Introduction
Photodynamic therapy (PDT) with photosensitizers,
including hematoporphyrin derivatives (HpD), photofrin and
5-aminolevulinic acid (ALA)-induced protoporphyrin IX (PpIX), has
been used in the treatment of several types of cancer (1–3). The
effectiveness of photosensitizers depends on two important
properties: The production of cytotoxic oxygen and fluorescence
upon excitation by laser irradiation (4–6). In
particular, 5-ALA has high affinity for malignant gliomas, and
thus, it is commonly used for fluorescence-guided tumor resection
in clinical neurosurgery (4).
Although several porphyrin compounds, including HpD and photofrin,
have been shown to act as radiosensitizers, the radiosensitizing
activity of 5-ALA-induced PpIX remains controversial (7–11).
We previously demonstrated that although the ability of
5-ALA-induced PpIX to radiosensitize glioma cell lines in
vitro was weak, multi-dose ionizing irradiation may be used to
enhance this radiosensitizing effect (12). The aim of the present study was to
further evaluate the radiosensitizing effects of 5-ALA-induced PpIX
in combination with multi-dose ionizing irradiation by assaying
this activity in a rat subcutaneous (s.c.) glioma model. The
potency of 5-ALA-induced PpIX as a radiosensitizer for cancer
therapy was also discussed.
Materials and methods
Chemicals
5-ALA was purchased from Cosmo Bio K.K. (Tokyo,
Japan) and was dissolved in phosphate-buffered saline (PBS; WAKO
Pure Chemical Industries, Ltd, Osaka, Japan) at a concentration of
100 mg/ml. The pH of the solution was adjusted to 6.0–6.3 with 10 N
sodium hydroxide (NaOH; WAKO Pure Chemical Industries, Ltd) and
checked using pH indicator paper. The solution was used within 10
min of preparation. 5-ALA was intravenously administered to rats
via the tail vein at a dose of 100 mg/kg body weight.
Brain tumor cell lines and animals
All the following experiments were performed in
accordance with an animal protocol approved by the Institutional
Animal Care and Use Committee (University of Occupational and
Environmental Health, Kitakyushu, Japan). The 9L gliosarcoma cell
line, which was generated from inbred Fischer rats, has been widely
used as a syngeneic rat model for experimental gliomas. Originally
produced by N-methyl-nitrosourea mutagenesis in Fischer rats
by Benda et al (13) and
Schmidek et al (14) at
Massachusetts General Hospital, the tumor was obtained by Barker at
the University of California, cloned and designated 9L gliosarcoma
due to the dual appearance of a glioblastoma and a sarcoma. 9L
gliosarcoma cells rapidly proliferate under in vitro and
in vivo conditions, and are the most widely used cells in
experimental rat glioma, for example in brain and subcutaneous
tumor models (6,15). 9L gliosarcoma cells were obtained
from Dr Tsutomu Tokuyama at the Hamamatsu University School of
Medicine (Hamamatsu, Japan) and cultured for several days in
RPMI-1640 medium (WAKO Pure Chemical Industries, Ltd) supplemented
with 10% fetal calf serum (FCS; Nichirei Biosciences Inc., Tokyo,
Japan) before use. Syngeneic male Fischer 344 rats (8 weeks of age;
mean body weight, 167 g) were purchased from SLC, Inc. (Hamamatsu,
Japan). A total of 32 rats were used for the present study. Animals
were inoculated with 9L gliosarcoma cells, as previously described
(3,6). Briefly, these cells
(1×106) were implanted into the dorsal skin of the
Fisher 344 rats, and thereby, a rat s.c. tumor model was prepared
for the following experiments. All animals were kept at a constant
room temperature of 24°C under a 12-h light/dark cycle (7 am to 7
pm) in the laboratory animal center of the University of
Occupational and Environmental Health. In addition, all animals
received sufficient food, which was sterilized and certified for
experimental animals (MF; Oriental Yeast Co., Ltd, Tokyo, Japan),
according to the animals requirements.
