Introduction
Heat stress is a severe environmental condition that
affects animals. Animals respond to environmental stress with a
series of reactions known as the ‘fight-or-flight’
response (1). Therefore, heat
stress may result in economic losses in animal husbandry due to a
decline in food consumption, growth rate, feeding efficiency and
survival of animals as the environmental temperature increases
(2–4). In addition, stressors, which can
cause general adaptation syndrome, were reported to be responsible
for eventual shock and sudden mortality of transported pigs and
poultry that were exposed to high temperatures (5). Previous studies have demonstrated
that chronic heat stress significantly altered physiological,
metabolic, biochemical and cellular responses in murine and poultry
models (6,7). However, animals have been shown to
exhibit protective measures against environmental challenges; these
include heat-shock proteins (Hsps), a set of proteins
synthesized in response to physical as well as chemical or
biological stresses, including heat exposure (8–10).
Hsps are ubiquitously expressed and highly conserved
in prokaryotes and eukaryotes (11). Hsps are divided into six families
of sequence-associated proteins on the basis of their
molecular size, structure and function as follows: Small Hsps,
Hsp40, Hsp60, Hsp70, Hsp90 and Hsp110 (12). Numerous Hsps were reported to be
effective in cell survival and adaptation; however, certain Hsps
exerted greater levels of cardiac protection than others (13), Hsp70 was suggested to have
important roles in protection against stress-induced cardiac
cell damage such as ischemia-reperfusion injury (14). In addition, Hsps were reported to
minimize the size of cardiac infarctions and improve contractility
of heart cells in humans and mice (15,16).
Hsp70 has been extensively studied and was revealed to be expressed
in the vertebrae, heart and brain, where it serves as a molecular
chaperone and has a significant role in protecting cells against
cellular stressors (17),
including heat (18,19), hypoxia (20), ultraviolet irradiation (21) and oxidative stress (22).
Previous studies have indicated that significant
increases of Hsp levels were the key step in the initiation of the
heat-shock response (23–25).
Hsp70 was also reported to be involved in the folding of nascent
and misfolded proteins under non-stressful conditions
(26). As a universal
cytoprotective protein, Hsp70 was suggested to enhance the
tolerance of cells to environmental changes or pathogenic
conditions, increase the survival rate of stressed cells as well as
have critical roles in cardiovascular disease, organism decay and
cellular aging (27). Furthermore,
one study demonstrated that Hsp70-overexpression accelerated ulcer
healing via the inhibition of apoptosis as well as the promotion of
proliferation and protein synthesis (28).
Stressful transports and increased environmental
temperature were confirmed to be responsible for the shock and
sudden mortality of pigs and broiler chickens, as a result of
sudden heart failure preceded by cardiovascular damage (29). However, due to the numerous
confounding environmental variables, investigating the mechanisms
by which stress induces cell damage and alters cellular metabolism
in vivo is challenging. The present study aimed to
investigate the correlation between Hsp70 levels and cellular
damage, using H9c2 cells subjected to heat stress in vitro
as a model system. In addition, the present study aimed to evaluate
the expression of Hsp70 in response to heat stress and examine its
potential protective role against hyperthermia-induced
cellular damage in vitro and in vivo.
Materials and methods
Heat-stress models of cell culture in
vitro and experimental rats in vivo
H9c2 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
11995-065; Gibco-BRL, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS; 16141079; Gibco-BRL), 100 U/ml
penicillin and 100 μg/ml streptomycin (Gibco-BRL) in a humidified
atmosphere of 5% CO2 and 95% air at 37°C. Cellular
viability of >95% was achieved and the cultured H9c2 cells were
divided into six groups, which were exposed to heat stress in a
water bath at 42°C for 20, 40, 60, 80 and 100 min. The control
group was maintained at normal environmental conditions.
All experiments were performed in accordance with
the guidelines of the Animal Ethics Committee of Jiangsu province
(Jiangsu, China) and were approved by the Institutional Animal Care
and Use Committee of Nanjing Agricultural University (Nanjing,
China). A total of 70 Sprague-Dawley (SD) rats, weighing
220±20 g, were purchased from Qinglongshan Farms (Nanjing, China).
The rats were housed in cages with access to food and water ad
libitum. Following three days of adaptation feeding at room
temperature (RT), the animals were placed in
controlled-climate chambers (RX8-500D, New Jiangnan
Co., Ltd., Ningbo, Zhejiang, China) and then exposed to 42°C for 0
(control), 20, 40, 60, 80 and 100 min. Each group consisted of ten
rats, with the exception of the 100 min group, which contained 20
rats. Mortality of the heat stressed rats in vivo was
>40% following 100 min of heat stress. All the experimental rats
were humanely sacrificed by decapitation at the end of the
heat-stress period.
Enzyme detection in vitro and in
vivo
Following exposure to heat stress, ~2 ml culture
supernatants and ~1.5 ml serum from the control and the
heat-stressed rats, respectively, were collected from each
group and the controls. The collected samples were then stored at
−80°C until further use. Enzymatic activities of aspartate
aminotransferase (AST; C010) and creatine kinase (CK; A032) were
measured using commercial kits (Nanjing Jiancheng Biochemical
Reagent Co., Ltd., Nanjing, China) according to the manufacturer’s
instructions and a microplate reader (Tecan Infinite M200PRO,
Grödig, Austria). Each sample was analyzed five times,
consecutively.
Cyto- and histopathological
observations
H9c2 cells were cultured on glass coverslips
(2–8×104 cells in 35-cm2 plates; Citotest
Labware Manufacturing Co., Ltd., Haimen, Jiangsu, China) coated
with poly-L-lysine (Sigma-Aldrich, St. Louis, MO,
USA). Myocardial H9c2 cells were cultured at 37±1°C were heat
stressed by incubating them in a water bath at 42±1°C for 0
(control), 20, 40, 60, 80 and 100 min. Following heat stress, cells
were fixed in 4% paraformaldehyde for 30 min at RT and washed with
phosophate-buffered saline (PBS) three times. Cells were
then stained with hematoxylin and eosin (H&E) and examined
using light microscopy (Axio Imager A2; Zeiss, Oberkochen,
Germany).
Heart samples were obtained from rats of each group
following heat stress and preserved in 10% formalin. Samples were
embedded in paraffin and then cut into 5-μm serial sections.
Sections were stained with H&E and the histopathological
changes among the groups were examined. Images were captured using
light microscopy.
Immunofluorescence staining
Following heat stress, myocardial H9c2 cells were
washed with PBS and fixed with 95 and 100% ethanol, for 15 min
each. Cells were washed with pre-cooled PBS for 15 min and
then permeabilized with 0.5% Triton™ X-100 (Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China) in PBS for 15
min. Non-specific protein binding of permeabilized cells was
blocked by incubation with 5% bovine serum albumin (BSA) for 30 min
at RT. Cells were then incubated with Hsp70-specific mouse
monoclonal antibodies (1:50; ADI-SPA-820-F;
Enzo Life Sciences, Inc., Farmingdale, NY, USA) diluted with 1% BSA
(Wuhan Boster Biological Technology, Ltd., Wuhan, Hubei, China) and
subsequently incubated with fluorescein isothiocyanate
(FITC)-conjugated antibody (BA1089; Wuhan Boster Biological
Technology, Ltd.) in 1% BSA (1:50). Fluorescence images were
obtained using light microscopy.
