase, cytochrome P450 and protein kinase C activities
(35). Despite these findings
however, the molecular mechanisms underlying the anticancer effects
of resveratrol remain largely unknown (6,25).
Previous studies have shown for the first time that resveratrol
exerts a strong inhibitory effect on HIF-1α and VEGF expression in
human ovarian cancer cells. These studies demonstrated that
resveratrol interferes with the protein translational machinery and
promotes HIF-1α protein degradation, rather than affecting the
HIF-1α mRNA level (6,22).
In this study, we focused on the regulation, by
resveratrol, of cell proliferation, cell invasion and of the EMT
process in the human osteosarcoma cell line Saos-2 under hypoxic
conditions. We demonstrate that resveratrol markedly reduces the
enhanced cell proliferation and migration in hypoxic conditions
through downregulation of the HIF-1α protein, and that it also
inhibits the epithelial-mesenchymal transition.
Materials and methods
Materials
The human osteosarcoma cell line Saos-2 was
purchased from the Shanghai Institutes for Biological Sciences
(Chinese Academy of Sciences, Shanghai, China); McCOY’s 5A, fetal
bovine serum (FBS), trypsin, methabenzthiazuron (MTT), and dimethyl
sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO,
USA); resveratrol was obtained from MP Biomedicals, Inc. (Aurora,
OH, USA). Antibodies were purchased from the following sources:
anti-human anti-HIF-1α, -E-cadherin and -vimentin from Cell
Signaling Technology (Boston, MA, USA); anti-human anti-β-actin,
goat-anti-mouse and anti-rabbit IgG, and rabbit anti-goat IgG from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). HIF-1α small
interfering (si)RNA and siRNA controls were obtained from Santa
Cruz Biotechnology, Inc.; Transwell chambers from EMD Millipore
(Billerica, MA, USA); Invitrogen® Lipofectamine 2000
from Thermo Fisher Scientific (Waltham, MA, USA); PCR mix from
Xi’an Runde Biotechnology (Xi’an, China); and PCR primer sets from
Dingguo Biotechnology Co., Ltd. (Beijing, China).
Cell cultures and transfection
The human osteosarcoma Saos-2 cell line was cultured
in McCOY’s 5A medium supplemented with 15% FBS, 100 μg/ml
ampicillin and 100 μg/ml streptomycin, at 37°C in a humidified
atmosphere containing 5% CO2. In experiments designed to
evaluate the role of hypoxia, cells were first incubated in
normoxic conditions to acquire the desired subconfluence level
(65–70%), and then cultured in controlled hypoxic conditions (3%
O2), as previously described (36,37)
for up to 24 h. Osteosarcoma cells at the exponential phase were
incubated in six orifice plates in McCOY’s 5A supplemented with 1%
FBS for 24 h. The drugs (or solvent only) were then added to the
medium containing 1% FBS, and cells were cultured for an additional
24 h prior to the Matrigel invasion assay.
Cell proliferation assay
Each group of cells was seeded onto a 96-well plate
at a density of 5,000–10,000 cells/well for 24 h prior to serum
starvation for 24 h. Following starvation, cells were incubated in
McCOY’s 5A medium supplemented with 15% FBS. After 12, 24, 48 or 72
h, the medium was removed, and the MTT reagent (5 mg/ml) was added
in each well for a 4-h incubation at 37°C. Optical densities (OD)
were measured at 490 nm on a microplate reader (BioTek Instruments,
Inc., Winooski, VA, USA). The proliferation rate was calculated as
OD (sample)/OD (control). Reported values are the average of
triplicate experiments.
Matrigel cell invasion assay
The invasive ability of each group of cells was
evaluated though a Transwell chamber-based invasion assay. Briefly,
the upper surface of the Transwell filter was coated with Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA). Before treatment, cells
at the exponential phase were incubated in McCOY’s 5A medium
supplemented with 1% FBS for 24 h in 6-well plates. The cells
(5×104) were resuspended in serum-free medium and
allowed to migrate towards a serum gradient (20%) in the lower
chamber for 24 h, in a humidified tissue culture incubator.
Non-invasive cells were removed from the top of the Matrigel by
scraping with a cotton-tipped swab. Invasive cells at the bottom
surface of the filter were fixed with 4% paraformaldehyde and
stained with Crystal Violet dye (Boster Biological Technology,
Ltd., Wuhan, China). The number of invasive cells was counted under
a light microscope (Axio Observer A1; Carl Zeiss Microscopy GmbH,
Jena Germany) at 10 random fields for each membrane, and pictures
were acquired at ×100 magnification with a AxiocaCam MRc5 camera
(Carl Zeiss Microscopy GmbH). The tumor cell invasion assay was
performed in triplicate.