Evaluation of the accumulation of
5-ALA-induced PpIX in the rat s.c
tumor model. Firstly, the accumulation of
5-ALA-induced PpIX was confirmed in the established rat s.c. tumor
model using high-performance liquid chromatography (HPLC) analysis
and fluorescence observation. Once the tumors had grown to ~1 cm in
diameter, the rats were given an intravenous (i.v.) injection of
5-ALA (100 mg/kg body weight) into the tail vein. Three hours
later, the rats were anesthetized and tumor specimens without the
dorsal skin cover were removed and immediately snap-frozen in
liquid nitrogen, then stored at −80°C in the dark for HPLC
analysis. According to the previously described method of HPLC
analysis of porphyrin metabolites (16,17),
tumor specimens (1-mm diameter) were treated with 200 μl of 0.1 M
NaOH and homogenized on ice with a Powermasher II (Assist, Tokyo,
Japan). An aliquot (10 μl) of the NaOH-treated samples was
withdrawn and used for a protein concentration assay (Quick Start™
Bradford Dye Reagent, Bio-Rad Laboratories, Inc., Hercules, CA,
USA), while the remaining 50 μl of cellular proteins were denatured
by the addition of 150 μl
N,N-dimethylformamide/isopropanol solution (100:1,
v/v; Nacalai Tesque, Inc., Kyoto, Japan). After overnight storage
in the dark, the prepared samples were subjected to HPLC analysis
performed as previously described (2,17)
with certain modifications. Briefly, porphyrins were separated
using a Prominance HPLC system (Shimadzu, Kyoto, Japan) equipped
with a reversed-phase C18 column (CAPCELL PAK, C18, SG300, 5 μm,
4.6 mmx250 mm; Shiseido, Tokyo, Japan) maintained at 40°C. The
elution solvents used were solvent A (1 M ammonium acetate
including 12.5% acetonitrile, pH 5.2) and solvent B (50 mm ammonium
acetate including 80% acetonitrile, pH 5.2; Kanto Chemical Co.,
Inc., Tokyo, Japan). Elution was performed with solvent A for 5 min
and subsequently with a linear gradient of solvent B (0–100%) for
25 min, followed by elution with solvent B for 10 min. The elution
flow was maintained at a constant rate through the use of a
fluorospectrometer (excitation at a wavelength of 404 nm, detection
at a wavelength of 624 nm; F7000; Hitachi, Tokyo, Japan). The
porphyrin concentrations in the samples were estimated using
calibration curves obtained with standard porphyrins
(Protoporphyrin IX; Sigma-Aldrich, St. Louis, MO, USA).
In addition, under anesthesia, the rat dorsal skin
covering the inoculated tumors was evaginated and the s.c. tumors
were observed underneath the skin at 3 h post-intravenous
administration of 100 mg/kg 5-ALA. Bright-field images of the s.c.
tumors were captured using a digital camera (D90, Nikon
Corporation, Tokyo, Japan) with a long-pass filter and an external
halogen lamp as the light source (C-FID, Nikon). Subsequently, the
same s.c. tumors were illuminated with ultraviolet light (410-nm
light-emitting diode illuminator, SBI Pharma CO., Ltd., Tokyo,
Japan), and tumor images were captured using a digital camera with
a long-pass filter.
Evaluation of the in vivo
radiosensitizing effects of 5-ALA with multi-dose ionizing
irradiation in a rat s.c
tumor model. Syngeneic Fischer 344 rats were
inoculated with 9L gliosarcoma cells, as previously described
(3,6). A previous study demonstrated that the
growth of s.c. 9L tumors in syngeneic Fischer rats was inhibited at
>10 Gy with single-dose ionizing irradiation (18). Other previous studies reported that
for PDT, the dose of 5-ALA used for a single i.v. administration
was 100–500 mg/kg for rodents (6,19–21).
Thus, in the present study, the maximal dose of ionizing
irradiation and 5-ALA administration used were 10 Gy and 500 mg/kg.
A previous study by our group confirmed that multi-dose ionizing
irradiation with 5-ALA inhibited tumor proliferation in
vitro (12). However, multiple
i.v. injection via the rat tail vein was technically difficult due
to injury and obstruction of vessels. According to preliminary
experiments by our group, up to 5 i.v. injections were possible.