Serial sections of the heart tissue were
immunostained using the standard avidin-biotin complex (ABC)
immunoperoxidase detection system (AR1022; Wuhan Boster Biological
Technology, Ltd.). Sections were then deparaffinized in xylene,
hydrated with ethanol and rinsed with distilled water. Endogenous
peroxidase was blocked using 3% H2O2 for 10
min at RT. The slides were blocked by incubation with 5% BSA for
~30 min at 37°C. Sections were then incubated with a 1:50 dilution
of the Hsp70-specific primary antibodies for 2 h at RT. Sections
were then washed with PBS and incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse immunoglobulin G
(IgG; heavy and light chain) secondary antibodies for 1 h at RT.
Following treatment with two drops of diaminobenzidine (DAB)
substrate chromogen solution (AR1022; Wuhan Boster Biological
Technology, Ltd.,) for 10 min, the reaction was terminated by
adding ~500 ml water. The sections were then counterstained with
hematoxylin and fluorescence images were obtained using light
microscopy. The corresponding negative controls were prepared in an
identical manner omitting the antibody.
Western blot analysis
Heat-stressed H9c2 cells were washed three
times with PBS and cell protein was extracted using an M-PER
mammalian protein extraction reagent (78501; Thermo Scientific,
Waltham, MA, USA) according to the manufacturer’s instructions.
Heart tissue samples were homogenized in 1 ml protein extraction
reagent (Thermo Scientific) on ice using an Ultra-Turrax
homogenizer (623003; Fluko Equipment Shanghai Co., Ltd., Shanghai,
China). Samples were then washed with ice-cold physiological
saline and the resultant homogenates were centrifuged at 12,000 ×g
for 20 min at 4°C to remove cellular debris. The supernatant was
collected and stored at −20°C until further use for protein
quantification. The protein concentration was measured using a
micro-bicinchoninic acid assay kit (23235; Thermo
Scientific).
The H9c2 cell protein extracts (20 μg) and heart
tissue protein extracts (80 μg) were separated using 10%
SDS-PAGE and transferred onto polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were incubated overnight with Tris-buffered saline
containing 0.05% Tween-20 (TBST) at 4°C. The membranes were
then washed with TBST and incubated with antibodies against Hsp70
(ADI-SPA-820-F; Enzo Life Sciences, Inc.) and
GAPDH (KC-5G4; Kangchen Bio-tech Inc., Shanghai, China) in
blocking buffer for 2 h at 37°C. Following washing with TBST, the
membranes were further incubated with HRP-conjugated goat
anti-mouse IgG antibody (Wuhan Boster Biological Technology,
Ltd.) at RT for 1 h. Antibody-antigen complexes were
detected using western blotting luminal reagent (Bio-Rad
Laboratories, Inc.). The bands were then quantified using Quantity
One software version 4.6.2 (Bio-Rad Laboratories). GADPH was
used as a loading control.
Statistical analysis
Statistical analysis of the differences between the
experimental groups and the control group were performed using the
one-way analysis of variance followed by the
least-significant-difference multiple comparison test
using Statistical Package for Social Sciences (SPSS) version 20.0
for Windows (International Business Machines, Armonk, NY, USA).
Values are expressed as the mean ± standard deviation of at least
three independent experiments. P<0.05 was considered to indicate
a statistically significant difference between values.
Results
Cell damage increases the levels of
enzymes AST and CK in vitro and in vivo
Significant differences in AST and CK levels were
observed in heat stressed rat myocardial cells compared with those
of the control group (Table I).
Following initiation of heat stress, enzymatic activity was
significantly increased in vitro and in vivo at 100
min (P<0.05). The culture supernatant from in vitro
myocardial cells showed a significant increase in AST levels from
40 min onwards and CK levels from 60 min onwards. However, in
vivo levels of these enzymes were not significantly increased
until 80 min for AST and 100 min for CK.
 | Table IActivity levels of AST and CK in
vitro and in vivo. |
Table I
Activity levels of AST and CK in
vitro and in vivo.
| Heat stress
group/time (min) |
|---|
|
|
|---|
| Enzyme | 0 | 20 | 40 | 60 | 80 | 100 |
|---|
| In
vitro |
| AST (U/l) | 9.17±0.98 | 9.67±1.37 | 11.17±0.75* | 11.33±2.06* | 12.83±2.13** | 15.83±2.14** |
| CK (U/l) | 8.67±1.19 | 10.17±2.12 | 10.33±1.79 | 11.67±2.51* | 11.83±2.56* | 12.00±2.45* |
| In vivo |
| AST (U/l) | 260.23±74.71 | 266.3±53.38 | 273.2±56.49 | 296.6±61.97 | 345.8±71.94* | 406.1±97.12** |
| CK (U/l) | 5596.2±1680.7 | 5864.5±2099.8 | 6057.8±1989.2 | 6496.5±1935.2 | 7064.5±1599.7 |
7924.8±1762.9* |
Cyto- and histopathological changes in
vitro and in vivo
Figs. 1 and
2 demonstrate the effect of
different durations of heat stress on cytological and
histopathological changes of myocardial cells in vitro and
in vivo, respectively.
