Introduction
Myocardial infarction (MI) is a common
cardiovascular disease, and one of the leading causes of mortality,
worldwide (1). MI is caused by
myocardial deprivation of oxygen and nutrients, which results in
the induction of the inflammatory response and apoptosis of
cardiomyocytes (2). Various
factors are involved in the process, including poly (ADP-ribose)
polymerase 1 (PARP1).
PARP1 is a nuclear enzyme, which functions as a DNA
damage sensor, and can be activated by DNA strand breaks (3). Activation of PARP1 leads to the
synthesis of poly(ADP-ribose) (PAR), from nicotinamide adenine
dinucleotide (NAD) and ATP, which is essential for DNA repair
(4). However, excessive activation
of PARP1 may result in the depletion of NAD and ATP, which leads to
cellular dysfunction and eventual cell death (5). Furthermore, PARP1 is required for
specific NF-κB-dependent gene activation, and acts as a
transcriptional co-activator of NF-κB (6). It has been shown to regulate various
key inflammatory cytokines, including monocyte chemotactic
protein-1, inducible nitric oxide synthase (iNOS), and adhesion
molecules, all of which are known to be regulated by NF-κB(7–9).
Excessive activation of PARP1 has been shown to be associated with
the pathogenesis of numerous diseases, including energetic failure
and vascular collapse in shock, diabetes, cerebral ischemia,
endothelial dysfunction in hypertension, atherosclerosis, and heart
failure (10–12). Martinet et al (13) previously provided evidence of
elevated levels of PARP1 in human atherosclerotic plaques. In
addition, PARP1 inhibition has been demonstrated to provide
protection against endothelial dysfunction in shock, hypertension,
and heart failure (14).
iNOS is a critical member among the inflammatory
cytokines regulated by PARP1 through the NF-κB pathway. Induction
of iNOS results in excessive production of nitric oxide (NO), which
reacts with superoxide anions to form peroxynitrite. The production
of peroxynitrite results in tyrosine nitration, DNA damage, and
activation of PARP1, which leads to changes in inflammatory
responses, and promotion of cell death by apoptosis and necrosis
(11,15). Numerous studies have demonstrated
that neutralization of peroxynitrite is an effective therapeutic
against cardiovascular, inflammatory, and neurodegenerative
diseases, by providing protection against cell death and
downregulating inflammatory responses (15).
In the present study, it was hypothesized that the
oxidative DNA damage, that results from the generation of reactive
species during the onset of MI, may cause excessive activation of
PARP1. Excessive PARP1 may result in an increased expression of
iNOS, and an imbalance of cell survival mechanisms that contribute
to the death of cardiomyocytes and aggravation of cardiac
functions. It may be hypothesized that pharmacological inhibition
of PARP1 or iNOS may protect cardiomyocytes from death, and improve
cardiac function. A rat model of MI was used to investigate the
potential role of PARP1 and iNOS in the process of MI, and to
examine the protective effects of their inhibition.
Materials and methods
Animals and surgery
A total of 40 male Wistar rats (Animal Experiment
Center of Shandong University, Jinan, China), 4 months old, were
housed and bred in a pathogen-free animal care facility at the Key
Laboratory of Cardiovascular Remodeling and Function Research
(Jinan, China). All of the rats were allowed full access to
standard mouse chow and water. All experiments were performed in
compliance with the Guide for the Care and Use of Laboratory
Animals, published by the US National Institutes of Health (NIH
Publication No. 85-23, revised 1985; NIH, Bethesda, MA, USA) and
Shandong University (Jinan, China).
The rats were anesthetized for a sham operation with
sodium pentobarbital (50 mg/kg). In order to induce an MI, the left
side of the chest was opened and the left anterior descending
coronary artery was occluded by ligation, using a 6-0 polypropylene
monofilament suture at the left atrial apex, as described by
previous methods (16).
Immediately following coronary ligation, all of the rats received a
single abdominal injection of either dimethylsulfoxide (DMSO; 100
μl; n=7),
3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone (DPQ;
10 mg/kg; n=8) (14), or
N-(1-naphthyl)ethylenediamine dihydrochloride (1400W; 10 mg/kg;
n=8) (17). Both DPQ and 1400W
were dissolved in 100 μl DMSO.
Measurement of cardiac function
The measurement was performed as previously
described (18). At three days,
and at two and four weeks following MI, the left ventricular (LV)
dimension and function were assessed by 2-D transthoracic
echocardiography on the isoflurane-anesthetized rats. A 12.5 mHz
linear-array probe was used, which has been specifically designed
for rat cardiac ultrasonic studies, with an HP Sonos 7500 Imaging
System (Philips Medical Systems, Andover, MA, USA). LV end-systolic
dimension (ESD) and end-diastolic dimension (EDD) were measured
from the short-axis view of the LV at the papillary muscle level.