Western blot assay
Saos-2 cells were washed with ice-cold
phosphate-buffered saline and lysed in situ with RIPA buffer
(50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% sodium deoxycholate, 1.0%
Triton X-100, 0.1% SDS), supplemented with protease inhibitors
(Roche Diagnostics, Penzberg, Germany) and phosphatase inhibitors
(Sigma-Aldrich). Following incubation on ice for 30 min, cell
lysates were centrifuged at 12,000 × g for 15 min at 4°C to remove
the debris. Proteins (100 μg) were separated by 10% SDS-PAGE and
were transferred to polyvinylidene difluoride (PVDF) membranes
(Roche Diagnostics). Then, the PVDF membranes were blocked with 5%
non-fat dry milk in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl,
0.05% Tween-20) and incubated with the primary antibodies overnight
at 4°C. After washing with TBST five times for 10 min each, the
PVDF membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies at room temperature for
2 h. The membranes were washed again with TBST and an enhanced
chemiluminescence (ECL) kit (USCN Life Science Inc., Wuhan, China)
was used to visualize the immunoblots.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNAs were extracted from osteosarcoma cells
with the Invitrogen® TRIzol reagent (Thermo Fisher
Scientific) according to the manufacturer’s protocol, and the RT
reaction was performed using a PrimeScript RT Reagent kit (Takara,
Dalian, China). cDNAs (1 μl for each sample) were amplified by PCR
using the following primer sequences: HIF-1α forward (F),
5′-AAGTCTAGGGATGCAGCA-3′, and reverse (R),
5′-CAAGATCACCAGCATCATG-3′; E-cadherin F,
5′-ATTCTGATTCTGCTGCTCTTG-3′, and R, 5′-AGTCCT GGTCCTCTTCTCC-3′;
vimentin F, 5′-AATGACCGCTTC GCCAAC-3′, and R,
5′-CCGCATCTCCTCCTCGTAG-3′; β-actin F, 5′-ATTCTGATTCTGCTGCTCTTG-3′,
and R, 5′-AGTCCTGGTCCTCTTCTCC-3′. β-actin was used as the
normalization control. The amplified products were observed by 1.5%
agarose gel electrophoresis. Images of the gel were acquired and
analyzed under a UV transilluminator. The RT-PCR assay was carried
out in triplicate.
RNA interference
siRNAs targeting the HIF-1α gene
(HIF-1α-Homo-2258: 5′-CCACCACUGAUGAAUUAAATT-3′ and
5′-UUUAAUUCAUCAGUGGUGGTT-3′) and negative control siRNAs (NC:
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′) were
purchased from Santa Cruz Biotechnology, Inc. Cells
(105/well) seeded in 6-well plates were transfected with
100 nM siRNA using the Lipofectamine 2000 reagent according to the
manufacturer’s instructions. The cells were used for further
experiments at 48 h after transfection.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Differences were evaluated using unpaired two-tailed
Student’s t-tests with unequal variance for multiple comparisons
using the SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
P-values <0.05 were considered to indicate statistically
significant differences. All experiments were independently
repeated at least three times.
Results
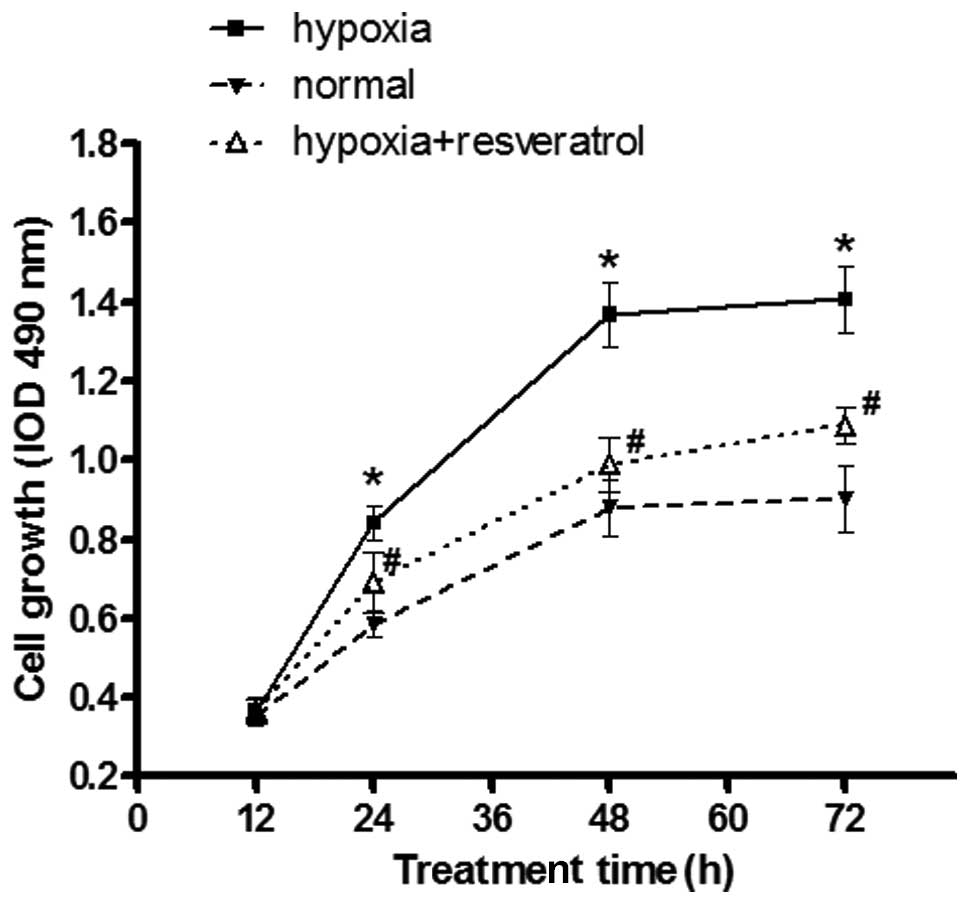
Resveratrol attenuates the effect of
hypoxia on increased proliferation of Saos-2 cells
To investigate the potential role of resveratrol on
osteosarcoma Saos-2 cell proliferation under hypoxic conditions,
normal (grown under normoxia) cells, hypoxic cells and hypoxic
cells pre-treated with resveratrol were seeded onto 96-well plates
at different time-points, and the proliferative rate of in each
group was investigated by the MTT assay. Hypoxia markedly increased
the proliferation rate of Saos-2 cells as compared to the normal
cell group, and resveratrol significantly reduced the proliferation
rate of the hypoxic cell group at different time-points (Fig. 1).