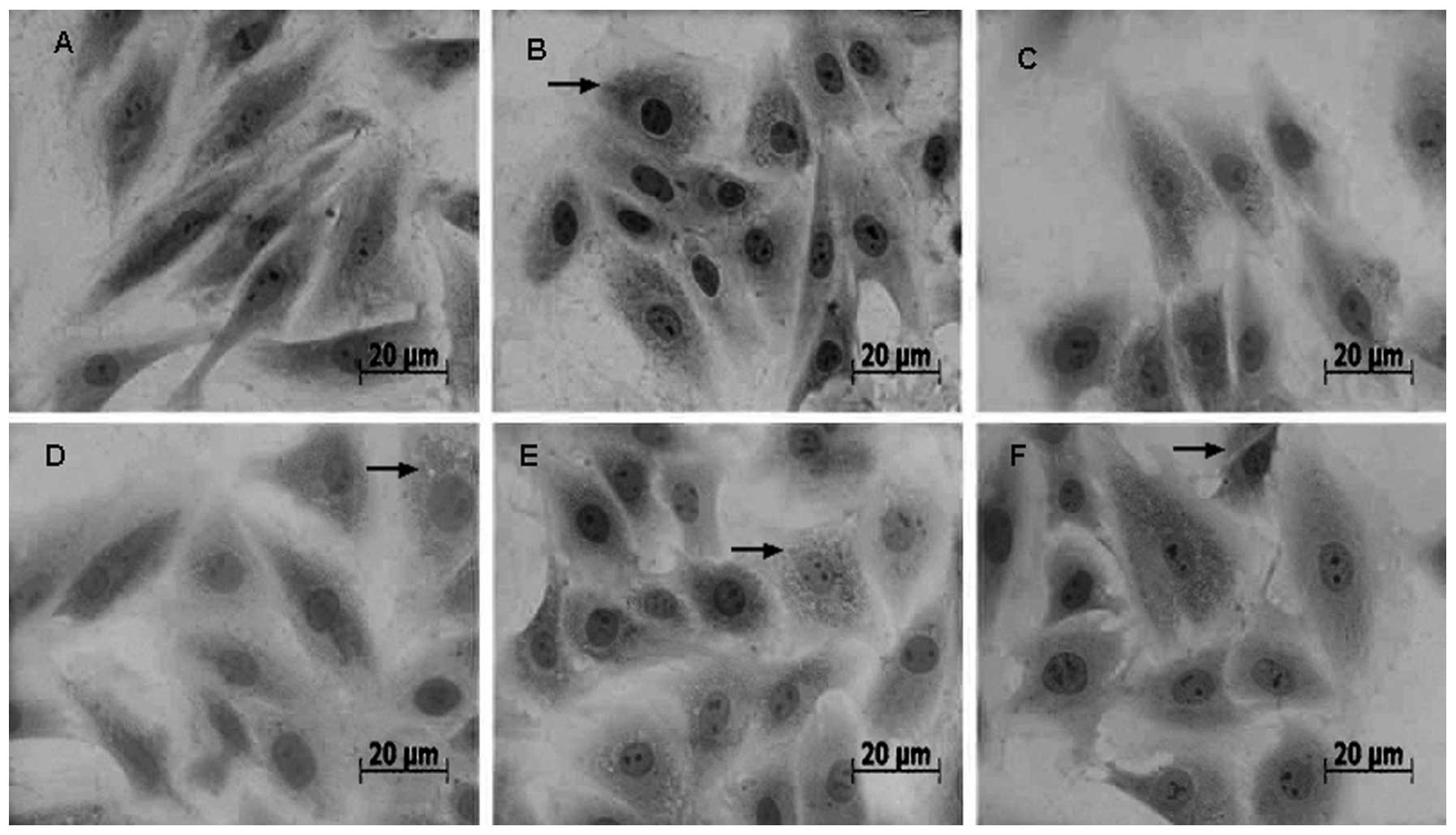
No obvious pathological changes were observed in the
control group (Fig. 1A). Following
20 min of heat stress, H9c2 cells exhibited acute degeneration with
enlarged cell size and numerous granular cytoplasmic particles
(Fig. 1B). Small granular
particles with deeply stained nuclear condensation became more
prominent in the cytoplasm of the enlarged myocardial cells
following 40 and 60 min of heat stress (Fig. 1C and D). In the 80 min group, the
primary indications of heat stress in myocardial cells were
cytomorphosis (enlarged size) and karyopyknosis (Fig. 1E and F).
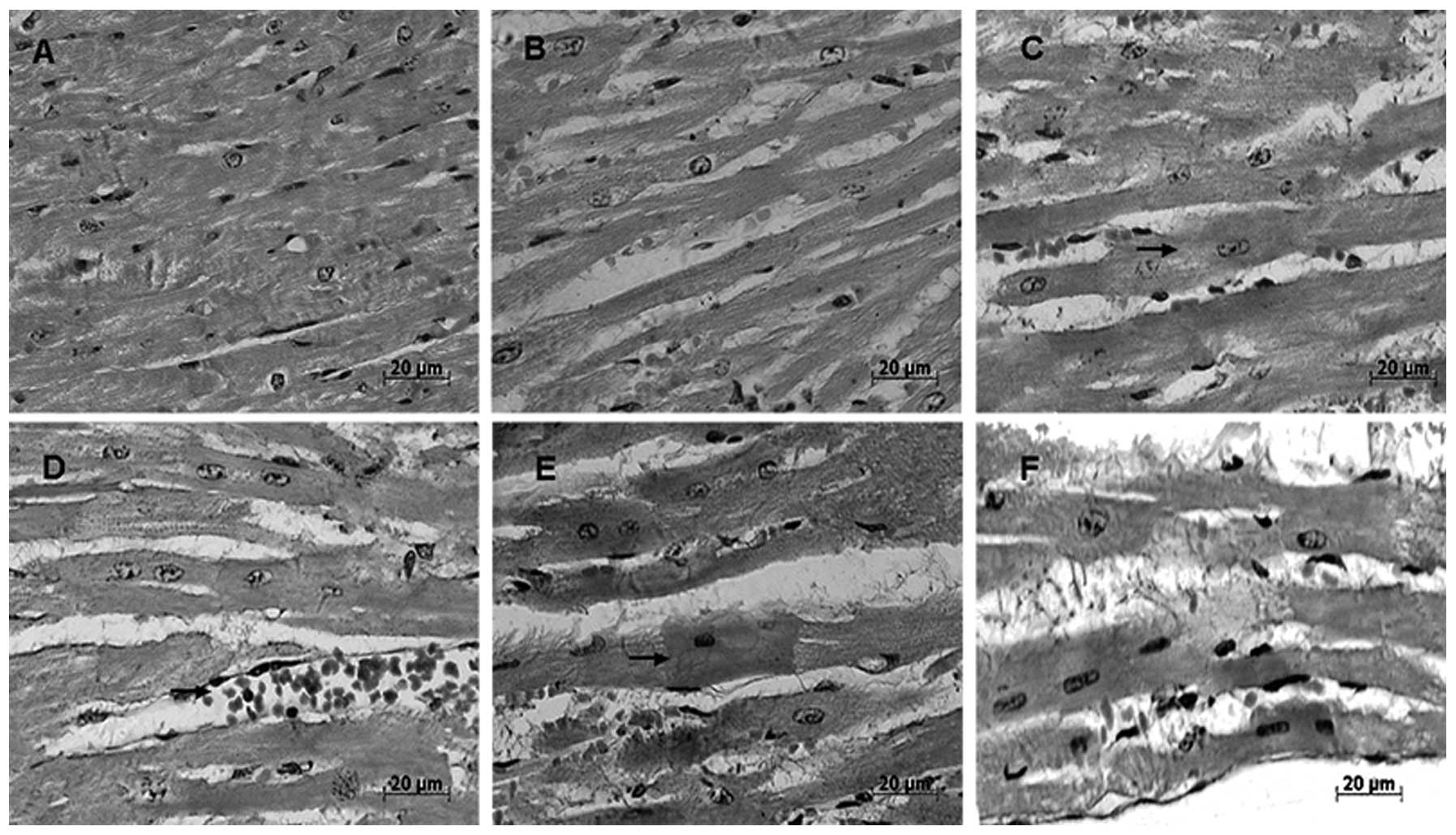
Following 20 min of heat stress in vivo, the
gaps between the myocardial fibers were widened. The number of
cytoplasmic granules in the intumesced myocardial cells of rats
gradually increased with each group from the initiation of heat
stress to its cessation at 100 min (Fig. 2B–F); of note, the number of
granules was increased at 60 min (Fig.
2D).