Fractional shortening (FS) was used as a sensitive marker of
systolic function, and was calculated using the following equation:
FS (%) = ((EDD-ESD)/EDD) × 100. All of the measurements were
averaged on three consecutive cardiac cycles, and analyzed by two
independent researchers. The mice were humanely euthanized under
anesthesia.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL)
Four weeks following MI, the rats were sacrificed
and the hearts were harvested, fixed in 4% paraformaldehyde,
embedded in paraffin, and cut into 5 μm sections. A TUNEL assay was
used to evaluate apoptotic activity. A commercially available
apoptosis detection kit (Roche Diagnostics GmbH, Mannheim, Germany)
was used. The terminal deoxynucleotidyl transferase (TdT) reaction
was carried out for 1 h at 37°C, and then 3,3′-diaminobenzidine
(DAB) chromogen was applied to the samples. Hematoxylin was used as
a counterstain. Myocardial apoptosis was assessed in the area at
risk (AAR), as described previously (16). Briefly, in each section, the number
of cardiomyocytes and the number of TUNEL-positive cardiomyocyte
nuclei, were analyzed under a bright field microscope (Olympus
Corporation, Tokyo, Japan). Image-Pro Plus v6.0 (Media Cybernetics,
Inc., Rockville, MD, USA) was used to quantify the apoptotic
activity, by randomly counting 10 fields of the section, at ×400
magnification.
Caspase-3 activity assay
Caspase-3 activity was measured using a commercial
kit, according to the manufacturer’s instructions (Beyotime
Institute of Biotechnology, Haimen, China). In the presence of
caspase-3, acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) is
hydrolysed to the yellow product, p-nitroaniline(pNA). The
concentration of pNA was measured at 405 nm, using a
spectrophotometer (Shanghai Optical Instrument Factory, Shanghai,
China).
Western blot analysis
The proteins were extracted from the frozen cardiac
tissue of each group using radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology). The protein samples
were quantified with a BCA Protein Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). Equal amounts of protein (50 μg)
were loaded onto 10% SDS-PAGE gels and separated by
electrophoresis. The blots were then transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were blocked at room temperature with 5% nonfat milk, in
Tris-buffered saline containing 0.1% Tween® 20 (TBST),
and then incubated at 4°C overnight with an appropriate primary
antibody: Rabbit anti-β-actin (1:2,000 dilution; Santa Cruz
Biotechnology, Dallas, TX, USA), rabbit anti-cleaved caspase-3
(1:1,000 dilution; Cell Signaling Technology, Danvers, MA, USA),
rabbit anti-cleaved PARP (1:500 dilution; Abcam, Cambridge, MA,
USA), or rabbit anti-iNOS (1:200 dilution; Santa Cruz
Biotechnology). The membranes were then washed with TBST and
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (1:5,000 dilution; Jingmei Biotech
Co., Ltd., Shenzhen, China) for 2 h at room temperature. Following
a further three washes with TBST, the blots were visualized using
Enhanced Chemiluminescence Plus reagents (EMD Millipore, Billerica,
MA, USA).
Immunofluorescence
The heart tissues were fixed in 4% polyformaldehyde
overnight and stored at −4°C. Serial cryosections (4–5 μm) embedded
in OCT compound were made by using a paraffin slicing machine. The
serial cryosections (6 μm) of the infarct-related area (IRA) were
permeabilized in phosphate-buffered saline (PBS), containing 0.5%
Triton X-100. After being blocked with normal goat serum, the
cryosections were incubated with the following primary antibodies,
overnight at 4°C: Rabbit anti-PAR antibody (1:200 dilution; Santa
Cruz Biotechnology), or rabbit anti-3NT (1:500 dilution; Abcam).
The cryosections were then incubated with a HRP conjugated
secondary antibody (1:5,000 dilution; Jingmei Biotech Co., Ltd.,
Shenzhen, China). As P3L2.. A small amount of Prolong Gold antifade
reagent, with 4′, 6-diamidino-2-phenylindole (DAPI; Invitrogen Life
Technologies, Carlsbad, CA, USA) was used to seal the coverslips.
The immunofluorescent images were captured using a P3L21 laser
scanning confocal microscope (LSM710; Carl Zeiss AG, Oberchoken,
Germany) and analyzed using Image Pro Plus 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA).
Superoxide anion
(O2−) production assay
Following treatment, the IRA of the cardiac tissues
were incubated with a fluorescent probe of
O2−, dihydroethidium (DHE), for 30 min at
37°C. The IRA samples were washed with PBS, and the fluorescent
signals were measured at 535 nm, using a laser scanning confocal
microscope. The fluorescence intensity was measured for ≥10 fields
per sample. Three independent experiments were performed.
Statistical analyses
All of the data are expressed as the means ±
standard deviation. SPSS for Windows version 16.0 (SPSS Inc,
Chicago, IL, USA) was used for statistical analyses. Intergroup
comparisons were performed using a two tailed student’s t test or a
one-way analysis of variance, followed by a test of least
significant difference (when equal variances were assumed) or a
Dunnett’s T3 (when equal variances were not assumed). A P<0.05
was considered to indicate a statistically significant
difference.
