Introduction
Renal cell carcinoma (RCC) is the second leading
cause of mortality among urological tumors, accounting for ~3% of
all adult malignancies (1). Clear
cell RCC is the most common malignant tumor of the kidney and is
associated with a poor prognosis (2). The reported incidence of RCC has
increased in the United States over the past two decades (3,4). In
>1/3 of patients RCC may have already metastasized prior to
diagnosis, and 50% of patients may suffer from recurrence, even
following nephrectomy (5).
Traditional chemotherapy and radiotherapy are not effective in the
treatment of advanced RCC. Furthermore, the molecular mechanisms
regulating the aggressive properties of RCC remain poorly
understood (6). Nevertheless, new
therapeutic strategies have emerged, based on molecular and
biological studies of RCC, including the potential applications of
microRNAs (miRNAs) in the diagnosis, prognosis and treatment of
tumors (7,8).
miRNAs are endogenous, non-coding 19–23 nucleotide
RNA molecules which are involved in post-transcriptional regulation
of gene expression (9). Mature
miRNAs bind to the 3′-untranslated regions (3′-UTR) of target
mRNAs, resulting in the degradation of mRNA or the blockade of
translation. Currently ~2,000 human miRNAs have been identified,
which are known to be involved in numerous biological processes,
including proliferation, apoptosis, migration and differentiation
(10). Numerous miRNAs have been
demonstrated to function as tumor suppressors or oncogenes
(11). It has been reported that
miRNA-451a (miR-451a) is widely dysregulated in numerous human
malignancies, including lung (12), liver (9) and breast cancer (13), and glioma (14), thus indicating that miR-451a may
have an important role in oncogenesis. However, knowledge is
currently limited on the mechanism of action of miR-451a in RCC. A
previous miRNA microarray chip analysis showed that miR-451a was
upregulated in RCC (15). The
expression and function of miR-451a in RCC requires further
investigation. The aim of the present study was to use quantitative
polymerase chain reaction (qPCR) to determine the relative
expression levels of miR-451a in paired RCC and normal tissues and
to analyze the effects of miR-451a on cell migration, proliferation
and apoptosis.
Materials and methods
Clinical sample collection and RNA
extraction
The present study was approved by the Institutional
Review Board and Ethical Committee of Peking University Shenzhen
Hospital (Shenzhen, China). All patients provided written informed
consent prior to the study. The RCC and matched normal adjacent
tissues were collected from the hospitals of Guangdong and Anhui.
The adjacent normal tissues were located 2.0 cm away from visible
RCC lesions. The fresh tissue samples were immediately immersed in
RNAlater (Qiagen, Hilden, Germany) following surgical resection,
stored at 4°C overnight and then frozen in liquid nitrogen and
stored at −80°C until further use. All tissue samples were reviewed
and classified with hematoxylin & eosin staining and disease
stages of the patients were classified according to the 2009
American Joint Committee on Cancer staging system. Total RNA was
extracted from 50 paired RCC samples and normal tissue using
TRIzol® (Invitrogen Life Technologies, Carlsbad, CA USA)
and were purified using the RNeasy® Maxi kit (Qiagen),
according to the manufacturer’s instructions. The clinical and
pathological characteristics of the 50 RCC patients, included in
the present study, are shown in Table
I.
 | Table IClinical characteristics of 50
patients with renal cancer. |
Table I
Clinical characteristics of 50
patients with renal cancer.
| Characteristic | N (%) |
|---|
| Ages (years) |
| ≥54 | 29(58) |
| <54 | 21(42) |
| Gender |
| Male | 30(60) |
| Female | 20(40) |
| Histological
type |
| Clear cell | 40(80) |
| Papillary | 10(20) |
| AJCC clinical
stage |
| I | 27(54) |
| II | 20(40) |
| III+IV | 3(6) |
qPCR
A previous miRNA microarray chip analysis showed
that hsa-miR-451a was highly expressed in RCC tissues, as compared
with the adjacent normal tissues (15). In order to validate the results of
the miRNA microarray chip analysis, qPCR was performed to detect
the relative expression levels of miR-451a in 50 paired RCC and
adjacent normal tissues. A total of 1 μg RNA was reverse
transcribed into cDNA using the miScript Reverse Transcription kit
(Qiagen), according to the manufacturer’s instructions. The qPCR
reaction of miR-451a was performed using a LightCycler®
480 Fluorescent Quantitative PCR system (Roche Diagnostics GmbH,
Mannheim, Germany) and miScript SYBR Green PCR kit (Qiagen),
according to the manufacturer’s instructions. Primers for the U6
non-coding small nuclear RNA were used as an internal control. The
20 μl reaction mixture contained 10 μl 2X QuantiTect SYBR Green PCR
Master Mix, 2 μl 10X miScript Universal Primer, 0.4 μl specific
microRNA primer, 1 μl cDNA template and RNase-free water. The
following primers were used: miR-451a forward,
5′-AAACCGTTACCATTACTGAGTT-3′ and the reverse miScript SYBR Green
PCR kit Universal Primer; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-ACGCTTCACGAATTTGCGT-3′ (Qiagen). The qPCR was performed
on tumor and normal cDNA in triplicate for each set. The PCR
reaction was performed as follows: 95° 15 min, followed by 40
cycles of 94°C 15 s, 55°C 30 s and 72°C 30 s.