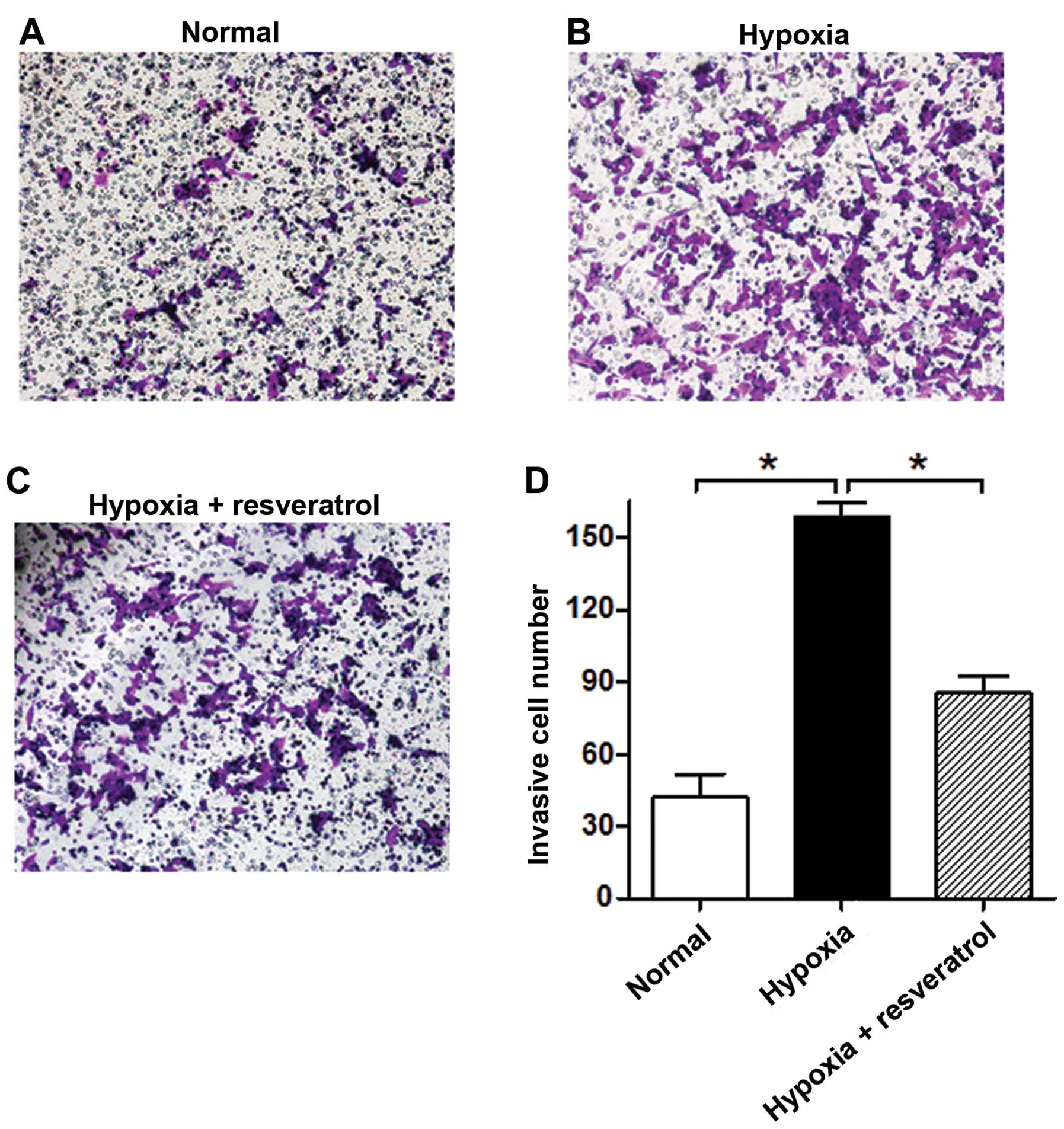
Resveratrol reverses the increased
invasive ability of Saos-2 cells under hypoxia
We further tested the effect of resveratrol on the
Saos-2 cell invasive ability under an hypoxic microenvironment. The
results showed that hypoxia significantly increases the number of
invasive Saos-2 cells as compared to normoxia, and resveratrol
markedly reduced the invasive ability of hypoxic cells (Fig. 2).
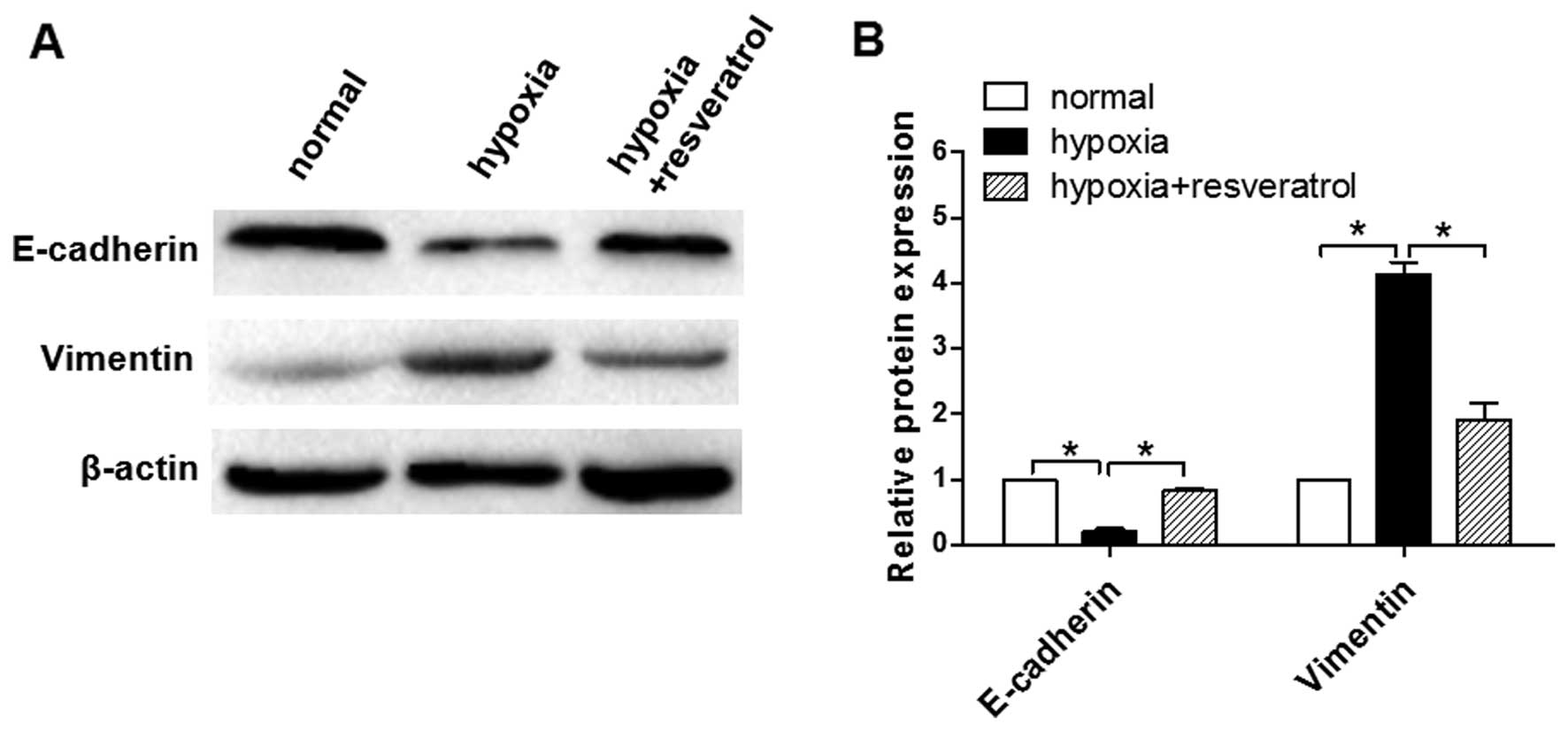
Resveratrol inhibits the EMT process
induced by hypoxia in Saos-2 cells
To investigate whether Saos-2 cells undergo EMT as a
result of exposure to hypoxia, we examined the expression of
markers of epithelial and mesenchymal phenotypes by western blot
analysis. Hypoxic cells displayed decreased E-cadherin and
increased vimentin expression (Fig.
3). However, when hypoxic cells were pre-treated with
resveratrol, the hypoxia-induced EMT process was reversed.
Resveratrol treatment caused a marked increase in the expression of
E-cadherin, and a significant decrease in the expression of
vimentin (Fig. 3).
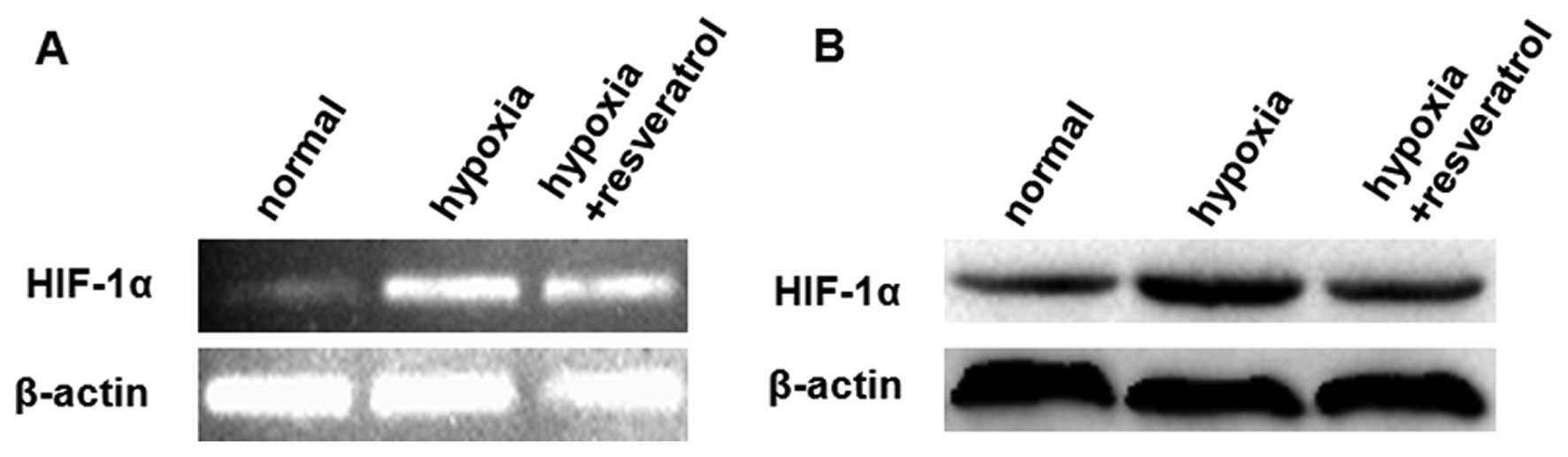
Resveratrol attenuates the effects of
hypoxia likely through downregulation of HIF-1α in Saos-2
cells
We further investigated whether resveratrol inhibits
HIF-1α expression, which was induced in tumor cells upon exposure
to hypoxic stress, a common condition in aggressive solid tumors.
We observed that pretreatment of Saos-2 cells with resveratrol
reduced the hypoxia-induced HIF-1α protein accumulation without
affecting the HIF-1α mRNA level (Fig. 4). Quantitative data from the three
repeated experiments, using β-actin as the normalized control,
showed the relative HIF-1α mRNA expression level (± standard
deviation) in each group was 0.136±0.042, 0.758±0.084 and
0.728±0.080 for the normal, hypoxia and hypoxia + resveratrol
groups, respectively. Furthermore, quantitative data from the three
repeated experiments revealed the relative HIF-1α protein
expression levels (± standard deviation), using β-actin as the
normalization control, were 0.323±0.050, 0.842±0.096 and
0.419±0.046 for the normal, hypoxia and hypoxia + resveratrol
groups, respectively. These results indicate that the HIF-1α
protein level was signincantly decreased in the hypoxia +
resveratrol group, when compared with the hypoxia group
(P<0.05), however, no significant difference in HIF-1α mRNA
expression was identified (P>0.05).