Results
Three rats in the MI group and two rats from both
the DPQ and 1400W groups succumbed to natural causes. Perioperative
mortality (<24 hours following coronary ligation) ranged between
20–30%, and was not affected by the surgery or the dose of the
drugs. There was no mortality following the first 24 hours, up to
the end of the experiment (4 weeks).
Inhibition of PARP1 and iNOS activity
improves cardiac function following MI
During the four weeks following coronary ligation,
the cardiac function of the rats was observed by serial
echocardiography, and the FS, LV EDD and ESD were determined
(Fig. 1A–C). The control group
maintained consistent cardiac function, during the four weeks. In
the MI group, the EDD and ESD increased by 19.30 and 16.65%,
respectively, four weeks after surgery, and the FS decreased by
26.87%, as compared with the control group (P<0.05). The echo
indices of FS and LV enlargement in the DPQ and 1400W groups,
following MI, were lower as compared with the control group;
however, the reduction was less severe, as compared with the MI
group (P<0.05; Fig. 1A–C).
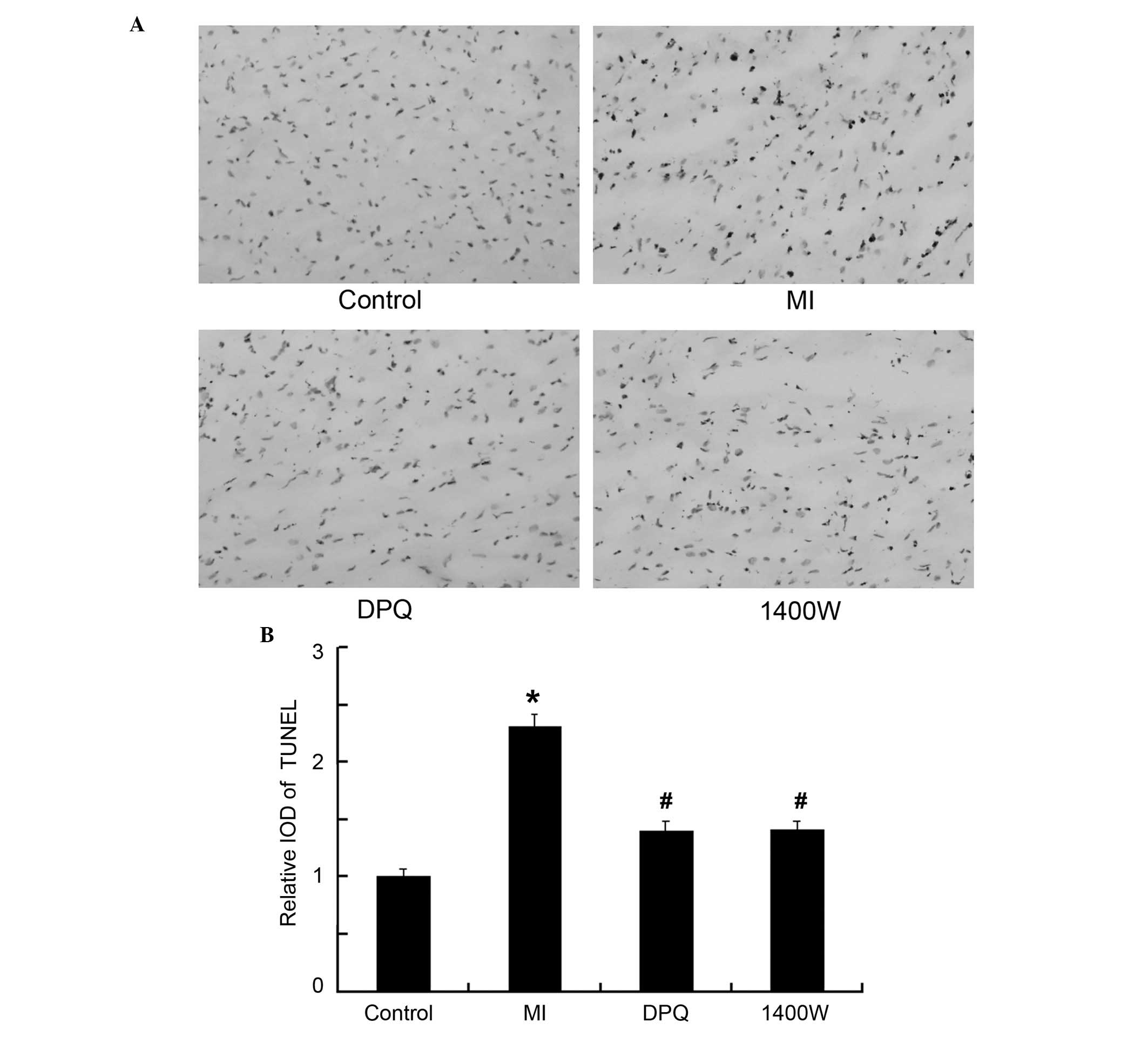
Inhibition of PARP1 and iNOS activity
reduces MI-induced apoptosis
The rate of apoptosis was measured histologically by
TUNEL assay (Fig. 2A). The average
integral optical density (IOD) for TUNEL-positive cardiomyocytes
within the myocardial AAR was significantly increased in the MI
group, as compared with the control group (4.05±0.41 vs 1.75±0.12,
P<0.05; Fig. 2B). The rate of
apoptosis was significantly reduced, by 39.71 and by 39.00% in the
DPQ and 1400W treatment groups, respectively, as compared with the
MI group (P<0.05, Fig. 2B).