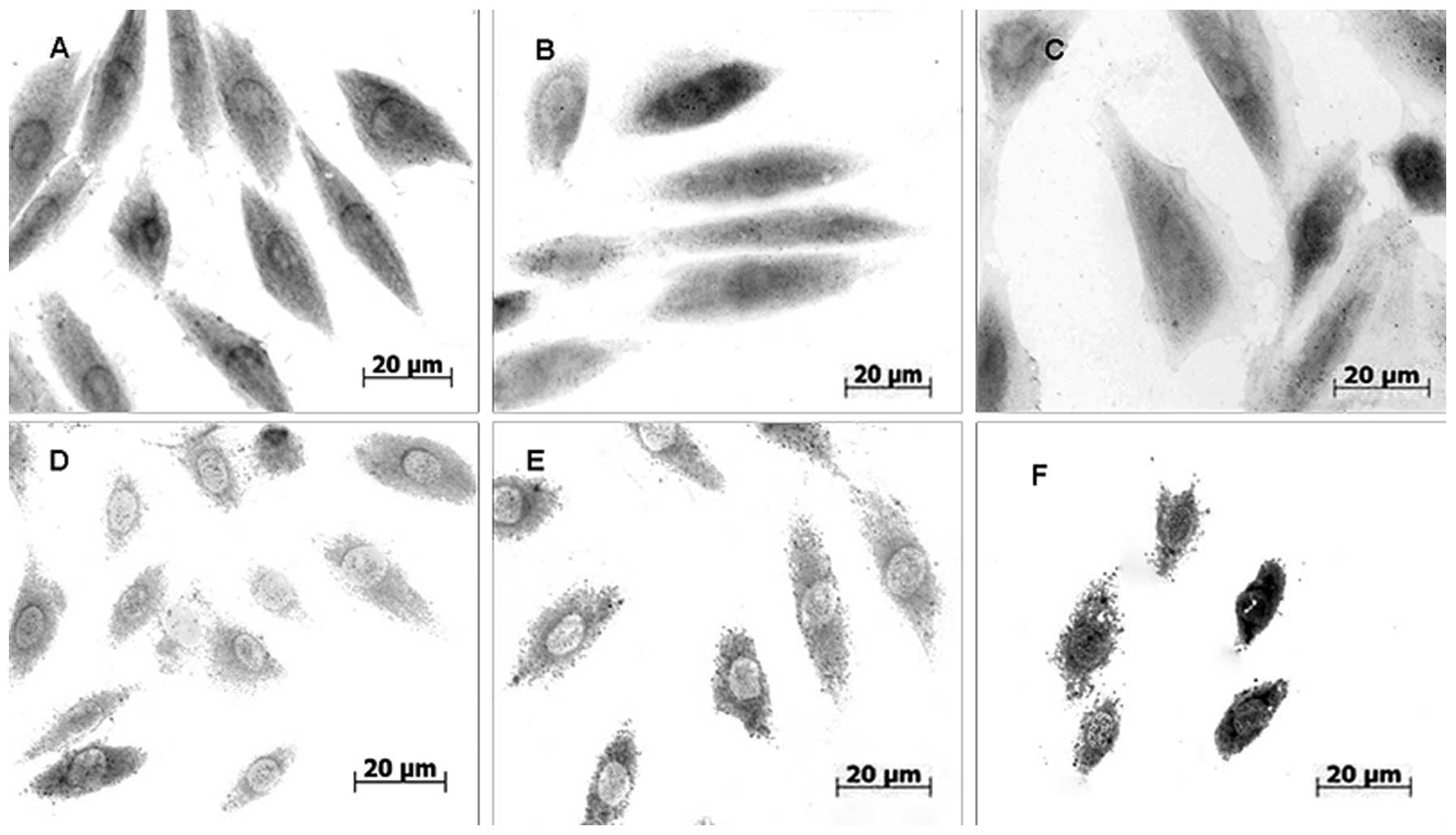
Localization of Hsp70 in heat-stressed
myocardial cells in vitro and in vivo
Figs. 3 and
4 reveal the distribution and
localization of Hsp70 in myocardial cells in vitro and in
vivo, respectively. Hsp70 was located in the nucleus and the
cytoplasm of myocardial cells following stress induction; however,
stronger Hsp70 signals were detected in the cytoplasm and signals
were identified in the heat-stressed groups as well as the
control groups (Fig. 3A–F).
Immunocytochemical staining revealed a decrease in cytoplasmic
Hsp70-positive signaling following 40 min of heat stress
(Fig. 3C) and the lowest density
of Hsp70 signals was observed in the cytoplasm of the myocardial
cells following 60 min of heat stress (Fig. 3D).
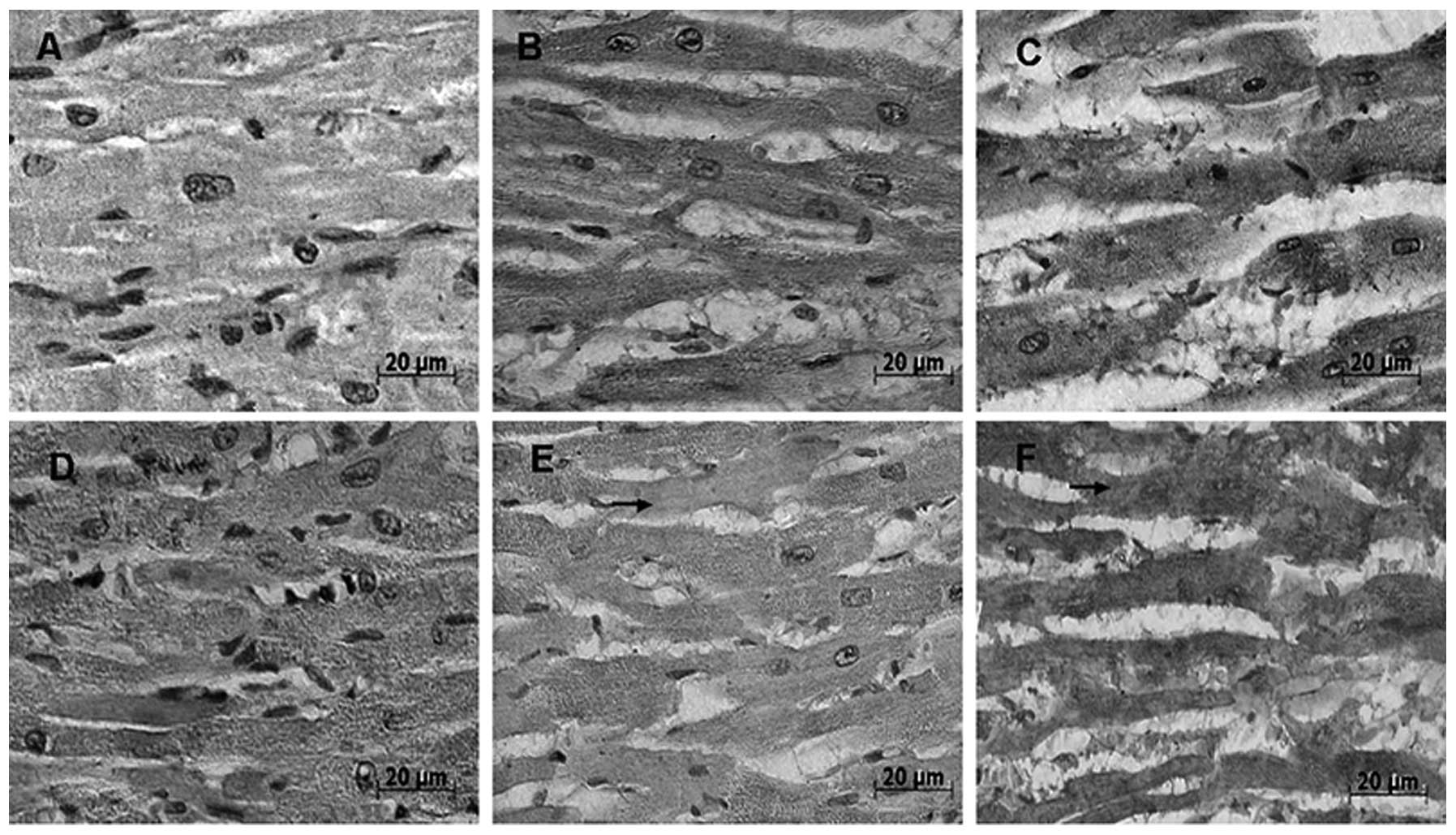
Immunohistochemical analysis revealed no observable
differences in the distribution of Hsp70 in the myocardial cells of
heat-stressed rats and normal control rats (Fig. 4). Hsp70-positive signals
were primarily detected in the cytoplasm of the myocardial cells
in vivo. Of note, following 60 min heat stress,
Hsp70-positive signals in the cytoplasm of the myocardial