Next, we investigated whether resveratrol attenuates
the effects of hypoxia through downregulation of the HIF-1α
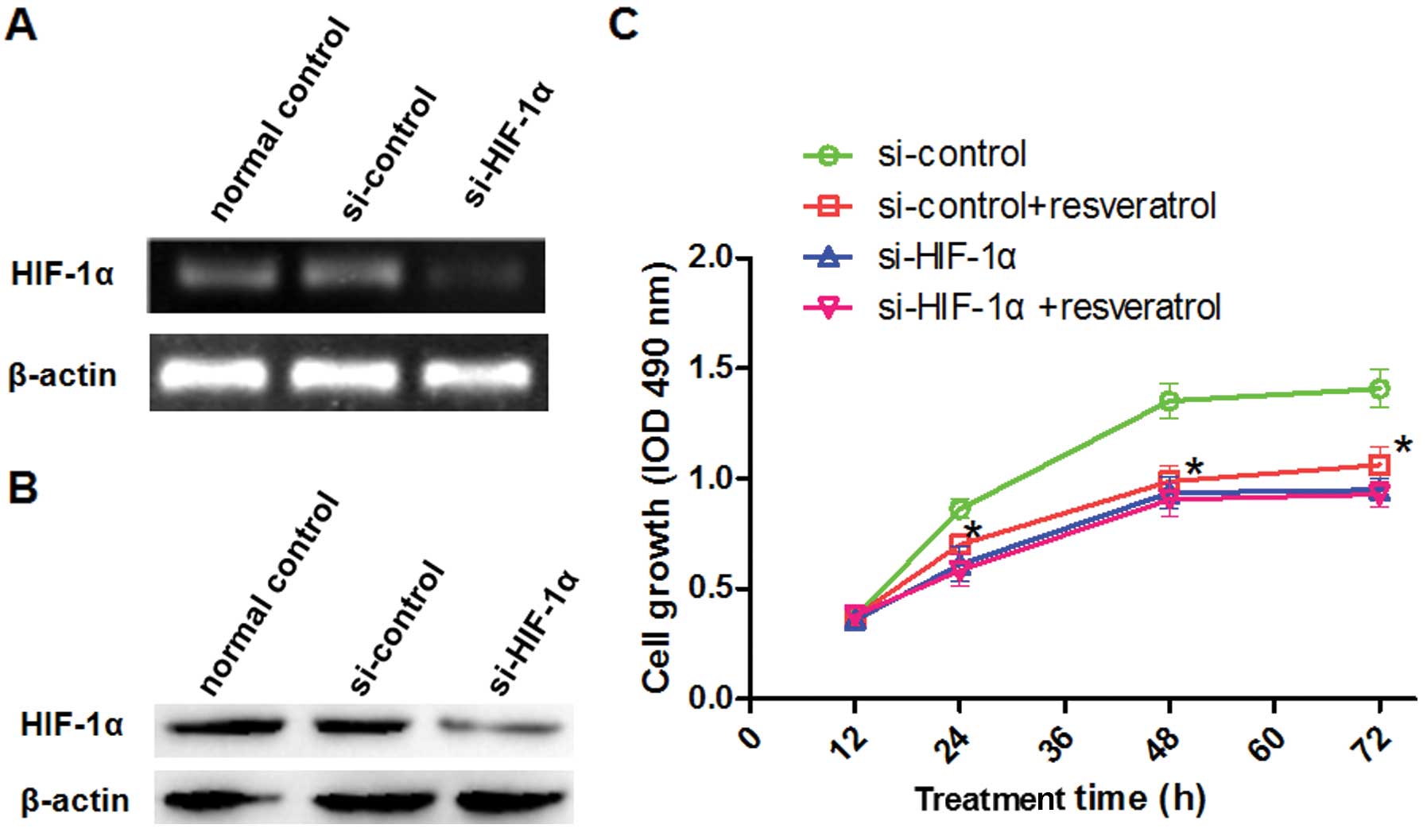
gene in Saos-2 cells. We transiently silenced HIF-1α and
analyzed its expression and cell growth under hypoxic conditions
(Fig. 5A and B). In the si-control
transfected group of cells exposed to hypoxic conditions, treatment
with resveratrol significantly reduced the proliferation rate
(Fig. 5C), increased the level of
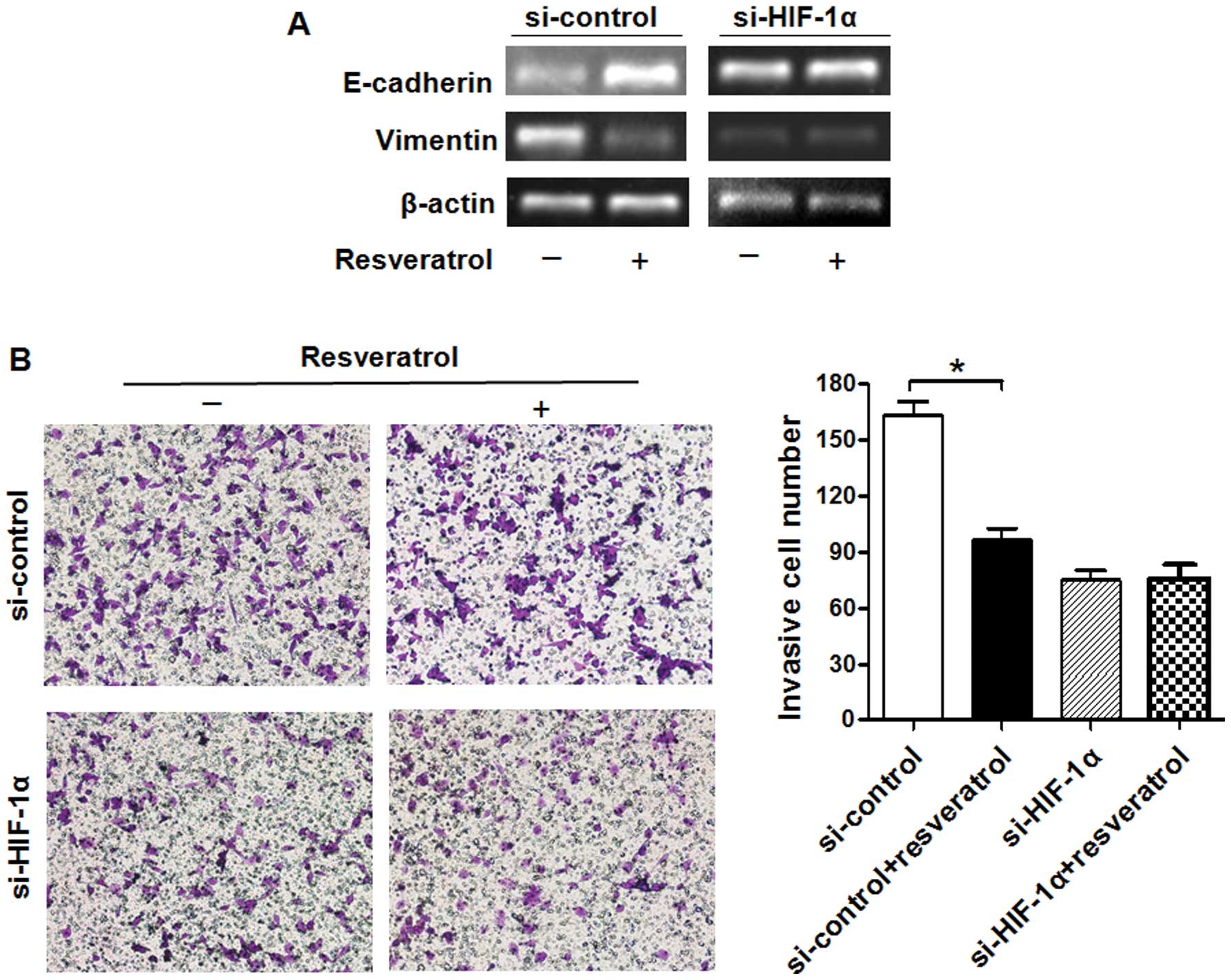
E-cadherin and decreased that of vimentin (Fig. 6A). Furthermore, resveratrol
significantly decreased the invasive ability of Saos-2 cells, which
was induced by hypoxia (Fig. 6B).
In the si-HIF-1α-transfected group, however, resveratrol had no
significant effect under hypoxic conditions (Figs. 5C and 6). These findings suggest that HIF-1α
expression plays a vital role in the resveratrol-abrogated effects
of hypoxia in Saos-2 cells.
Discussion
Invasive solid tumors often present a characteristic
hypoxic microenvironment, which leads to an abnormality in the
microvasculature and a rapid expansion of the tumor mass. Previous
studies have demonstrated that the effects induced by hypoxia is
mainly mediated by HIF-1α (9).
HIF-1α overexpression was observed in numerous solid tumors
including glioma, pancreatic cancer and prostate carcinoma cells,
as well as human tongue squamous cell carcinomas (9,38–41).
Previous studies have shown that HIF-1α overexpression, either as a
result of intratumoral hypoxia or genetic alterations, increases
the transcription of downstream genes, which contributes to cancer
cell invasion (41–43). These findings indicate that there
is an association between hypoxia or HIF-1α overexpression and
tumor invasion or patient mortality (43). In the present study, we showed that
resveratrol markedly inhibits the promoting effect of hypoxia on