There were no significant differences between the rate of apoptosis
in the DPQ and 1400W groups.
Inhibition of PARP1 and iNOS attenuates
apoptosis in the AAR by regulating the expression levels of cleaved
caspase-3 and PARP1
The main functions of caspase-3 and PARP1 are in
apoptosis. The protein expression levels of caspase-3 and PARP1
were examined (Fig. 3). The
activity of caspase-3 was 2.60-fold higher in the MI group, as
compared with the control group (P<0.05, Fig. 3A). Treatment with DPQ or 1400W
significantly decreased caspase-3 activity, as compared with the MI
group (P<0.05, Fig. 3A). There
was no difference in caspase-3 activity between the two treatment
groups. Similar results were determined by western blot analysis
(Fig. 3B), and the enhancement of
cleaved caspase-3 and PARP1 induced by MI was attenuated by
treatment with DPQ or 1400W (P<0.05; Fig. 3C). These results were concordant
with the rate of apoptosis detected in each of the groups.
DPQ and 1400W effectively inhibit
MI-induced PARP1 and iNOS activity
In order to verify the respective inhibition
efficiency of DPQ and 1400W on PARP1 and iNOS, the expression
levels of PAR (active PARP) and iNOS were detected in the
myocardium of the various groups (Fig.
4). The activity of PAR was detected by immunofluorescence
(Fig. 4A). The average IOD was
enhanced 3.20-fold in the MI group, as compared with the control
(P<0.05, Fig. 4B); however,
treatment with DPQ or 1400W reduced MI-induced PAR activity by
39.38 and 38.75%, respectively (P<0.05; Fig. 4B). Simultaneously, the expression
levels of iNOS were enhanced 4.14-fold following MI, as compared
with the control group, as determined by western blotting
(P<0.05, Fig. 4C and D). The
inhibition efficiency of DPQ and 1400W was 51.14 and 51.84%
respectively, as compared with the MI group (P<0.05, Fig. 4C and D). Notably, DPQ and 1400W
could interact with each other in the inhibition of PARP1 and
iNOS.
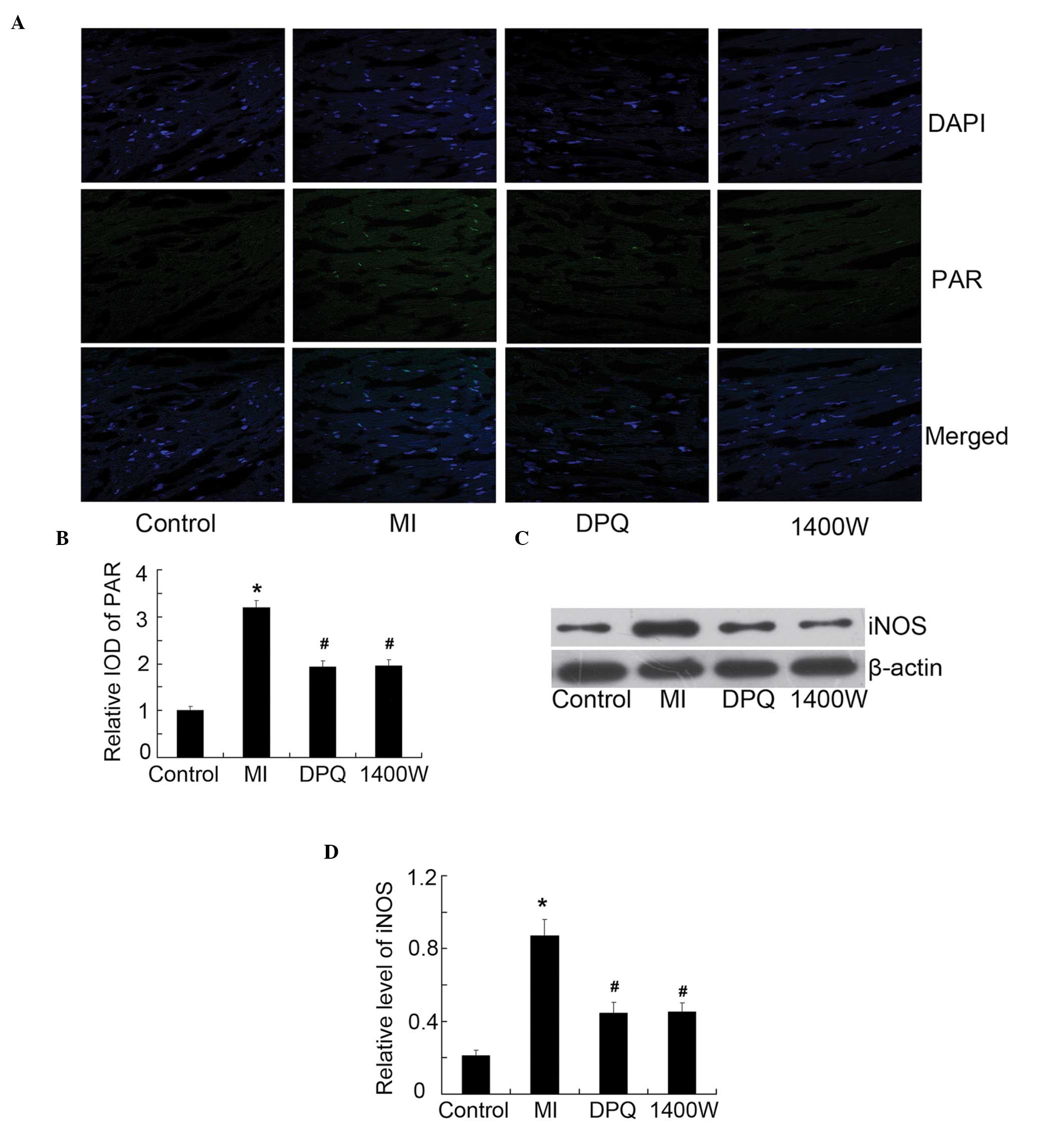 | Figure 4The protein expression levels of poly