Therefore, the optimal ionizing irradiation schedule was determined
to be 2 Gy and 5-ALA administration (100 mg/kg) per day, for five
consecutive days. Once the s.c. tumors had grown to a diameter of
6–8 mm, the animals were randomly divided into four groups and
treated as follows: Control group, no further treatment (n=5);
5-ALA administration (n=5); ionizing irradiation (RT) (n=7); and
ionizing irradiation with 5-ALA administration (5-ALA + RT) (n=7).
In the 5-ALA administration group, the animals received 5-ALA (100
mg/kg) alone for five consecutive days without ionizing
irradiation. In the ionizing irradiation with 5-ALA administration
group, the animals were anesthetized 3 h post-5-ALA administration
and the s.c. tumors were irradiated with 2 Gy in the dark using an
X-ray irradiator (MBR-1520R; HITACHI Engineering & Service Co.,
Ltd., Japan) at a rate of 0.65 Gy/min. The animals were completely
covered with an X-ray shield sheet, except for the tumor regions,
to avoid excessive exposure of the rest of the body to the ionizing
irradiation. This procedure was performed for five consecutive
days, resulting in exposure to a total of 10 Gy (2 Gy/day, 5 days).
In the ionizing irradiation group, the s.c. tumors were irradiated
in an identical manner, without the administration of 5-ALA. To
avoid photochemical effects, all animals were kept in the dark for
12 h after 5-ALA administration. Thereafter, direct exposure of the
animals to light was avoided. Tumor growth was assessed every 2
days until 16 days post-treatment. Tumor volumes were calculated
using the formula V = a2b/2, where a and b are the
shortest and longest perpendicular diameters, respectively
(22). Sixteen days after
treatment, the animals were sacrificed under deep anesthesia using
equithesin (0.4 ml/100g), which was composed of chloral hydrate,
magnesium sulfate, ethanol and propylene glycol, all purchased from
WAKO Pure Chemical Industries Ltd, as well as pentobarbital sodium
salt (Tokyo Chemical Industry Co., Ltd, Tokyo, Japan). All tumor
specimens were immediately removed with the dorsal skin cover and
fixed in 20% formaldehyde/PBS for further pathological
examination.
Pathological examination
Following fixation all tumor specimens were cut at
the center of the tumor in the direction of the longest axis.
Sections were stained with hematoxylin and eosin and ionized
calcium-binding adapter molecule 1 (Iba1) for macrophage detection,
and all staining processes were outsourced to Pathology Institute
Corp, Toyama, Japan. Briefly, after a water bath pretreatment (40
min, 95°C), deparaffinized sections were washed with KN buffer
(KN-09002; Pathology Institute Corp., Toyama, Japan). The sections
were incubated with goat polyclonal anti-Iba1 (1:3,000, ab107159;
Abcam, Cambridge, UK) for 30 min, followed by a further wash with
KN buffer. Subsequently, the sections were incubated with Simple
Stain™ MAX-PO (G) (H1301; Nichirei Bioscience Inc.) for 30 min.
Following a final wash with KN buffer, color development was
performed with diaminobenzidine (DAB; DAKO, Glostrup, Denmark) for
10 min and the sections were counterstained with hematoxylin (WAKO
Pure Chemical industries, Ltd). Thereafter, all tumor specimens
were evaluated in our laboratory. Based on a previous study
(23), microdensitometry for
quantitative evaluation of Iba1 immunohistochemistry was performed
on digital microphotographs (Colorio EP-705A; Seiko Epson Corp.,
Suwa, Japan) using the public domain software ImageJ 1.46r
(National Institutes of Health, Bethesda, MD, USA), with certain
modifications of the original method. In brief, all Iba1-stained
tumor specimens were scanned using a color scanner (Colorio
EP-705A) and then photographed. To perform quantification of the
immunohistochemical DAB color signal of Iba1, image data of all
samples were transferred to ImageJ and converted to an 8-bit
grey-scale image. The freehand tool was used to delineate the
whole-tumor section as the region of interest (ROI) for in each
tumor specimen, and the mean gray value (MGV) within the ROI was
plotted on a graph. The representative value was defined as the
average MGV of all the five tumor specimens in the control group
and the relative intensity of the MGV of the other groups was
calculated according to the representative value.