cells were more prominent in intact areas than in degenerated areas
(Fig. 3D–F). At 80 min of heat
stress, the Hsp70-positive signals were unevenly distributed
in favor of the cytoplasm of myocardial cells in vitro
(Fig. 3E and F). Increasingly
stronger cytoplasmic Hsp70-positive signals were observed in
myocardial cells in vivo following 40 and 60 min of heat
stress, respectively (Fig. 4C and
D). In addition, following 80 min of heat stress, Hsp70
staining was observably lower in the cytoplasm of degenerated areas
(Fig. 4E). As shown in Fig. 5, partial myocardial fiber collapse
was observed in several rats (Fig.
5A), whereas no lesions were observed in the myocardium of
normal rats. Hsp70-positive signals markedly decreased in
the cytoplasm and were unevenly distributed in the myocardial cells
of the rats (Fig. 5B).
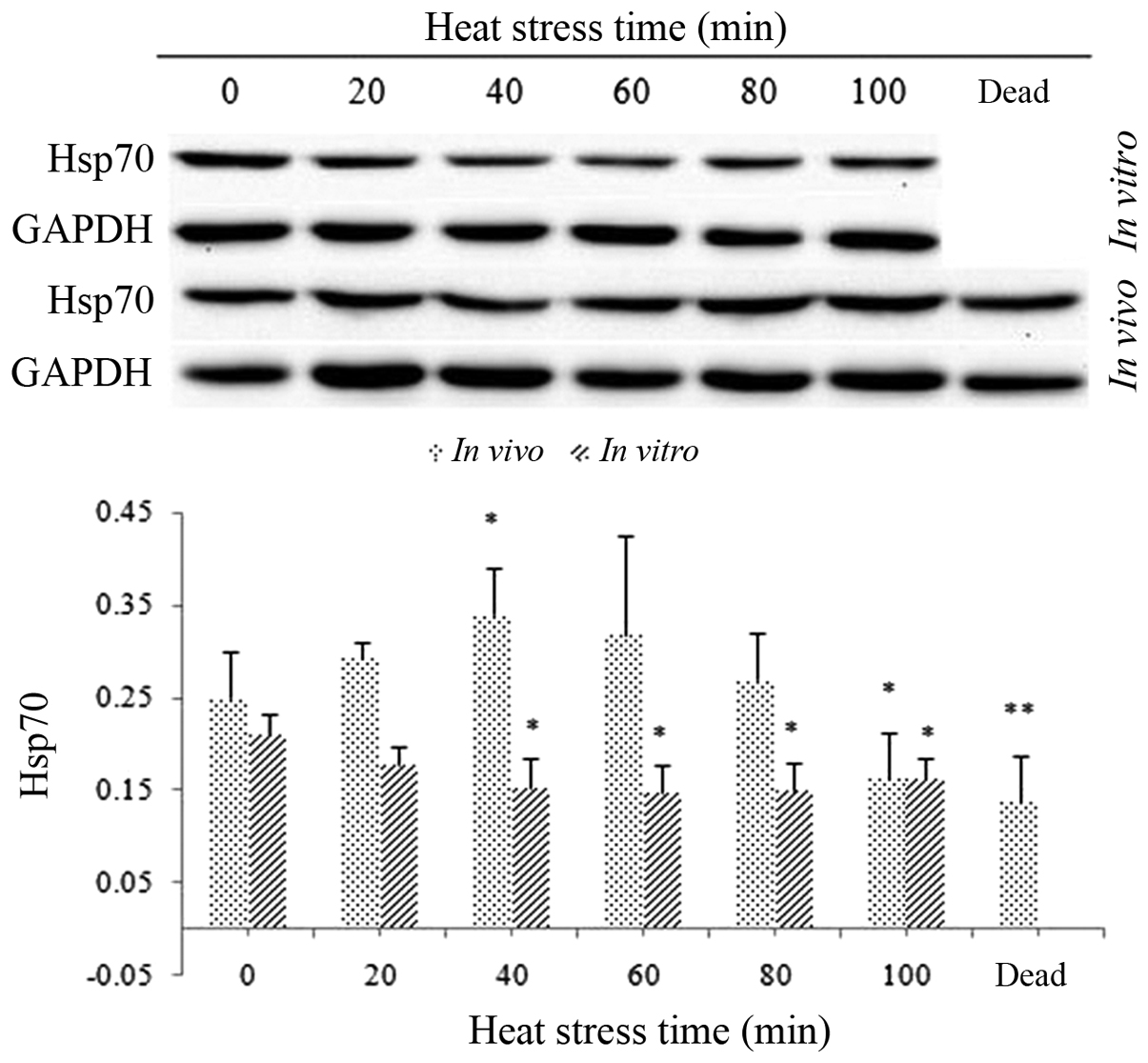
Hsp70 expression levels in heat-stressed
H9c2 cells in vitro and in heart tissues in vivo
Hsp70 expression in H9c2 cells in vitro
decreased slightly, but not significantly, following exposure to
heat stress; however, by 40 min, this decrease was significant and
remained low until the cessation of heat stress (P<0.05)
(Fig. 6).