(ADP-ribose) (PAR) (active PAR polymerase), and inducible nitric
oxide synthase (iNOS), in the myocardium of the various groups. (A)
Representative fluorescent images of the myocardium, acquired by
laser scanning confocal microscopy (magnification, ×400); PAR
staining is green, and 4′, 6-diamidino-2-phenylindole (DAPI)
staining for the cell nuclei is blue. (B) Quantitative analysis of
PAR, expressed as a fold increase over the control group. (C) The
protein expression levels of iNOS were examined by western blot
analysis, with the indicated antibody. (D) Quantitative analysis of
iNOS, relative to β-actin. Control, n=10; MI, n=7; DPQ, n=8; 1400W,
n=8.*P<0.05 vs the control group; #P<0.05 vs the MI group.
DPQ, 3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)- isoquinolinone;
1400W, N-(1-naphthyl)ethylenediamine dihydrochloride; IOD, integral
optical density. MI, myocardial infarction. |
Inhibition of PARP1 and iNOS repressed
O2− and nitrotyrosine (3-NT), induced by
MI
To further elucidate the signaling pathways involved
in MI-induced apoptosis, the expression of
O2− and the production of NO (3-NT) was
determined. The production of intracellular
O2− was significantly increased in the MI
group, as compared with the control group, as determined by DHE
detection (P<0.05, Fig. 5A and
B). Similarly, the expression of 3-NT was significantly higher
in the MI group, as compared with the control group, as determined
by immunofluorescence (P<0.05, Figure 6A, B). Treatment with DPQ or 1400W
effectively reversed the high O2− levels
induced by MI, by 53.07 and 51.43% (<0.05, Fig. 5A and B), and reduced 3-NT
expression by 44.87 and 41.38% (P<0.05, Fig. 6A and B), respectively.
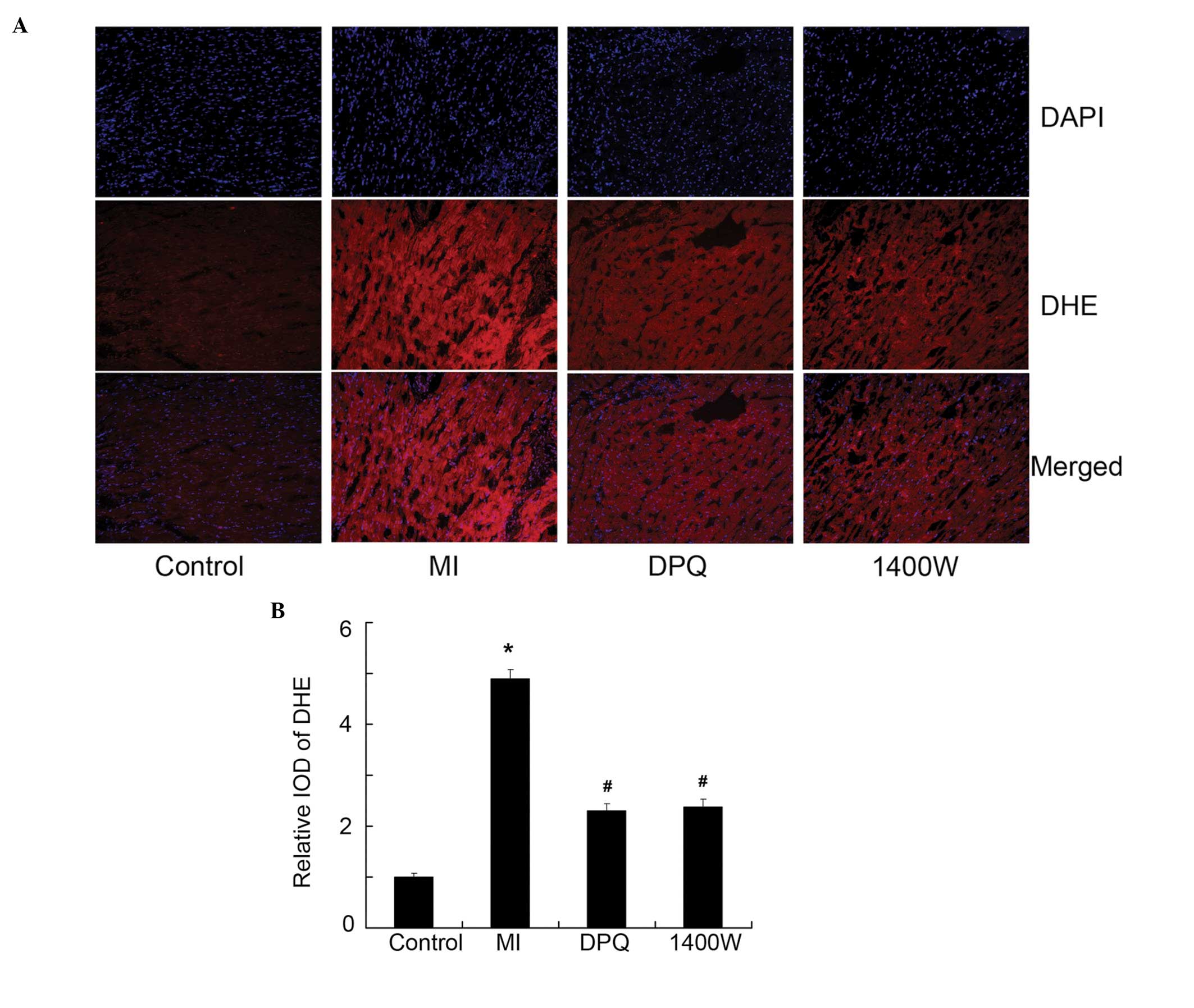 | Figure 5The expression of
O2− detected by dihydroethidium (DHE). (A)
The representative images of O2− detected by
DHE (red) in the myocardium, 4′, 6-diamidino-2-phenylindole (DAPI)
staining for the cell nuclei is blue. (B) Quantitative analysis of
DHE, expressed as a fold increase over the control group. Control,