the invasive ability of Saos-2 cells.
The epithelial to mesenchymal transition (EMT) is
considered a dynamic and reversible biological process. It has now
became apparent that EMT plays important roles in the progression
of cancer (44). Hypoxia is
associated with the EMT process, and HIF-1α was shown to mediate
the hypoxia-induced changes in the EMT (42). Recently, resveratrol was reported
to have antitumor activities, including the ability to inhibit the
EMT process in tumors. A number of studies have shown that
resveratrol can suppress the EMT process through reactivating p53
and by inducing miR-145 and miR-200c, suppressing the
PI3K/Akt/nuclear factor-κB (NF-κB) pathway, downregulating the
PI3K/Akt and Wnt/β-catenin signaling cascades, or inhibiting
transforming growth factor-β1 (TGF-β1) in vitro. In
addition, resveratrol treatment reduced the levels of EMT markers
in xenograft tumors in vivo (45–50).
In our experiments, resveratrol reversed the hypoxia-enhanced EMT
process by affecting the expression of related proteins.
Several in vitro studies have demonstrated
that resveratrol can inhibit the proliferation of a number of tumor
types, such as leukemia, prostate, breast and colon cancers, as
well as osteosarcoma, under normoxic conditions (23,51–53).
The results from the present study provide evidence that
resveratrol also has an inhibitory effect on hypoxia-enhanced
proliferation of osteosarcoma (Saos-2) cells.
Resveratrol exerts a strong inhibitory effect on
HIF-1α expression in certain human cancer cells (8,22).
These studies showed that resveratrol interferes with the protein
translational machinery and promotes HIF-1α protein degradation
rather than affecting the HIF-1α mRNA levels. In the present
study, we showed that resveratrol markedly inhibits HIF-1α protein
expression without affecting the HIF-1α mRNA level.