Statistical analyses
Data are presented as the mean ± the standard error
of the mean. Statistical analyses were performed using StatView 5.0
software (SAS Institute, Cary, NC, USA) The mean tumor volume was
analyzed using unpaired two-sample t-tests, and the relative
intensity of MGV of Iba1 was calculated using Fisher’s exact
probability tests. Statistical significance was defined as
P<0.05.
Results
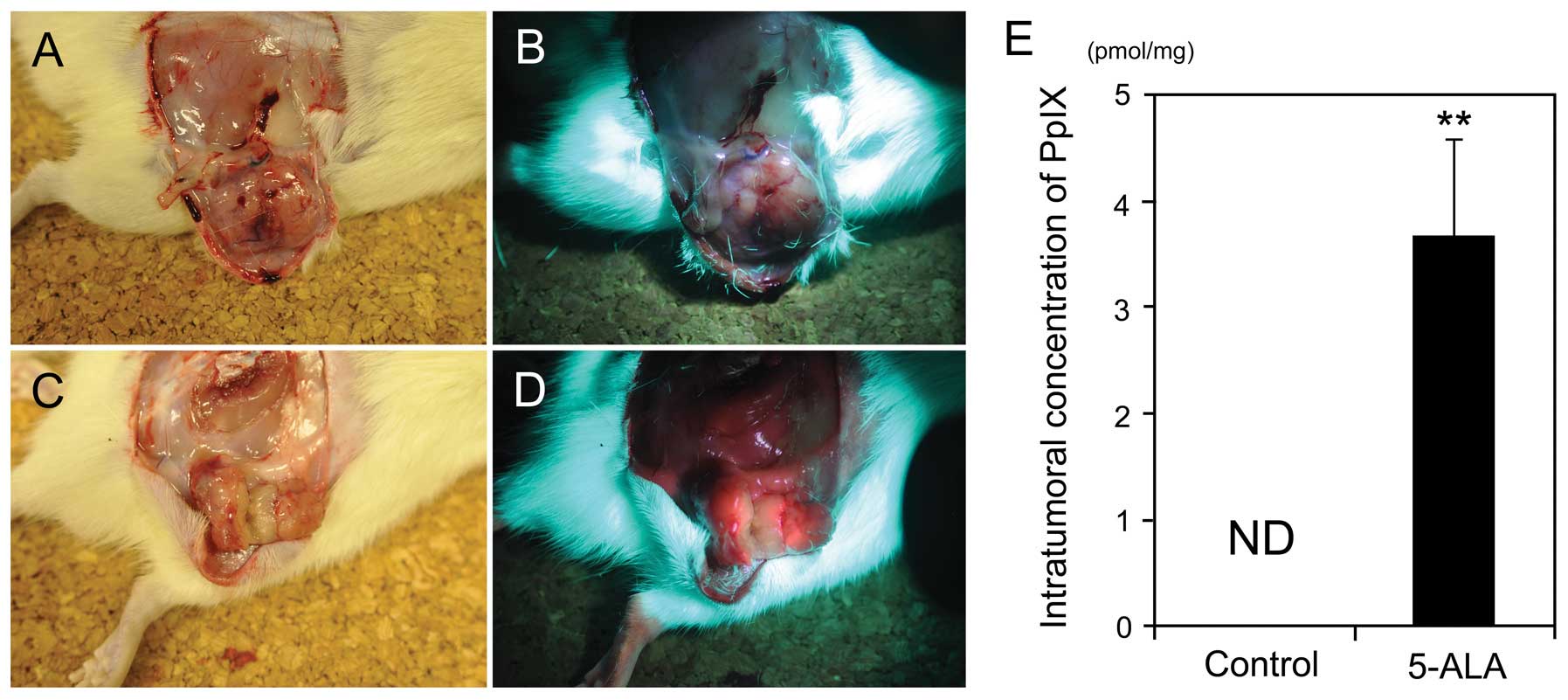
Intratumoral accumulation of
5-ALA-induced PpIX in the rat s.c
tumor model. In the established rat s.c. tumor
model, tumors demonstrated strong fluorescence 3 h after the
intravenous administration of 5-ALA, compared with that of the
control tumors without 5-ALA administration (Fig. 1). Furthermore, HPLC analysis
revealed that the amount of 5-ALA-induced PpIX in tumors was
3.66±0.91 pmol/mg-protein 3 h after the intravenous administration
of 5-ALA, which was significantly higher than that of the control
(P<0.01) (Fig. 1E). By
contrast, 5-ALA-induced PpIX was not detected in the control tumors
without 5-ALA administration (<0.1 pmol/mg-protein).