n=10; MI, n=7; DPQ, n=8; 1400W, n=8. *P<0.05 vs the
control group; #P<0.05 vs the MI group. DPQ,
3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)- isoquinolinone;
1400W, N-(1-naphthyl)ethylenediamine dihydrochloride; IOD, integral
optical density. MI, myocardial infarction. |
 | Figure 6The relative levels of nitrotyrosine
(3-NT) in the myocardium. (A) Fluorescent micrographs showing the
relative level of 3-NT (red) in the myocardium, 4′,
6-diamidino-2-phenylindole (DAPI)staining for the cell nuclei is
blue. (B) Quantitative analysis of 3-NT, expressed as a fold
increase over the control group. Control, n=10; MI, n=7; DPQ, n=8;
1400W, n=8. *P<0.05 vs the control group;
#P<0.05 vs the MI group. DPQ,
3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)- isoquinolinone;
1400W, N-(1-naphthyl)ethylenediamine dihydrochloride; IOD, integral
optical density. MI, myocardial infarction. |
Discussion
The present study tested the hypothesis that PARP1
and iNOS contribute to the deleterious effects on cardiac function,
exerted by cardiomyocyte apoptosis, following MI. PARP1 and iNOS
were shown to be upregulated, alongside an increased rate of
caspase-3-dependent apoptosis, and deterioration of cardiac
function in rats, following an MI. The PARP inhibitor DPQ and the
iNOS inhibitor 1400W attenuated these effects. Furthermore, the
reduction in the rate of apoptosis, by DPQ and 1400W, was
associated with the reduced production of O2−
and 3-NT in the ischemic myocardium.
Previous studies have evaluated cardiac function
following induction of MI by serial echocardiography (2,19),
and demonstrated that it is associated with apoptosis and
inflammation in the AAR (20). In
concordance with previous reports, the results of the present study
showed that apoptosis following MI and production of iNOS were
markedly elevated, with deterioration of cardiac function.
Considering the involvement of PARP1 in various cardiovascular and
inflammatory diseases, these results emphasize its role in the
pathogenesis of MI.