Silencing of HIF-1α reversed all the above-mentioned effects
of hypoxia, which indicates that the effect of resveratrol on
hypoxia may be attributed to its inhibitory effects on HIF-1α
protein expression. However, additional studies are needed to
identify the associated genes that are directly or indirectly
involved in resveratrol-regulated cancer cell invasion in response
to hypoxia and/or HIF-1α overexpression.
Acknowledgements
We thank all laboratory technicians in the
Translational Medicine Research Center of Suzhou University School
for their guidance in molecular biology techniques.
References
|
1
|
Stiller CA: International patterns of
cancer incidence in adolescents. Cancer Treat Rev. 33:631–645.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Link MP, Goorin AM, Miser AW, et al: The
effect of adjuvant chemotherapy on relapse-free survival in
patients with osteosarcoma of the extremity. N Engl J Med.
314:1600–1606. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harting MT, Blakely ML, Jaffe N, et al:
Long-term survival after aggressive resection of pulmonary
metastases among children and adolescents with osteosarcoma. J
Pediatr Surg. 41:194–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyers PA, Heller G, Healey JH, et al:
Osteogenic sarcoma with clinically detectable metastasis at initial
presentation. J Clin Oncol. 11:449–453. 1993.PubMed/NCBI
|
|
5
|
Geller DS and Gorlick R: Osteosarcoma: a
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.
|
|
6
|
Michieli P: Hypoxia, angiogenesis and
cancer therapy: to breathe or not to breathe? Cell Cycle.
8:3291–3296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pennacchietti S, Michieli P, Galluzzo M,
Mazzone M, Giordano S and Comoglio PM: Hypoxia promotes invasive
growth by transcriptional activation of the met protooncogene.
Cancer Cell. 3:347–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteban MA, Tran MG, Harten SK, et al:
Regulation of E-cadherin expression by VHL and hypoxia-inducible
factor. Cancer Res. 66:3567–3575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng ZX, Sun B, Wang SJ, et al: Nuclear
factor-κB-dependent epithelial to mesenchymal transition induced by
HIF-1α activation in pancreatic cancer cells under hypoxic
conditions. PLoS One. 6:e237522011. View Article : Google Scholar
|
|
11
|
Semenza GL: HIF-1 and tumor progression:
pathophysiology and therapeutics. Trends Mol Med. 8:S62–S67. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai T, Horiuchi A, Wang C, et al: Hypoxia
attenuates the expression of E-cadherin via up-regulation of SNAIL
in ovarian carcinoma cells. Am J Pathol. 163:1437–1447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang MH and Wu KJ: TWIST activation by
hypoxia inducible factor-1 (HIF-1): implications in metastasis and
development. Cell Cycle. 7:2090–2096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta GP and Massague J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Overexpression of hypoxia-inducible
factor 1alpha is a marker for an unfavorable prognosis in
early-stage invasive cervical cancer. Cancer Res. 60:4693–4696.
2000.PubMed/NCBI
|
|
17
|
Birner P, Schindl M, Obermair A,
Breitenecker G and Oberhuber G: Expression of hypoxia-inducible
factor 1alpha in epithelial ovarian tumors: its impact on prognosis
and on response to chemotherapy. Clin Cancer Res. 7:1661–1668.
2001.PubMed/NCBI
|
|
18
|
Aebersold DM, Burri P, Beer KT, et al:
Expression of hypoxia-inducible factor-1alpha: a novel predictive
and prognostic parameter in the radiotherapy of oropharyngeal
cancer. Cancer Res. 61:2911–2916. 2001.PubMed/NCBI
|
|
19
|
Koukourakis MI, Giatromanolaki A,
Skarlatos J, et al: Hypoxia inducible factor (HIF-1α and HIF-2α)
expression in early esophageal cancer and response to photodynamic
therapy and radiotherapy. Cancer Res. 61:1830–1832. 2001.PubMed/NCBI
|
|
20
|
Soleas GJ, Diamandis EP and Goldberg DM:
Wine as a biological fluid: history, production, and role in
disease prevention. J Clin Lab Anal. 11:287–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
22
|
Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J
and Le AD: Resveratrol inhibits hypoxia-induced accumulation of
hypoxia-inducible factor-1alpha and VEGF expression in human tongue
squamous cell carcinoma and hepatoma cells. Mol Cancer Ther.
4:1465–1474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kundu JK, Chun KS, Kim SO and Surh YJ:
Resveratrol inhibits phorbol ester-induced cyclooxygenase-2
expression in mouse skin: MAPKs and AP-1 as potential molecular
targets. Biofactors. 21:33–39. 2004. View Article : Google Scholar
|
|
25
|
Cao Z, Fang J, Xia C, Shi X and Jiang BH:
trans-3,4,5′-Trihydroxystibene inhibits hypoxia-inducible factor
1alpha and vascular endothelial growth factor expression in human
ovarian cancer cells. Clin Cancer Res. 10:5253–5263. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aziz MH, Kumar R and Ahmad N: Cancer
chemoprevention by resveratrol: In vitro and in vivo studies and
the underlying mechanisms (review). Int J Oncol. 23:17–28.
2003.PubMed/NCBI
|
|
27
|
Jiang H, Zhang L, Kuo J, et al:
Resveratrol-induced apoptotic death in human U251 glioma cells. Mol
Cancer Ther. 4:554–561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang TT, Schoene NW, Kim EK and Kim YS:
Pleiotropic effects of the sirtuin inhibitor sirtinol involves
concentration-dependent modulation of multiple nuclear
receptor-mediated pathways in androgen-responsive prostate cancer
cell LNCaP. Mol Carcinog. 52:676–685. 2013. View Article : Google Scholar
|
|
29
|
Juan ME, Alfaras I and Planas JM:
Colorectal cancer chemoprevention by trans-resveratrol. Pharmacol
Res. 65:584–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu P, Wang X, Hu C and Hu T: Inhibition
of proliferation and induction of apoptosis by trimethoxyl stilbene
(TMS) in a lung cancer cell line. Asian Pac J Cancer Prev.