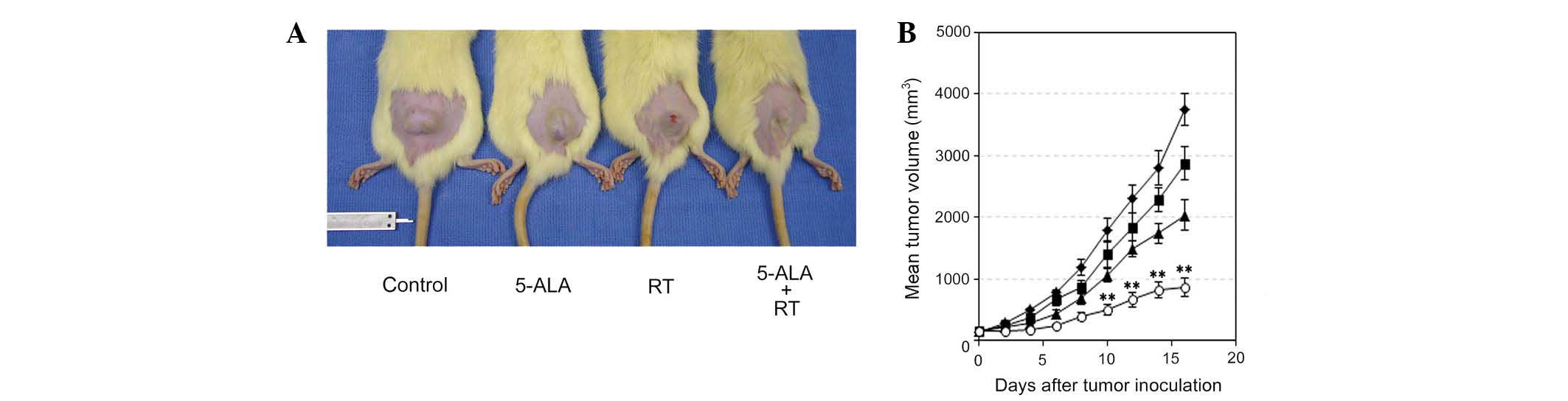
Radiosensitizing effect of 5-ALA with
multi-dose ionizing irradiation in vivo
The subcutaneously inoculated 9L gliosarcomas grew
at a near exponential rate (Fig.
2, Table I). On the first day
(day 0) of tumor treatment, there was no difference in tumor size
between the RT and 5-ALA + RT groups (P=0.8457, Table I). Treatment with multi-dose
ionizing irradiation and 5-ALA significantly inhibited tumor
growth, compared with that of tumors treated with irradiation alone
(P<0.01, day 10) (Fig. 2 and
Table I). On day 16, the mean
inhibition of tumor growth in the 5-ALA + RT group was 42.5% of
that in the RT group (Fig. 2A and
Table I). OF note, tumor growth
was inhibited by 5-ALA administration without treatment with
ionizing irradiation as compared with that of the control group
(P=0.0448 at day 16).
 | Table IEffect of 5-ALA and multi-dose RT on
tumor growth. |
Table I
Effect of 5-ALA and multi-dose RT on
tumor growth.