Previous evidence has suggested that PARP may be
excessively activated by reactive oxygen and nitrogen species in
cardiomyocytes and endothelial cells, during myocardial
ischemia/reperfusion injury, various forms of heart failure or
cardiomyopathies, circulatory shock, cardiovascular aging, diabetic
complications, myocardial hypertrophy, atherosclerosis, and
vascular remodeling following injury (21). Furthermore, severe DNA damage may
lead to initiation of the second apoptotic pathway, in which
caspases inactivate PARP1, by cleaving it into two fragments,
therefore preventing it from responding to DNA strand breaks
(22,23). Reactive oxygen species, which have
been implicated in cardiac pathophysiology, can trigger apoptosis
of myocytes by upregulating pro-apoptotic proteins, such as
Bcl-2-associated X protein and caspases, and the
mitochondria-dependent pathway (24). The results of the present study
indicated that inhibition of PARP1 with DPQ, could reduce
O2− and ONOO− in rats following
MI, and reduce caspase-3-dependent apoptosis. These results were
concordant with previous findings that the PARP1 inhibitor PJ34
limited myocardial damage, due to post-ischemic reperfusion, by
decreasing NAD+ and ATP, and the caspase-dependent
pathway (25).
Another role of PARP1 is its regulation of
inflammation at the transcriptional level (e.g., iNOS,
intracellular adhesion molecule-1, cyclooxygenase-2). The absence
of functional PARP-1 has been shown to decrease the expression
levels of proinflammatory mediators, including cytokines,
chemokines, adhesion molecules and enzymes, such as iNOS (26). Previous results have suggested that
iNOS expression contributes to PARP activation in cerebral
ischemia, and a previously unrecognized deleterious interaction
between iNOS and PARP has been identified (27). The present study demonstrated that
inhibition of PARP1 reduced the expression of iNOS, meanwhile the
inhibitor of iNOS, 1400W, attenuated the expression of PARP1 and
resulted in the reduction of apoptosis. The interaction between
PARP1 and iNOS requires further study.
iNOS is one of the most important NO donors, and is
associated with numerous important pathophysiological processes in
various conditions, including MI and heart failure (28,29).
Cell apoptosis and NT formation have previously been attenuated by
selective iNOS inhibitors, or in iNOS knockout mice (30). Peroxynitrite is a reactive oxidant
which is formed by reaction of NO with the superoxide anion
(11). ONOO− has been
shown to function as an important trigger of myocardial necrosis
and apoptosis in various cardiac pathologies, by inducing oxidative
DNA damage, which may lead to the activation of the DNA repair
enzyme PARP. PARP subsequently consumes cellular NAD+,
ultimately leading to ATP depletion and necrotic cell death
(21,31,32).
The results of the present study showed that the iNOS inhibitor,
1400W, could attenuate NT formation and apoptosis, and could reduce
the activity of PARP1.
In conclusion, the present study provided novel
evidence that pharmacological inhibition of PARP1 with DPQ and iNOS
with 1400W, improved cardiac function in rats, following MI, by
attenuating cell apoptosis and inflammation. The predicted pathway
of these effects is that DPQ may downregulate the inflammatory
factor iNOS and its effects on O2− and
ONOO− in MI rats, and reduce the rate of
caspase-3-dependent apoptosis. In addition, inhibition of iNOS
decreased the levels of reactive oxygen and nitrogen species,
resulting in the reduction of DNA single strand breaks and PARP1
activiation, leading to similar protection in ischemic myocardium.
The effects of PARP1 and iNOS inhibitors may be exploited for the
experimental therapy of disease.
Acknowledgements
The present study was supported by the Seed Fund of
The Second Hospital of Shandong University (no. S2013010010).
References
|
1
|
Ozaki K, Sato H, Inoue K, et al: SNPs in
BRAP associated with risk of myocardial infarction in Asian
populations. Nat Genet. 41:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chacko SM, Khan M, Kuppusamy ML, et al:
Myocardial oxygenation and functional recovery in infarct rat
hearts transplanted with mesenchymal stem cells. Am J Physiol Heart
Circ Physiol. 296:H1263–H1273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen-Armon M: PARP-1 activation in the
ERK signaling pathway. Trends Pharmacol Sci. 28:556–560. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hassa PO, Haenni SS, Elser M and Hottiger
MO: Nuclear ADP-ribosylation reactions in mammalian cells: where
are we today and where are we going? Microbiol Mol Biol Rev.
70:789–829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szabó C, Zingarelli B, O’Connor M and
Salzman AL: DNA strand breakage, activation of poly (ADP-ribose)
synthetase, and cellular energy depletion are involved in the
cytotoxicity of macrophages and smooth muscle cells exposed to
peroxynitrite. Proc Natl Acad Sci USA. 93:1753–1758. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ullrich O, Diestel A, Eyüpoglu IY and
Nitsch R: Regulation of microglial expression of integrins by
poly(ADP-ribose) polymerase-1. Nat Cell Biol. 3:1035–1042. 2001.
View Article : Google Scholar
|
|
7
|
Sharp C, Warren A, Oshima T, Williams L,
Li JH and Alexander JS: Poly ADP ribose-polymerase inhibitors
prevent the upregulation of ICAM-1 and E-selectin in response to
Th1 cytokine stimulation. Inflammation. 25:157–163. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hassa PO and Hottiger MO: The functional
role of poly (ADP-ribose)polymerase 1 as novel coactivator of
NF-kappaB in inflammatory disorders. Cell Mol Life Sci.