Cell culture and transfection
786-O and ACHN human RCC cell lines, were obtained
from the Guangdong and Shenzhen Key Laboratory of Male Reproductive
Medicine and Genetics. The cells were cultured in Dulbecco’s
modified Eagle’s medium (Invitrogen Life Technologies),
supplemented with 10% fetal bovine serum (Shanghai ExCell Biology
Inc., Shanghai, China), and maintained in a humidified incubator
containing 5% CO2, at 37°C. The miRNA oligonucleotide
was chemically synthesized by GenePharma Company, Ltd. (Shanghai,
China). The sequences were as follows: hsa-miR-451a inhibitor,
5′-AACUCAGUAAUGGUAACGGUUU-3′; and the hsa-miR-451a inhibitor
negative control, 5′-CAGUACUUUUGUGUAGUACAA-3′. The cells were grown
to 60–80% confluence, followed by a transfection with the miR-451a
inhibitor or negative control using Lipofectamine® 2000
reagent (Invitrogen Life Technologies), according to the
manufacturer’s instructions. The transfection efficiency and
miR-451a expression changes were confirmed by fluorescence
microscopy (DMIRB; Leica Microsystems GmbH, Wetzlar, Germany) and
qPCR. The culture medium was refreshed 6 h after transfection, and
the transfection efficiency was observed in the cells that were
transfected with green fluorescent protein Fam-labeled miR-451a
inhibitor-negative control (GenePharma Co., Ltd.). The cells were
harvested and the total RNA was extracted for qPCR analysis 24 h
after transfection with a miR-451a inhibitor or the control.
Migration scratch assay in vitro
The migration scratch assay was used to assess the
migratory ability of 786-O and ACHN cells in vitro.
Approximately 600,000 cells were seeded per 6-well dish and
transfected after 24 h with 100 pmol of either the miR-451a
inhibitor or a negative control, using Lipofectamine®
2000. Following 6 h of transfection, a sterile 20 μl pipette tip
and markers were used to make a scratch, at the same point in each
of the samples, in the cell monolayers. The cells were then rinsed
with phosphate-buffered saline (PBS) and cultured at 37°C. Images
of the scratches were acquired, using a digital camera system, 0
and 24 h after the scratches were made. The migration distances
(μm) were measured using MIAS-2000 software and the experiments
were performed in triplicate and repeated ≥3 times.
Assessment of cell proliferation by MTT
assay
The cellular proliferation potential was determined
by MTT assay, as previously described (16). Briefly, 786-O and ACHN cells were
seeded into 96-well plates, at a cell density of 8,000 cells/well,
in growth medium and transfected with 10 pmol of either the
miR-451a inhibitor or a negative control. The cell growth was
measured by adding 20 μl of MTT (5 mg/ml, Sigma-Aldrich, St Louis,
MO, USA) to each well, followed by an incubation at 37°C for 4 h.
The proliferation assay was performed for three days and the cell
growth was measured at 24 h intervals. The reaction was stopped by
the addition of 150 μl dimethyl sulfoxide (Sigma-Aldrich).
Following agitation for 15 min at room temperature, the optical
density (OD) of each sample was measured at a wavelength of 490 nm,
using an Enzyme Immunoassay Instrument (Model 680; Bio-Rad,
Hercules, CA, USA).
Flow cytometric analysis of
apoptosis
786-O and ACHN cells (~3,000) were cultured at 37°C
and 5% CO2 in 6-well plates. Once the cells had reached
~60% confluence, they were transfected with either the miR-451a
inhibitor or a negative control. For the apoptosis assays, floating
and adherent cells were harvested 48 h after transfection, combined
and washed twice with cold PBS, followed by resuspension in 1X
binding buffer (Invitrogen Life Technologies). A total of 5 μl
Alexa Fluor® 488 Annexin V (Invitrogen Life
Technologies) and 3 μl propidium iodide (Invitrogen Life
Technologies), was added to 500 μl of the cell suspension, and the
samples were analyzed within 30 min of staining. The fluorescence
was measured using a flow cytometer (Beckman Coulter, Miami, FL,
USA) at an excitation of 488 nm, according to the manufacturer’s
instructions (8).
Statistical analysis
Statistical significance was determined using the
Student’s t-test. For the comparison of miR-451a expression levels
between the matched RCC and normal tissue samples, a paired
two-tailed t-test was used. A P<0.05 was considered to indicate
a statistically significant difference. Statistical analyses were
carried out using SPSS version 16.0 (SPSS, Inc., Chicago, IL,
USA).