| Group | Day 0 | Day 8 | Day 10 | Day 12 | Day 14 | Day 16 |
|---|
| Control (n=5) | 142.3±13.8 | 1191.5±128.8 | 1793.2±199.2 | 2296.8±233.3 | 2799.0±281.9 | 3747.1±254.1 |
| ALA (n=5) | 143.9±17.4 | 865.4±107.2 | 1403.0±224.3 | 1842.6±218.8 | 2276.3±192.8 | 2869.4±267.9 |
| RT (n=7) | 149.9±14.9 | 683.3±99.4 | 1059.0±101.7 | 1485.8±133.4 | 1736.9±162.9 | 2035.2±245.9 |
| ALA + RT (n=7) | 146.0±12.9 | 385.3±59.7 | 495.6±91.1 | 667.6±119.7 | 819.0±135.5 | 863.1±147.4 |
| P-valuea | 0.8457 | 0.0246 | 0.0014 | 0.0007 | 0.001 | 0.0015 |
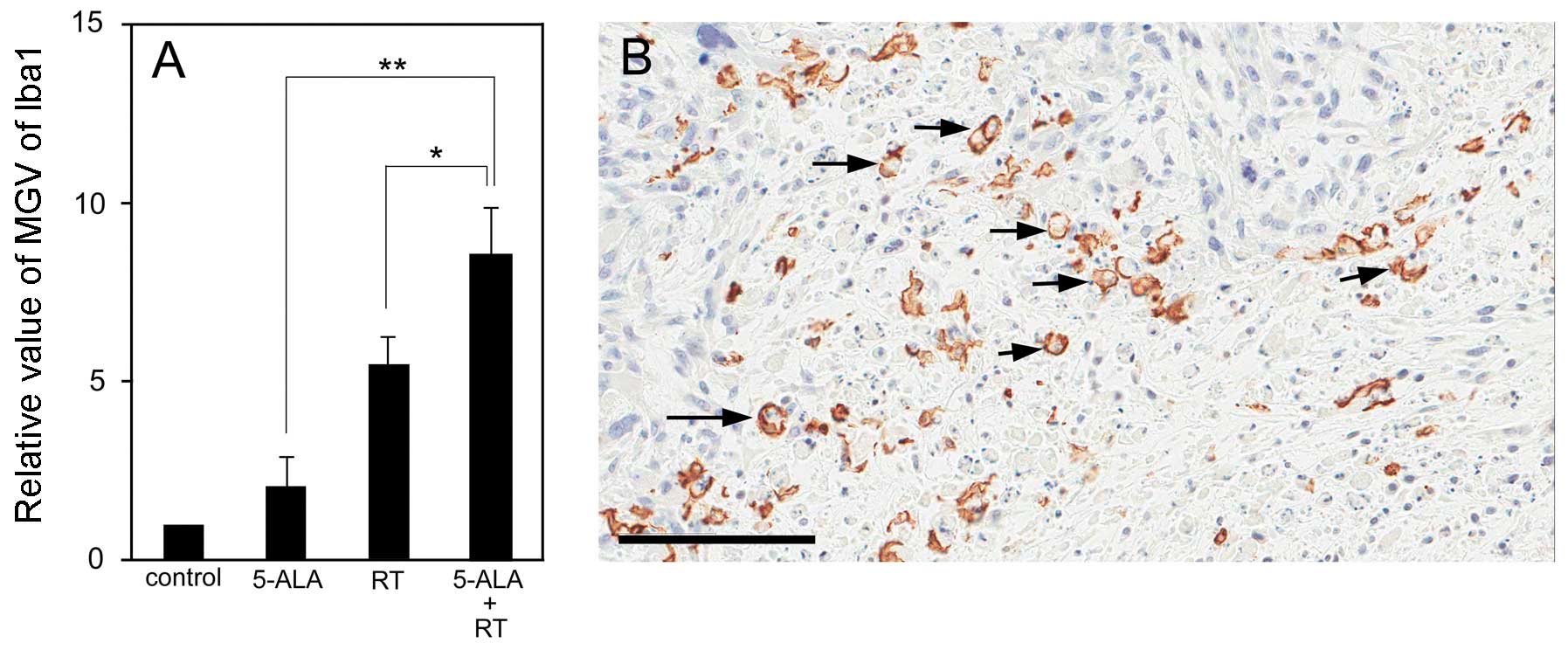
Histological evaluation of s.c. 9L
gliosarcomas after multi-dose ionizing irradiation
The tumor specimens showed coagulative necrosis
within the tumors, with variance among the groups (Fig. 3A–D). Of note, the Iba1-positive
macrophages also showed a certain variance among the groups
(Fig. 3E–L). The control group
showed a very low accumulation of Iba1-positive macrophages in the
s.c. tumors (Fig. 3E and I). In
the 5-ALA group, Iba1-positive macrophages mainly gathered at the
surface of the s.c. tumors, only slightly invading the tumors
(Fig. 3E and J). Similarly,
numerous Iba1-positive macrophages gathered at the surface of and
within the s.c. tumors following treatment with ionizing
irradiation (Fig. 3G and K) and in
particular following treatment with ionizing irradiation in
combination with 5-ALA (Fig. 3H and
L). Microdensitometric analysis showed that significantly more
Iba1-positive macrophages gathered in the s.c. tumors of the
ionizing irradiation + 5-ALA group compared with those of the other
groups (P<0.05 vs. RT; P<0.01 vs. 5-ALA) (Fig. 4A). Analysis of the distribution of
the Iba1-positive macrophages within the tumors revealed that the
Iba1-positive macrophages did not gather within the zone of
coagulation necrosis, but primarily gathered in the boundary zones
between the coagulation necrosis and the surviving tumor cells. In
particular, a number of Iba1-positive macrophages displayed
features of the phagocytic process (Fig. 4B).