Hsp70 expression levels in the myocardial cells of
heat-stressed rats showed a slight, but not significant increase at
20 min following heat stress. Hsp70 expression in these cells
peaked and was statistically significant at 40 min following heat
stress; however, expression levels then decreased until 100 min at
which point they were significantly decreased compared to those of
the control group (P<0.05). Of note, the protein levels of Hsp70
in the heart tissues of rats post-mortem were also significantly
lower than those of the control rats (P<0.01) (Fig. 6).
Discussion
Variations in CK and AST activities were reported to
be associated with liver and heart disease (30,31).
However, the present study revealed that activity levels of the two
enzymes differed between the in vivo and in vitro
experiments; in addition, activity levels of AST and CK in the
supernatants of heat-stressed H9c2 cells as well as the sera
of the heat-stressed rats exhibited significant variations.
In H9c2 cells, the enzyme activities, markedly AST activity,
increased immediately following exposure to heat stress.
Furthermore, acute degeneration was observed in the
heat-stressed cells in vitro and in vivo,
characterized by increased cell size, cytoplasmic granular
degradation as well as nuclear condensation. Altered AST levels
were found to correspond with the pathological changes that
occurred following heat stress in the cytoplasm of rat myocardial
cells in vitro and in vivo. This therefore indicated
that H9c2 cells and myocardial cells were protected from damage,
particularly at the beginning of heat stress. These results were
comparable with the findings of previous studies (32,33).
It has been reported that increased levels of AST and LDH were
associated with heart disease (34), the proposed mechanism of this is
thought to be due to external stresses that may damage myocardial
cell membranes, leading to the release of CK into the supernatant
in vitro (35). AST, which
was used as a heat stress indicator in these experiments, may be
beneficial for the further elucidation of the correlation between
enzymatic activity induction and cardiac tissue damage (36).
Cytopathological results of the present study
demonstrated acute degeneration, with increased cell size and
numerous granular particles in the cytoplasm of H9c2 cells
following heat stress for 20 min. Increased damage was observed
with the duration of heat exposure. H9c2 cell cytoplasmic changes
were consistent with the cytopathological changes in the myocardial
cells in vivo. In addition, histopathological analysis
revealed degeneration of the heart tissue and disordering of
myocardial fibers following 20 min of heat stress as well as cell
karyopyknosis in vivo; furthermore, following 60 min of heat
stress exposure the damage observed had worsened. These results
confirmed that heat stress at 42°C injured the myocardial cells
in vitro as well as in vivo.
Hsps are important endogenous, protective proteins
that have a significant role in the cellular response to stress
(37). Hsps from different
families exhibit different roles and functions and it was
hypothesized that the localization of Hsps may be associated with
the protection function of molecular chaperones (38). In the present study,
immunocytochemical analysis revealed that Hsp70 was primarily
distributed in the cytoplasm of myocardial cells, which was
partially consistent with previous reports of Hsp family members
being localized in the nucleus as well as the cytoplasm (39,40).
However, in the present study, the cytoplasm of H9c2 cells
demonstrated a lower density of Hsp70-positive signals in
vitro following 60 min of exposure to heat stress. This
therefore indicated that Hsp70 consumption may exceed its
production prior to 60 min of heat stress, following which the
cells produced sufficient Hsp60 in order to protect them against
heat stress. In addition, immunohistochemical analyses revealed
that Hsp70 was primarily expressed in the cytoplasm of muscle
fibers in vivo; this was consistent with the results of
previous studies where Hsp70 was detected in the muscle fibers of
transported pigs. The present study reported that following 60 min
of heat stress, Hsp70-positive signals in the cytoplasm of
the heart cells were more prominent in intact areas than in
degenerated areas; furthermore, the density of
Hsp70-positive signals was reduced following 60 min of heat
stress. These results were also consistent with those of previous
studies (32,41,42).
Of note, the present study demonstrated that Hsp70-positive
signals with lower densities were unevenly distributed throughout
the cytoplasm of myocardial cells of rats subjected to heat stress
post mortem; however, these changes were not consistent with the
in vivo and in vitro experiments. This inconsistency
may be due to the different regulatory mechanisms in vivo
and in vitro.
The expression of Hsp70 in the presence and absence
of external stressors indicated the potential role of these
proteins in physiological adaptation (43). The results of the present study
showed that Hsp70 levels in vitro decreased following the
initiation of heat stress and reached the lowest levels at 60 min,
gradually increasing thereafter until the heat stress experiment
was terminated at 100 min. These results were comparable with the
results of the immunocytochemical analysis in the present study. By
contrast, the Hsp70 levels in the heart cells of rats in
vivo gradually increased from the initiation of heat stress and
decreased sharply at 100 min of heat stress; this was not
consistent with the results of the H9c2 cells in vitro,
which may be due to different regulatory mechanisms in vivo
and in vitro. It was reported that increased Hsp expression
occurs in the majority of cells in response to stress (44) or exposure to high temperatures
(45). In addition, overexpression
of Hsp70 was shown to confer substantial heat resistance and
protect cells and proteins from thermal damage via the efficient
recognition of denatured proteins (46). By contrast, decreased Hsp70 levels
were reported to have a reduced protective effect on myocardial
cells (47).
Hsps are synthesized in response to increased
temperature in order to repair or degrade damaged proteins as a
defense strategy to ensure cell survival (48–50).
Hsp70 family members refold degenerated proteins by recognizing and
binding to the cytoskeletal myosin heavy chain and actin in damaged
gastric mucosa (51). In addition,
Hsp70 has an important role in cytoprotection in numerous cell
types by preventing denaturation as well as enhancing the proper
assembly of cellular proteins and structure. Induction of Hsp70 was
also associated with myocardial protection (52,53).
In the present study, increased expression of Hsp70
was observed in damaged heart tissue and myocardial cells,
characterized by acute cell degeneration and release of specific
enzymes. Hsp70 expression levels were compared with
histopathological changes in myocardial cells and the results
demonstrated that following heat stress, increased levels of
specific enzymes and tissue damage were accompanied by the
reduction of Hsp70 expression. Of note, within 100 min of heat
stress there was a 45% mortality rate, accompanied by sustained
severe heat-induced damage of myocardial cells in rats as
well as a low uneven distribution of Hsp70 in the cytoplasm. This
therefore indicated that heat stress injured myocardial cells in
vivo and in vitro. However, the altered expression
levels of Hsp70 in H9c2 cells in vitro did not correspond
with those of rat myocardial cells in vivo following heat
stress.
Hsp70 expression in myocardial cells was
hypothesized to enable the organism to resist the adverse effects
of stress. When exposed to heat stress, the heart rate is increased
(54). Increased expression of
Hsp70 in transgenic mouse hearts was reported to increase
resistance to ischemia/reperfusion injury and significantly improve
recovery of cardiac function (55). Furthermore, increased cellular
levels of Hsp70 via gene transfer was demonstrated to significantly
increase resistance in myocardial cells in vitro as well as
in transgenic animals in vivo (55). A previous study reported that Hsp72
overexpression reduced the size of cardiac infarctions in in
vivo transgenic mouse models of myocardial ischemia and
reperfusion (56). These studies
therefore indicated that enhanced Hsp70 expression may be a
mechanism to improve cell survival in response to stressful
environments, which may proceed via protecting proteins from
degradation and facilitating their refolding (57,58).