Results
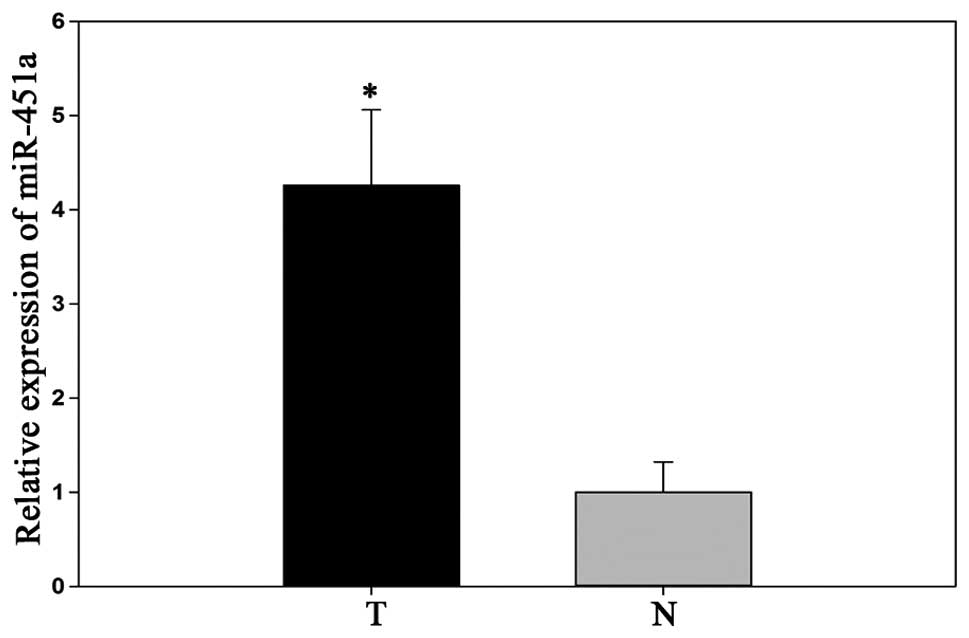
miR-451a is significantly upregulated in
RCC tissue
Previous research determined that the expression of
miR-451a was upregulated in RCC tissues (15). To confirm the results of the
previous miRNA microarray chip analysis, the expression of miR-451a
was determined in 50 matched RCC and adjacent normal tissues, by
qPCR. The results demonstrated that miR-451a relative expression
levels were significantly higher in RCC tissues, as compared with
the adjacent normal tissues (P<0.05; Fig. 1).
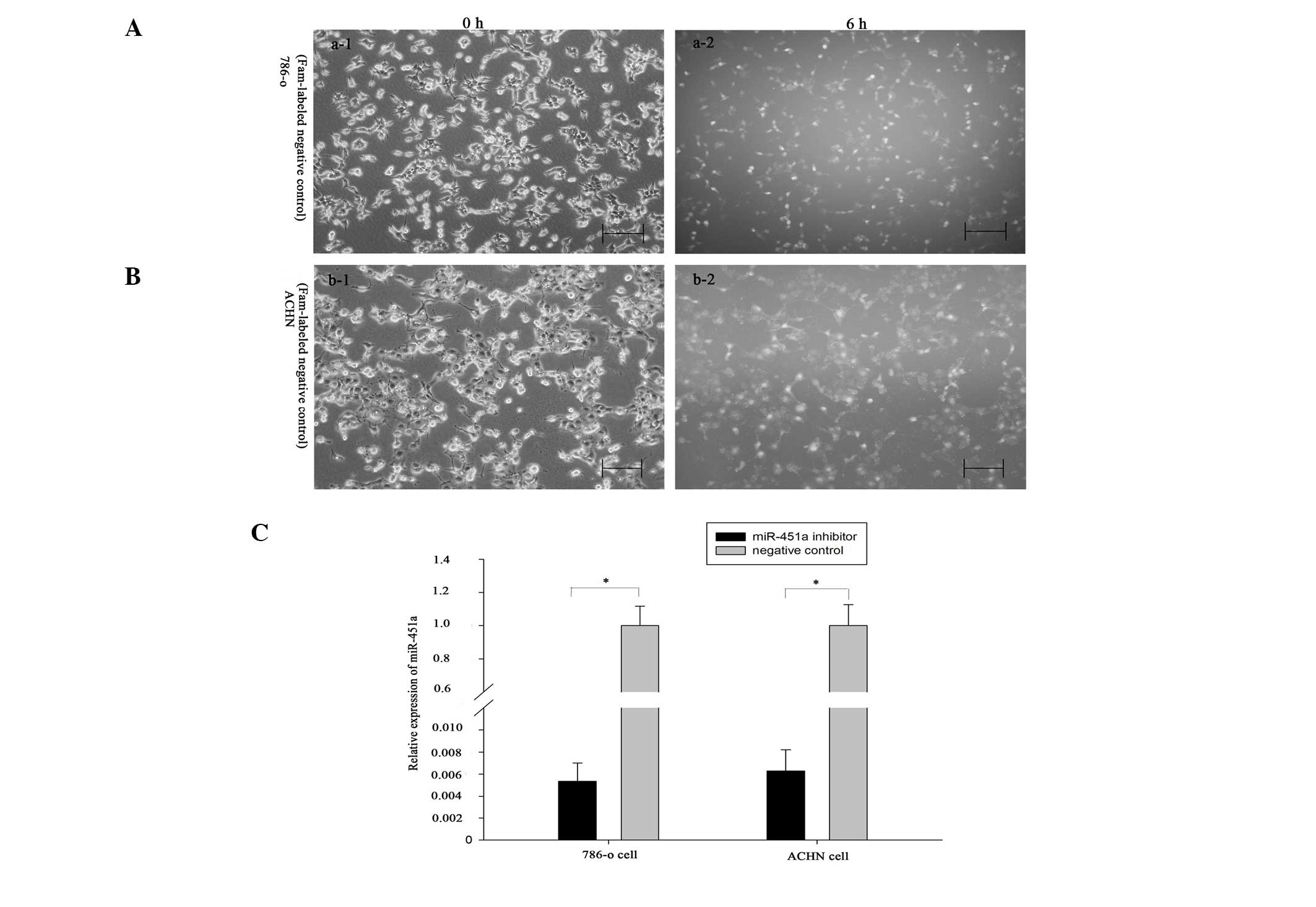
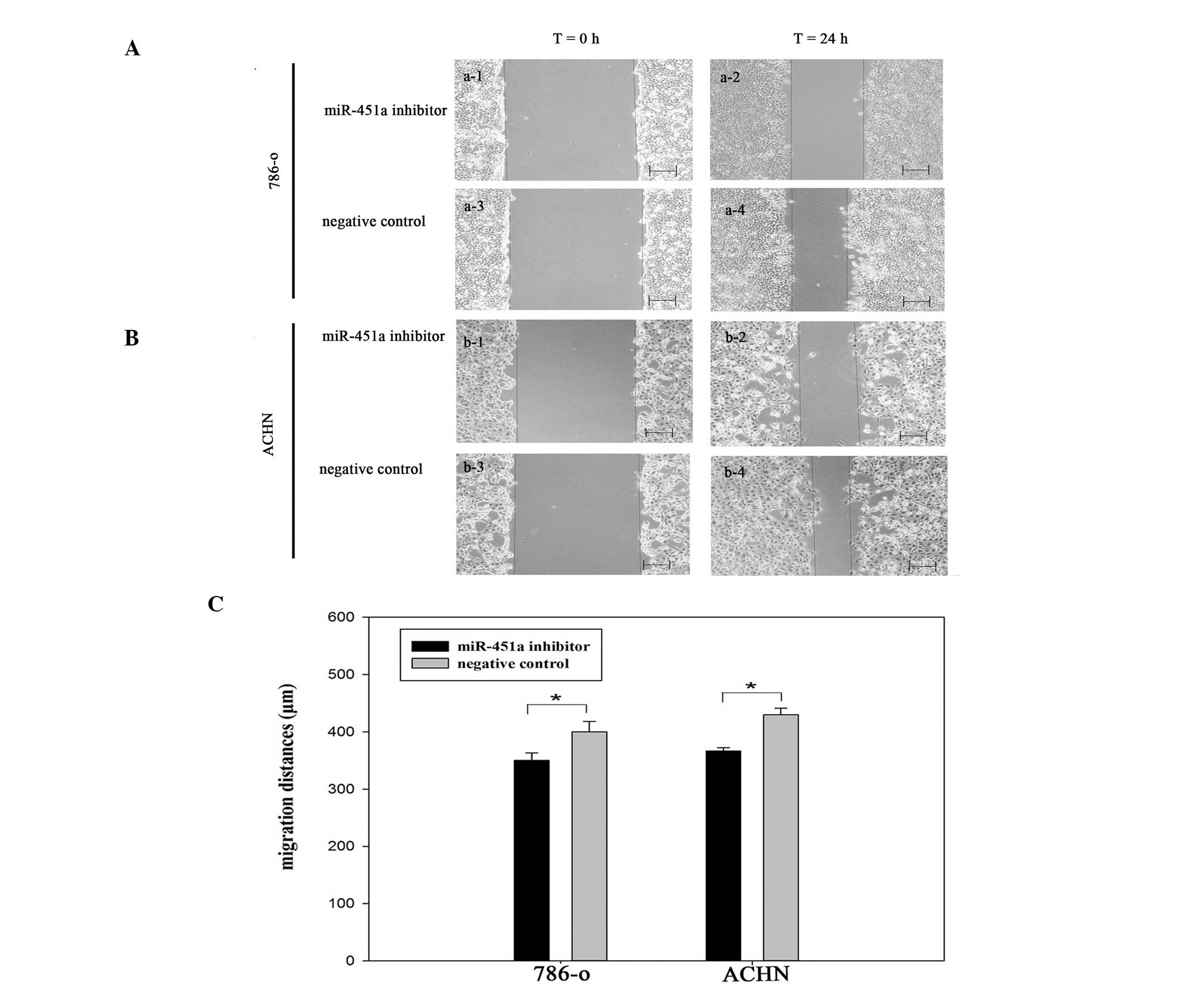
Reduction of miR-451a inhibits RCC cell
migration
To explore the functions of miR-451a in renal
cancer, a miR-451a inhibitor or a negative control was transfected
into 786-O and ACHN cells. The Fam-labeled negative control was
transfected into the cells, and the transfection efficiency was
analyzed by fluorescence microscopy 6 h after transfection. As
shown in Fig. 2A and B, the
transfection efficiency was ~90 and 85% in 786-O and ACHN cells,
respectively. In addition, the fold changes to the expression
levels of miR-451a, as determined by qPCR, were 198.1 and 157.9 in
the 786-O and ACHN cells, respectively (Fig. 2C, P<0.05) (Fig. 2C, P<0.05). Scratch assays were
performed to observe the function of miR-451a in cell migration.