59:1534–1553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carrillo A, Monreal Y, Ramírez P, et al:
Transcription regulation of TNF-alpha-early response genes by
poly(ADP-ribose) polymerase-1 in murine heart endothelial cells.
Nucleic Acids Res. 32:757–766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szabó C, Zanchi A, Komjáti K, et al:
Poly(ADP-Ribose) polymerase is activated in subjects at risk of
developing type 2 diabetes and is associated with impaired vascular
reactivity. Circulation. 106:2680–2686. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pacher P and Szabó C: Role of
poly(ADP-ribose) polymerase-1 activation in the pathogenesis of
diabetic complications: endothelial dysfunction, as a common
underlying theme. Antioxid Redox Signal. 7:1568–1580. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinet W, Knaapen MW, De Meyer GR,
Herman AG and Kockx MM: Elevated levels of oxidative DNA damage and
DNA repair enzymes in human atherosclerotic plaques. Circulation.
106:927–932. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szabó C: Pharmacological inhibition of
poly(ADP-ribose) polymerase in cardiovascular disorders: future
directions. Curr Vasc Pharmacol. 3:301–303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szabó C, Ischiropoulos H and Radi R:
Peroxynitrite: biochemistry, pathophysiology and development of
therapeutics. Nat Rev Drug Discov. 6:662–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahmet I, Lakatta EG and Talan MI:
Pharmacological stimulation of beta2-adrenergic receptors (beta2AR)
enhances therapeutic effectiveness of beta1AR blockade in rodent
dilated ischemic cardiomyopathy. Heart Fail Rev. 10:289–296. 2005.
View Article : Google Scholar
|
|
17
|
Matsuhisa S, Otani H, Okazaki T, et al:
Angiotensin II type 1 receptor blocker preserves tolerance to
ischemia-reperfusion injury in Dahl salt-sensitive rat heart. Am J
Physiol Heart Circ Physiol. 294:H2473–H2479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Zhang G, Hou Y, et al:
Transplantation of microencapsulated Schwann cells and mesenchymal
stem cells augment angiogenesis and improve heart function. Mol
Cell Biochem. 366:139–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khan M, Meduru S, Mohan IK, et al:
Hyperbaric oxygenation enhances transplanted cell graft and
functional recovery in the infarct heart. J Mol Cell Cardiol.
47:275–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmet I, Tae HJ, Juhaszova M, et al: A
small nonerythropoietic helix B surface peptide based upon
erythropoietin structure is cardioprotective against ischemic
myocardial damage. Mol Med. 17:194–200. 2011. View Article : Google Scholar :
|
|
21
|
Pacher P and Szabó C: Role of
poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases:
the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev.
25:235–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levrand S, Vannay-Bouchiche C, Pesse B, et
al: Peroxynitrite is a major trigger of cardiomyocyte apoptosis in
vitro and in vivo. Free Radic Biol Med. 41:886–895. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Virag L and Szabó C: The therapeutic
potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev.
54:375–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar D and Jugdutt BI: Apoptosis and
oxidants in the heart. J Lab Clin Med. 142:288–297. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fiorillo C, Ponziani V, Giannini L, et al:
Protective effects of the PARP-1 inhibitor PJ34 in
hypoxic-reoxygenated cardiomyoblasts. Cell Mol Life Sci.
63:3061–3071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szabó C: Poly(ADP-ribose) polymerase
activation by reactive nitrogen species - relevance for the
pathogenesis of inflammation. Nitric Oxide. 14:169–179. 2006.
View Article : Google Scholar
|
|
27
|
Sarkar D and Fisher PB: Molecular
mechanisms of aging-associated inflammation. Cancer Lett.
236:13–23. 2006. View Article : Google Scholar
|
|
28
|
Gilson WD, Epstein FH, Yang Z, et al:
Borderzone contractile dysfunction is transiently attenuated and
left ventricular structural remodeling is markedly reduced
following reperfused myocardial infarction in inducible nitric
oxide synthase knockout mice. J Am Coll Cardiol. 50:1799–807. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu YH, Carretero OA, Cingolani OH, et al:
Role of inducible nitric oxide synthase in cardiac function and
remodeling in mice with heart failure due to myocardial infarction.
Am J Physiol Heart Circ Physiol. 289:H2616–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mukhopadhyay P, Rajesh M, Bátkai S, et al:
Role of superoxide, nitric oxide, and peroxynitrite in
doxorubicin-induced cell death in vivo and in vitro. Am J Physiol
Heart Circ Physiol. 296:H1466–H1483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Wijk SJ and Hageman GJ:
Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell
death after ischemia/reperfusion. Free Radic Biol Med. 39:81–90.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Zhang X, Park TS and Gidday JM:
Cerebral endothelial cell apoptosis after ischemia-reperfusion:
role of PARP activation and AIF translocation. J Cereb Blood Flow
Metab. 25:868–877. 2005. View Article : Google Scholar : PubMed/NCBI
|