In the present study, Hsp70 levels decreased in vivo at the
later stages of heat stress exposure, which may be due to the rats
developing a tolerance to heat following several hours of exposure
to heat stress, or due to material deficiencies following
long-term stress. It has been previously reported that
caloric restriction increased the induction of Hsp70 transcription
and improved thermotolerance (59). It was also reported that inorganic
phosphate deficiencies may affect the major cellular biochemical
pathways controlling Hsp protein expression (60–63).
In conclusion, comprehensive comparisons of enzymes,
cell morphology and Hsp70 levels indicated that decreased levels of
Hsp70 were associated with the reduced protective effect on
myocardial cells in vitro and in vivo. However,
further studies are required in order to fully elucidate the
mechanisms underlying the interaction between Hsp70 and tissue
damage in heat-stressed cells in vivo and in
vitro.
Acknowledgements
The present study was supported by grants from the
National Key Basic Research Program of China (973 Program), the
National Natural Science Foundation of China and the National
Department Public Benefit Research Foundation (Agriculture; nos.
2014CB138502, 31372403 and 201003060-11, respectively) as
well as the Priority Academic Program Development of Jiangsu Higher
Education Institutions, Graduate Research and Innovation Projects
in Jiangsu Province and the Sino-German Agricultural
Cooperation Project of the Federal Ministry of Food, Agriculture
and Consumer Production (Berlin, Germany).
References
|
1
|
Banerjee Mustafi S, Chakraborty PK, Dey RS
and Raha S: Heat stress upregulates chaperone heat shock protein 70
and antioxidant manganese superoxide dismutase through reactive
oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones.
14:579–589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Hel W, Verstegen MW, Pijls L and
van Kampen M: Effect of two-day temperature exposure of neonatal
broiler chicks on growth performance and body composition during
two weeks at normal conditions. Poult Sci. 71:2014–2021. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geraert PA, Padilha JC and Guillaumin S:
Metabolic and endocrine changes induced by chronic heat exposure in
broiler chickens: growth performance, body composition and energy
retention. Br J Nutr. 75:195–204. 1996.PubMed/NCBI
|
|
4
|
Mashaly MM, Hendricks GL III, Kalama MA,
Gehad AE, Abbas AO and Patterson PH: Effect of heat stress on
production parameters and immune responses of commercial laying
hens. Poult Sci. 83:889–894. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee WC, Lin KY, Chiu YT, et al:
Substantial decrease of heat shock protein 90 in ventricular
tissues of two sudden-death pigs with hypertrophic cardiomyopathy.
FASEB J. 10:1198–1204. 1996.PubMed/NCBI
|
|
6
|
Hu Y, Jin H, Du X, et al: Effects of
chronic heat stress on immune responses of the foot-and-mouth
disease DNA vaccination. DNA Cell Biol. 26:619–626. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Q, Wen J and Zhang H: Effect of chronic
heat exposure on fat deposition and meat quality in two genetic
types of chicken. Poult Sci. 86:1059–1064. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCormick PH, Chen G, Tlerney S, Kelly CJ
and Bouchier-Hayes DJ: Clinically relevant thermal preconditioning
attenuates ischemia-reperfusion injury. J Surg Res. 109:24–30.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ganter MT, Ware LB, Howard M, et al:
Extracellular heat shock protein 72 is a marker of the stress
protein response in acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 291:L354–L361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staib JL, Quindry JC, French JP, Criswell
DS and Powers SK: Increased temperature, not cardiac load,
activates heat shock transcription factor 1 and heat shock protein
72 expression in the heart. Am J Physiol Regul Integr Comp Physiol.
292:R432–R439. 2007. View Article : Google Scholar
|
|
11
|
Li Z and Srivastava P: Heat-shock
proteins. Curr Protoc Immunol. Appendix 1: Appendix 1T. 2004.
View Article : Google Scholar
|
|
12
|
Lindquist S and Craig EA: The heat-shock
proteins. Annu Rev Genet. 22:631–677. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Zhang X, Qian B, et al:
Over-expression of heat shock protein 27 attenuates
doxorubicin-induced cardiac dysfunction in mice. Eur J Heart Fail.
9:762–769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lepore DA, Knight KR, Anderson RL and
Morrison WA: Role of priming stresses and Hsp70 in protection from
ischemia-reperfusion injury in cardiac and skeletal muscle. Cell
Stress Chaperones. 6:93–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knowlton AA, Kapadia S, Torre-Amione G, et
al: Differential expression of heat shock proteins in normal and
failing human hearts. J Mol Cell Cardiol. 30:811–818. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gray CC, Amrani M and Yacoub MH: Heat
stress proteins and myocardial protection: experimental model or
potential clinical tool? Int J Biochem Cell Biol. 31:559–573. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oyake J, Otaka M, Matsuhashi T, et al:
Over-expression of 70-kDa heat shock protein confers protection
against monochloramine-induced gastric mucosal cell injury. Life
Sci. 79:300–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cvoro A, Dundjerski J, Trajković D and
Matić G: Heat stress affects the glucocorticoid receptor
interaction with heat shock protein Hsp70 in the rat liver. Biochem
Mol Biol Int. 46:63–70. 1998.PubMed/NCBI
|
|
19
|
Evdonin AL, Guzhova IV, Margulis BA and
Medvedeva ND: Extracellular heat shock protein 70 mediates heat
stress-induced epidermal growth factor receptor transactivation in
A431 carcinoma cells. FEBS Lett. 580:6674–6678. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dwyer BE, Nishimura RN and Brown IR:
Synthesis of the major inducible heat shock protein in rat
hippocampus after neonatal hypoxia-ischemia. Exp Neurol. 104:28–31.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li HM, Niki T, Taira T, Iguchi-Ariga SM
and Ariga H: Association of DJ-1 with chaperones and enhanced
association and colocalization with mitochondrial Hsp70 by
oxidative stress. Free Radic Res. 39:1091–1099. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Drummond IA and Steinhardt RA: The role of
oxidative stress in the induction of Drosophila heat-shock
proteins. Exp Cell Res. 173:439–449. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morimoto RI: Cells in stress:
transcriptional activation of heat shock genes. Science.
259:1409–1410. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sorger PK: Heat shock factor and the heat
shock response. Cell. 65:363–366. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu C: Heat shock transcription factors:
structure and regulation. Annu Rev Cell Dev Biol. 11:441–469. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beckmann RP, Mizzen LA and Welch WJ:
Interaction of Hsp 70 with newly synthesized proteins: implications
for protein folding and assembly. Science. 248:850–854. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Njemini R, Bautmans I, Lambert M, Demanet
C and Mets T: Heat shock proteins and chemokine/cytokine secretion
profile in ageing and inflammation. Mech Ageing Dev. 128:450–454.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pierzchalski P, Krawiec A, Ptak-Belowska
A, Barańska A, Konturek SJ and Pawlik WW: The mechanism of
heat-shock protein 70 gene expression abolition in gastric
epithelium caused by helicobacter pylori infection. Helicobacter.