Images of the scratches were captured at 0 and 24 h after
transfection, using a camera coupled to a fluorescence microscope
(Fig. 3). The results demonstrated
that the migration distances of the cells transfected with miR-451a
inhibitor were markedly shorter, as compared with the negative
control group (P<0.05). These results indicate that the
reduction of miR-451a expression, inhibited the migration of RCC
cells (Fig. 3C).
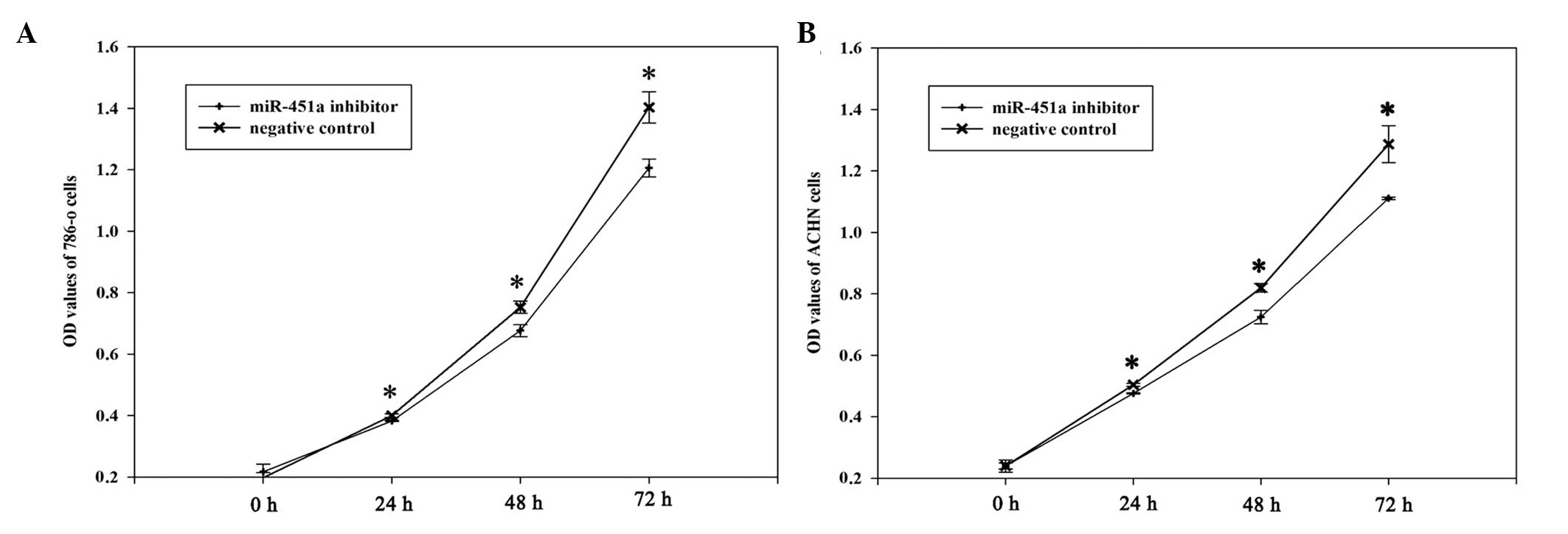
miR-451a inhibitor suppresses RCC cell
proliferation
To determine the potential role of miR-451a on the
proliferation of RCC cells, MTT assays were performed. The miR-451a
inhibitor and negative control groups were measured at 0, 24, 78
and 72 h after transfection. The OD values demonstrated that the
proliferation of 786-O cells was decreased by 4.6 (P<0.05),
11.25 (P<0.05) and 16.31% (P<0.05), and the proliferation of
ACHN cells was decreased by 5.8 (P<0.05), 13.12 (P<0.05) and
15.84% (P<0.05) at 24, 48 and 72 h after transfection,
respectively. These results suggest that the miR-451a inhibitor
reduced the growth of 786-O and ACHN cells, as compared with the
negative control inhibitor (Fig.