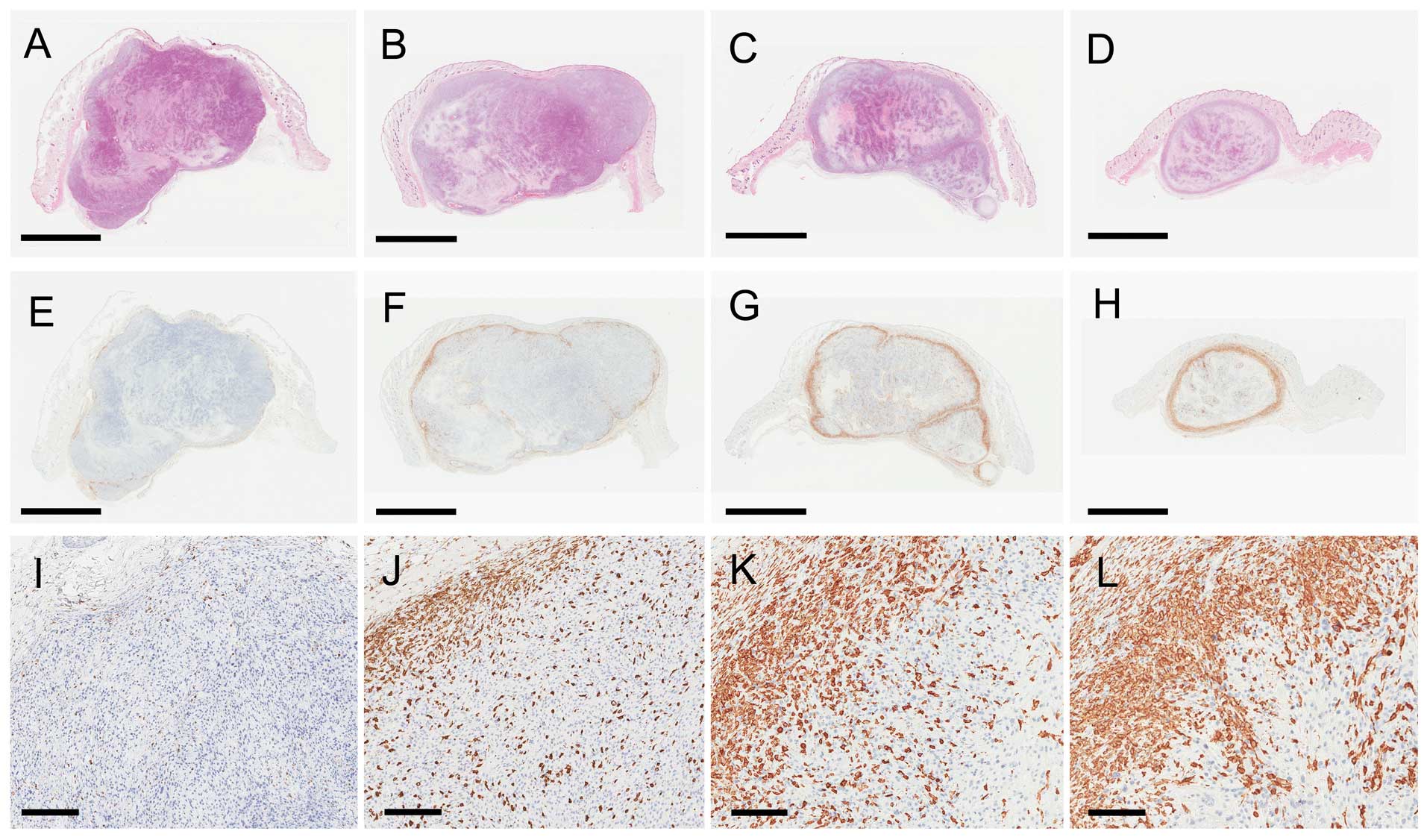 | Figure 3Pathology results of sc tumors at day
16 after treatment in the (A, E, I) control, (B, F, J) 5-ALA, (C,
G, K) multi-dose RT, and (D, H, L) multi-dose RT with 5-ALA groups.