11:96–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee WC, Lin KY, Chiu YT, et al:
Substantial decrease of heat shock protein 90 in ventricular
tissues of two sudden-death pigs with hypertrophic cardiomyopathy.
FASEB J. 10:1198–1204. 1996.PubMed/NCBI
|
|
30
|
Jabiry-Zieniewicz Z, Bobrowska K, Kaminski
P, Wielgos M, Zieniewicz K and Krawczyk M: Low-dose hormonal
contraception after liver transplantation. Transplant Proc.
39:1530–1532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Radosavljević T, Mladenović D, Vucević D,
et al: Effect of acute lindane and alcohol intoxication on serum
concentration of enzymes and fatty acids in rats. Food Chem
Toxicol. 46:1739–1743. 2008. View Article : Google Scholar
|
|
32
|
Yu J, Bao E, Yan J and Lei L: Expression
and localization of Hsps in the heart and blood vessel of
heat-stressed broilers. Cell Stress Chaperones. 13:327–335. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang S, Lv Y, Chen H, et al: Comparative
analysis of αB-crystallin expression in heat-stressed myocardial
cells in vivo and in vitro. PLoS One. 9:e869372014. View Article : Google Scholar
|
|
34
|
Mitchell MA and Sandercock DA: Creatine
kinase isoenzyme profiles in the plasma of the domestic fowl
(Gallus domesticus): effects of acute heat stress. Res Vet Sci.
59:30–34. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Britton CV, Hernandez A and Roberts R:
Plasma creatine kinase isoenzyme determinations in infants and
children. Characterization in normal patients and after cardiac
catheterization and surgery. Chest. 77:758–760. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang M, Xin L, Bao E, Hartung J and Yue
Z: Variation in the expression of Hsp27, αB-crystallin mRNA and
protein in heart and liver of pigs exposed to different transport
times. Res Vet Sci. 90:432–438. 2011. View Article : Google Scholar
|
|
37
|
Gullo CA and Teoh G: Heat shock proteins:
to present or not, that is the question. Immunol Lett. 94:1–10.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Georgopoulos C and Welch WJ: Role of the
major heat shock proteins as molecular chaperones. Annu Rev Cell
Biol. 9:601–634. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Easton DP, Kaneko Y and Subjeck JR: The
hsp110 and Grp1 70 stress proteins: newly recognized relatives of
the Hsp70s. Cell Stress Chaperones. 5:276–290. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Geum D, Son GH and Kim K:
Phosphorylation-dependent cellular localization and
thermoprotective role of heat shock protein 25 in hippocampal
progenitor cells. J Biol Chem. 277:19913–19921. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kervinen H, Huittinen T, Vaarala O, et al:
Antibodies to human heat shock protein 60, hypertension and
dyslipidemia. A study of joint effects on coronary risk.
Atherosclerosis. 169:339–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bao E, Sultan K, Nowak B and Hartung J:
Expression and distribution of heat shock proteins in the heart of
transported pigs. Cell Stress Chaperones. 13:459–466. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kilgore JL, Musch TI and Ross CR: Physical
activity, muscle, and the HSP70 response. Can J Appl Physiol.
23:245–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Locke M and Noble EG: Stress proteins: the
exercise response. Can J Appl Physiol. 20:155–167. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lindquist S and Petersen R: Selective
translation and degradation of heat-shock messenger RNAs in
Drosophila. Enzyme. 44:147–166. 1990.PubMed/NCBI
|
|
46
|
Simard JP, Reynolds DN, Kraguljac AP,
Smith GS and Mosser DD: Overexpression of HSP70 inhibits cofilin
phosphorylation and promotes lymphocyte migration in heat-stressed
cells. J Cell Sci. 124(Pt 14): 2367–2374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matokanovic M, Barisic K, Filipovic-Grcic
J and Maysinger D: Hsp70 silencing with siRNA in nanocarriers
enhances cancer cell death induced by the inhibitor of Hsp90. Eur J
Pharm Sci. 50:149–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Benjamin IJ and McMillan DR: Stress (heat
shock) proteins molecular chaperones in cardiovascular biology and
disease. Circ Res. 83:117–132. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ciocca DR, Oesterreich S, Chamness GC,
McGuire WL and Fuqua SA: Biological and clinical implications of
heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst.
85:1558–1570. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Harrington HM, Dash S, Dharmasiri N and
Dharmasiri S: Heat-shock proteins: a search for functions. Aust J
Plant Physiol. 21:843–855. 1994. View Article : Google Scholar
|
|
51
|
Otaka M, Odashima M, Tamaki K and Watanabe
S: Expression and function of stress (heat shock) proteins in
gastrointestinal tract. Int J Hyperthermia. 25:634–640. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Locke M and Tanguay RM: Diminished heat
shock response in the aged myocardium. Cell Stress Chaperones.
1:251–260. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Plumier JC and Currie RW: Heat
shock-induced myocardial protection against ischemic injury: a role
for Hsp70? Cell Stress Chaperones. 1:13–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Knowlton AA: Heat-shock proteins, stress,
and the heart. Ann NY Acad Sci. 723:128–137. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Plumier JC, Ross BM, Currie RW, et al:
Transgenic mice expressing the human heat shock protein 70 have
improved post-ischemic myocardial recovery. J Clin Invest.
95:1854–1860. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hutter JJ, Mestril R, Tam EK, Sievers RE,
Dillmann WH and Wolfe CL: Overexpression of heat shock protein 72
in transgenic mice decreases infarct size in vivo. Circulation.
94:1408–1411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pratt W: The role of heat shock proteins
in regulating the function, folding, and trafficking of the
glucocorticoid receptor. J Biol Chem. 268:21455–21458.
1993.PubMed/NCBI
|
|
58
|
Hartl FU: Molecular chaperones in cellular
protein folding. Nature. 381:571–579. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Heydari AR, Wu B, Takahashi R, Strong R
and Richardson A: Expression of heat shock protein 70 is altered by
age and diet at the level of transcription. Mol Cell Biol.
13:2909–2918. 1993.PubMed/NCBI
|
|
60
|
Edens FW, Hill CH and Wang S: Heat shock
protein response in phosphorus-deficient heat-stressed broiler
chickens. Comp Biochem Physiol B. 103:827–831. 1992.PubMed/NCBI
|
|
61
|
Belay T and Teeter RG: Effects of ambient
temperature on broiler mineral balance partitioned into urinary and
faecal loss. Br Poult Sci. 37:423–433. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mahmoud KZ, Beck MM, Scheideler SE, Forman
MF, Anderson KP and Kachman SD: Acute high environmental
temperature and calcium-estrogen relationships in the hen. Poult
Sci. 75:1555–1562. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mahmoud KZ, Edens FW, Eisen EJ and
Havenstein GB: The effect of dietary phosphorus on heat shock
protein mRNAs during acute heat stress in male broiler chickens
(Gallus gallus). Comp Biochem Physiol C Toxicol Pharmacol.
137:11–18. 2004. View Article : Google Scholar : PubMed/NCBI
|