4).
Downregulation of miR-451a induces RCC
cell apoptosis
The effects of miR-451a on apoptosis were
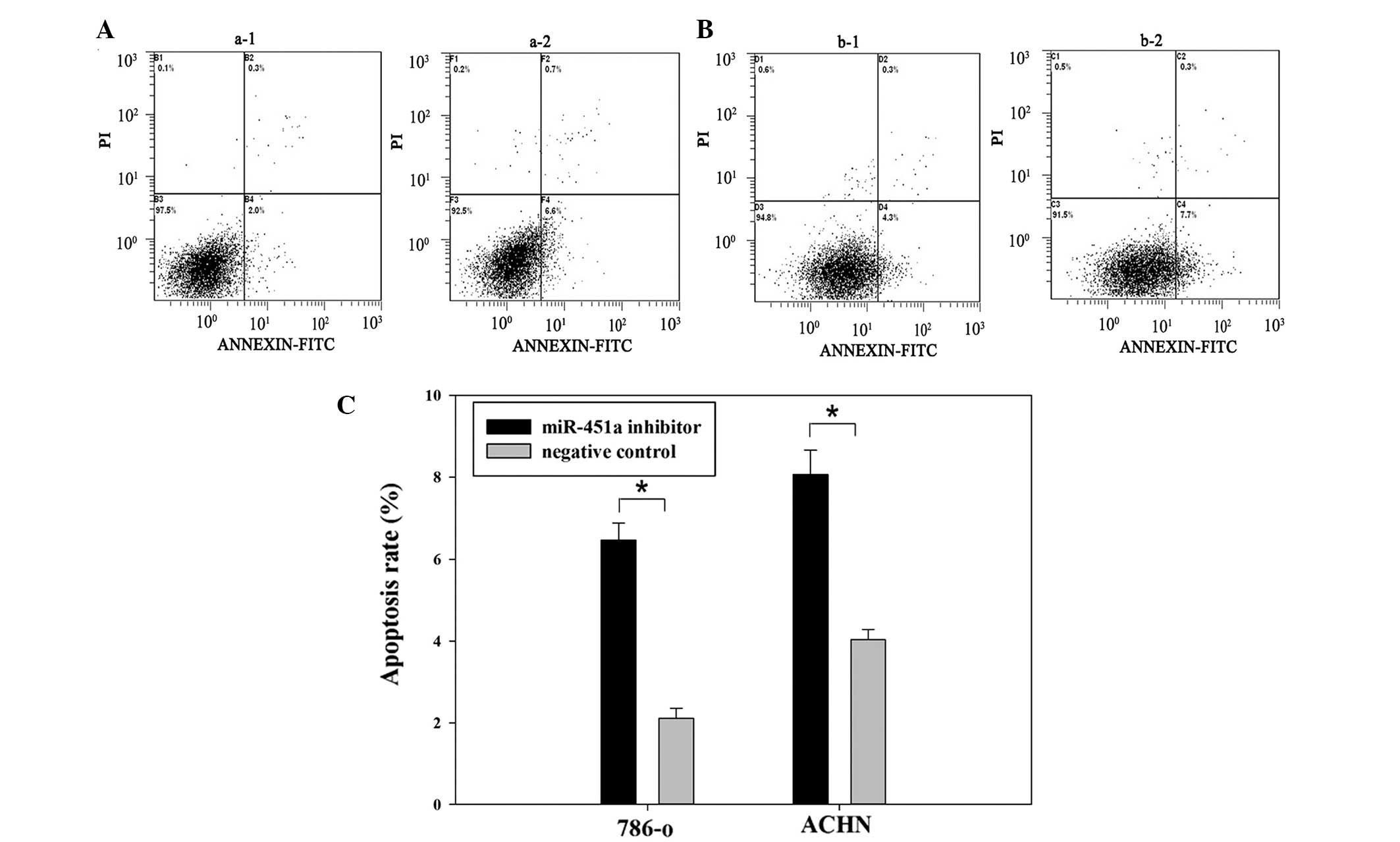
determined. 786-O and ACHN cells were transfected with either the
miR-451a inhibitor or a negative control for 48 h. Flow cytometric
analysis demonstrated that the apoptotic rate of 786-O cells
transfected with miR-451a inhibitor or a negative control was 6.6
vs. 2% (P<0.05) and the apoptotic rate of ACHN cells was 7.7 vs.
4.3% (P<0.05). These data demonstrate that downregulation of
miR-451a promoted RCC cell apoptosis (Fig. 5).
Discussion
miRNAs are a class of small, non-coding RNAs that
can regulate the expression of protein-coding genes through various
mechanisms, including targeted mRNA degradation and translational
inhibition (7,17). Previous evidence has suggested that
miRNAs have a crucial role in carcinogenesis and cancer
progression. By altering cell proliferation, differentiation,
invasion and apoptosis, miRNAs can function as either tumor
suppressors or oncogenes (18–20).