12:2263–2269. 2011.
|
|
31
|
Zhou JH, Cheng HY, Yu ZQ, He DW, Pan Z and
Yang DT: Resveratrol induces apoptosis in pancreatic cancer cells.
Chin Med J (Engl). 124:1695–1699. 2011.
|
|
32
|
Yu HB, Zhang HF, Zhang X, et al:
Resveratrol inhibits VEGF expression of human hepatocellular
carcinoma cells through a NF-kappa B-mediated mechanism.
Hepatogastroenterology. 57:1241–1246. 2010.
|
|
33
|
Li Y, Bäckesjö CM, Haldosén LA and
Lindgren U: Resveratrol inhibits proliferation and promotes
apoptosis of osteosarcoma cells. Eur J Pharmacol. 609:13–18. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Z, Li Y and Yang R: Effects of
resveratrol on vascular endothelial growth factor expression in
osteosarcoma cells and cell proliferation. Oncol Lett. 4:837–839.
2012.PubMed/NCBI
|
|
35
|
Gusman J, Malonne H and Atassi G: A
reappraisal of the potential chemopreventive and chemotherapeutic
properties of resveratrol. Carcinogenesis. 22:1111–1117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aleffi S, Petrai I, Bertolani C, et al:
Upregulation of proinflammatory and proangiogenic cytokines by
leptin in human hepatic stellate cells. Hepatology. 42:1339–1348.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Novo E, Cannito S, Zamara E, et al:
Proangiogenic cytokines as hypoxia-dependent factors stimulating
migration of human hepatic stellate cells. Am J Pathol.
170:1942–1953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zagzag D, Zhong H, Scalzitti JM, Laughner
E, Simons JW and Semenza GL: Expression of hypoxia-inducible factor
1alpha in brain tumors: association with angiogenesis, invasion,
and progression. Cancer. 88:2606–2618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong H, Chiles K, Feldser D, et al:
Modulation of hypoxia-inducible factor 1alpha expression by the
epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP
pathway in human prostate cancer cells: implications for tumor
angiogenesis and therapeutics. Cancer Res. 60:1541–1545.
2000.PubMed/NCBI
|
|
40
|
Zhang Q, Zhang ZF, Rao JY, et al:
Treatment with siRNA and antisense oligonucleotides targeted to
HIF-1alpha induced apoptosis in human tongue squamous cell
carcinomas. Int J Cancer. 111:849–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akakura N, Kobayashi M, Horiuchi I, et al:
Constitutive expression of hypoxia-inducible factor-1alpha renders
pancreatic cancer cells resistant to apoptosis induced by hypoxia
and nutrient deprivation. Cancer Res. 61:6548–6554. 2001.PubMed/NCBI
|
|
42
|
Lei J, Ma J, Ma Q, et al: Hedgehog
signaling regulates hypoxia induced epithelial to mesenchymal
transition and invasion in pancreatic cancer cells via a
ligand-independent manner. Mol Cancer. 12:662013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krishnamachary B, Berg-Dixon S, Kelly B,
et al: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143.
2003.PubMed/NCBI
|
|
44
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi XP, Miao S, Wu Y, et al: Resveratrol
sensitizes tamoxifen in antiestrogen-resistant breast cancer cells
with epithelial-mesenchymal transition features. Int J Mol Sci.
14:15655–15668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen YA, Lin CH, Chi WH, et al:
Resveratrol impedes the stemness, epithelial-mesenchymal
transition, and metabolic reprogramming of cancer stem cells in
nasopharyngeal carcinoma through p53 activation. Evid Based
Complement Alternat Med. 2013:5903932013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li W, Ma J, Ma Q, et al: Resveratrol
inhibits the epithelial-mesenchymal transition of pancreatic cancer
cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr Med
Chem. 20:4185–4194. 2013. View Article : Google Scholar
|
|
48
|
Tsai JH, Hsu LS, Lin CL, et al:
3,5,4′-Trimethoxystilbene, a natural methoxylated analog of
resveratrol, inhibits breast cancer cell invasiveness by
downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and
reversal of epithelial-mesenchymal transition. Toxicol Appl
Pharmacol. 272:746–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang H, Zhang H, Tang L, et al:
Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal
transition and suppresses lung cancer invasion and metastasis.
Toxicology. 303:139–146. 2013. View Article : Google Scholar
|
|
50
|
Hu FW, Tsai LL, Yu CH, Chen PN, Chou MY
and Yu CC: Impairment of tumor-initiating stem-like property and
reversal of epithelial-mesenchymal transdifferentiation in head and
neck cancer by resveratrol treatment. Mol Nutr Food Res.
56:1247–1258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mitchell SH, Zhu W and Young CY:
Resveratrol inhibits the expression and function of the androgen
receptor in LNCaP prostate cancer cells. Cancer Res. 59:5892–5895.
1999.PubMed/NCBI
|
|
52
|
Schneider Y, Vincent F, Duranton B, et al:
Anti-proliferative effect of resveratrol, a natural component of
grapes and wine, on human colonic cancer cells. Cancer Lett.
158:85–91. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Estrov Z, Shishodia S, Faderl S, et al:
Resveratrol blocks interleukin-1beta-induced activation of the
nuclear transcription factor NF-kappaB, inhibits proliferation,
causes S-phase arrest, and induces apoptosis of acute myeloid
leukemia cells. Blood. 102:987–995. 2003. View Article : Google Scholar : PubMed/NCBI
|