Various miRNAs have previously been shown to be significantly
upregulated in RCC, including miR-210, miR-34a and miR-21, which
were correlated with pathological processes, including oncogenesis
(21–23). Conversely, miR-141, miR-224,
miR-200c have been shown to be decreased in human RCC tissues,
implying that they possess tumor suppressive activity (24,25).
In the present study, the expression of miR-451a was shown to be
upregulated in RCC tissues.
Previous studies have provided evidence of the
effects of miR-451a, demonstrating its ability to inhibit cell
proliferation and induce apoptosis in numerous cancer cell lines.
Wang et al (26) reported
that ectopic miR-451a significantly suppressed the proliferation of
non-small cell lung carcinoma cells in vitro; this effect
was shown to be partially due to the downregulation of ras-related
protein 14. Bandreset et al (27) reported that hsa-miR-451a was
significantly underexpressed in gastric and colorectal cancer, as
compared with adjacent normal tissues, and showed that the
overexpression of miR-451a in gastric and colorectal cancer cells
inhibited cell proliferation. The downregulation of miRNA-451a was
also shown to be associated with a worse prognosis in these
cancers. Previous research has also demonstrated that miR-451a may
regulate LKB1/AMPK signaling and promote proliferation and
migration of glioma cells (28).
There is numerous evidence demonstrating that miR-451a may be up or
downregulated in various cancers and function as either a tumor
suppressor or oncogene. Furthermore, the present study demonstrated
that miR-451a expression was upregulated in RCC tissues, as
compared with adjacent nonmalignant tissues. These results suggest
that miR-451a may be characterized as an oncogene in RCC.
In the present study, qPCR was used to detect the
relative miR-451a expression levels in 50 paired RCC and adjacent
normal tissues. The results were consistent with the previous miRNA
microarray chip analysis, which demonstrated that miR-451a
expression was significantly upregulated in RCC (15). Furthermore, the functions of
miR-451a on cell migration, proliferation and apoptosis were
analyzed by transfecting a miR-451a inhibitor into 786-O and ACHN
RCC cell lines. The results demonstrated that cells transfected
with a miR-451a inhibitor had reduced cell migration and
proliferation, and increased apoptosis, as compared with the cells
transfected with a negative control. To the best of our knowledge,
the results of the present study provide a novel insight into the
roles and possible mechanisms of miR-451a in the occurrence and
development of RCC.
Observed phenotypical changes may be mediated by
miRNA-regulated genes. Previous research has determined that miRNAs
post-transcriptionally regulate the expression of >30% of
protein coding genes by translational repression. miRNAs have also
been shown to regulate the expression of numerous putative target
genes, by binding to a complementary sequence found predominantly
in the UTR, however the binding sequences are not always completely
complementary, especially in mammals (6,29).
miRNA-associated post-transcriptional regulation, within the
context of tumor development, has been reported for the regulation
of genes that have an impact on cell differentiation, apoptosis and
neoplastic transformation (30,31).
The results of the present study may seem contradictory to other
research, as miR-451a has previously been characterized as a tumor
suppressor in some cancers and an oncogene in others. This
contradiction may be explained by the ‘imperfect complementarity’
of the interactions between miRNAs and target genes (8). Further research should be conducted
to determine the roles and target genes of miR-451a in RCC.
In conclusion, the results of the present study
demonstrate that miR-451a may be significantly upregulated in human
RCC tissues and be involved in cell proliferation, migration and
apoptosis in RCC cell lines. In addition, the data suggests tha
miR-451a may be a promising therapeutic target for the treatment of
RCC. Further research is required to explore the roles and target
genes of miR-451a.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81101922), Medical
Scientific Research Foundation of Guangdong Province of China (nos.
A2012584 andA2013606) and the Science and Technology Development
Fund Project of Shenzhen (no. JCYJ20130402114702124).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leroy X, Zini L, Buob D, Ballereau C,
Villers A and Aubert S: Renal cell carcinoma with rhabdoid
features: an aggressive neoplasm with overexpression of p53. Arch
Pathol Lab Med. 131:102–106. 2007.PubMed/NCBI
|
|
3
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel C, Ahmed A and Ellsworth P: Renal
cell carcinoma: a reappraisal. Urol Nurs. 32:182–190.
2012.PubMed/NCBI
|
|
5
|
Wei C, Wu S, Li X, et al: High expression
of FER tyrosine kinase predicts poor prognosis in clear cell renal
cell carcinoma. Oncol Lett. 5:473–478. 2013.PubMed/NCBI
|
|
6
|
Yu XY, Zhang Z, Liu J, Zhan B and Kong CZ:
MicroRNA-141 is downregulated in human renal cell carcinoma and
regulates cell survival by targeting CDC25B. Onco Targets Ther.
6:349–354. 2013.PubMed/NCBI
|
|
7
|
Zhai Q, Zhou L, Zhao C, et al:
Identification of miR-508-3p and miR-509-3p that are associated
with cell invasion and migration and involved in the apoptosis of
renal cell carcinoma. Biochem Biophys Res Commun. 419:621–626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu Z, Ni L, Chen D, et al: Identification
of miR-7 as an oncogene in renal cell carcinoma. J Mol Histol.
44:669–677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang XW, Zhang LJ, Huang XH, et al:
miR-145 suppresses cell invasion in hepatocellular carcinoma cells:
miR-145 targets ADAM17. Hepatol Res. 4:551–559. 2014. View Article : Google Scholar
|
|
10
|
Xi Y: MicroRNA: A New Player for Cancer
Chemoprevention. J Integr Oncol. 2:2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xi JJ: MicroRNAs in Cancer. Cancer Treat
Res. 158:119–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bian HB, Pan X, Yang JS, Wang ZX and De W:
Upregulation of microRNA-451 increases cisplatin sensitivity of
non-small cell lung cancer cell line (A549). J Exp Clin Cancer Res.
30:202011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bergamaschi A and Katzenellenbogen BS:
Tamoxifen downregulation of miR-451 increases 14-3-3ζ and promotes
breast cancer cell survival and endocrine resistance. Oncogene.
31:39–47. 2012. View Article : Google Scholar
|
|
14
|
Rani SB, Rathod SS, Karthik S, Kaur N,
Muzumdar D and Shiras AS: MiR-145 functions as a tumor-suppressive
RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro
Oncol. 15:1302–1316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yi Z, Fu Y, Zhao S, Zhang X and Ma C:
Differential expression of miRNA patterns in renal cell carcinoma
and nontumorous tissues. J Cancer Res Clin Oncol. 136:855–862.
2010. View Article : Google Scholar
|
|
16
|
Yoshino H, Enokida H, Itesako T, et al:
Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in
renal cell carcinoma. Cancer Sci. 104:1567–1574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou L, Chen J, Li Z, et al: Integrated
profiling of microRNAs and mRNAs: microRNAs located on Xq27.3
associate with clear cell renal cell carcinoma. PloS One.
5:e152242010. View Article : Google Scholar
|
|
18
|
Zhang J, Yang Y, Yang T, et al:
microRNA-22, downregulated in hepatocellular carcinoma and
correlated with prognosis, suppresses cell proliferation and
tumourigenicity. Br J Cancer. 103:1215–1220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kefas B, Godlewski J, Comeau L, et al:
microRNA-7 inhibits the epidermal growth factor receptor and the
Akt pathway and is down-regulated in glioblastoma. Cancer Res.
68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun H, Li QW, Lv XY, et al: MicroRNA-17
post-transcriptionally regulates polycystic kidney disease-2 gene
and promotes cell proliferation. Mol Biol Rep. 37:2951–2958. 2010.
View Article : Google Scholar
|
|
21
|
Yamamura S, Saini S, Majid S, et al:
MicroRNA-34a suppresses malignant transformation by targeting c-Myc
transcriptional complexes in human renal cell carcinoma.
Carcinogenesis. 33:294–300. 2012. View Article : Google Scholar :
|
|
22
|
Redova M, Poprach A, Besse A, et al:
MiR-210 expression in tumor tissue and in vitro effects of its
silencing in renal cell carcinoma. Tumour Biol. 34:481–491. 2013.
View Article : Google Scholar
|
|
23
|
Zhang H, Guo Y, Shang C, Song Y and Wu B:
miR-21 downregulated TCF21 to inhibit KISS1 in renal cancer.
Urology. 80:1298–1302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakada C, Matsuura K, Tsukamoto Y, et al:
Genome-wide microRNA expression profiling in renal cell carcinoma:
significant down-regulation of miR-141 and miR-200c. J Pathol.
216:418–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boguslawska J, Wojcicka A,
Piekielko-Witkowska A, Master A and Nauman A: MiR-224 targets the
3′UTR of type 1 5′-iodothyronine deiodinase possibly contributing
to tissue hypothyroidism in renal cancer. PloS One. 6:e245412011.
View Article : Google Scholar
|
|
26
|
Wang R, Wang ZX, Yang JS, Pan X, De W and
Chen LB: MicroRNA-451 functions as a tumor suppressor in human
non-small cell lung cancer by targeting ras-related protein 14
(RAB14). Oncogene. 30:2644–2658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bandres E, Bitarte N, Arias F, et al:
microRNA-451 regulates macrophage migration inhibitory factor
production and proliferation of gastrointestinal cancer cells. Clin
Cancer Res. 15:2281–2290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Godlewski J, Nowicki MO, Bronisz A, et al:
MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to
metabolic stress in glioma cells. Mol Cell. 37:620–632. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim VN: MicroRNA biogenesis: coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hammond SM: RNAi, microRNAs, and human
disease. Cancer Chemother Pharmacol. 58(Suppl 1): s63–s68. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Drakaki A and Iliopoulos D: MicroRNA gene
networks in oncogenesis. Curr Genomics. 10:35–41. 2009. View Article : Google Scholar : PubMed/NCBI
|