(A–D) (HE) staining and (E–L) Iba1 staining. (E–H) The Iba1-stained
sections correspond to the HE-stained sections in each group. (I–L)
Magnified views of the Iba1 staining of the surface of the s.c.
tumors in each group. (A–D) The s.c. tumors showed coagulative
necrosis in each group. (H, L) Iba1-positive macrophages gathered
at the surface of, and within, the s.c. tumors in the multi-dose
ionizing irradiation with 5-ALA group. In contrast, (E, I)
Iba1-positive macrophages were scarcely observed in the control
group. Scale bar: (A–H) 5 mm and (I–L) 200 μm. 5-ALA,
5-aminolevulinic acid; RT, ionizing irradiation; HE, hematoxylin
and eosin; Iba1, ionized calcium-binding adapter molecule 1. |
Discussion
The results of the present study confirmed that
5-ALA-induced PpIX has a radiosensitizing effect in experimental
glioma. It was determined that tumor growth was significantly
inhibited in 5-ALA-treated irradiated rats compared with rats
exposed to multi-dose ionizing irradiation in the absence of 5-ALA.
This was in agreement with a previous in vitro study by our
group (12). To the best of our
knowledge, the present study was the first to show the
radiosensitizing effects of 5-ALA-induced PpIX and to confirm the
immunological effects in experimental glioma in vivo.
The mechanism underlying the radiosensitizing
effects of porphyrin compounds remains elusive. Using confocal
laser scanning microscopy, it was previously demonstrated that
intracellular 5-ALA-induced PpIX has an important role in the
production of reactive oxygen species and in radiosensitization
(12). This radiosensitizing
effect was found to depend on the intracellular concentrations of
porphyrin compounds, including HpD and photofrin, which were low
following 5-ALA administration (6). Therefore, high intracellular
concentrations of HpD and photofrin may have a strong
radiosensitizing effect with single-dose ionizing irradiation,
comparable with that of 5-ALA-induced PpIX in vitro and
in vivo (8–10, 24). In certain cell lines, however,
5-ALA-induced PpIX was shown to have a low sensitizing effect with
single-dose ionizing irradiation (12,25).
In addition, another study indicated that ionizing irradiation
increased, rather than inhibited, ALA-induced synthesis of PpIX in
human colon adenocarcinoma cells in vitro (9). In the present study, 5-ALA-induced
PpIX was found to significantly enhance tumor sensitivity to
multi-dose ionizing irradiation. Therefore, repeated administration
of 5-ALA, combined with ionizing irradiation, may enhance the
radiosensitizing effect of 5-ALA-induced PpIX, thereby strongly
inhibiting tumor growth in experimental glioma.
In addition, the immunological response to
5-ALA-induced PpIX and multi-dose ionizing irradiation was measured
by performing immunohistochemical staining with Iba1 for
macrophages. The combination of 5-ALA-induced PpIX with multi-dose
ionizing irradiation resulted in a strong aggregation of
Iba1-positive macrophages at the surface of and within the s.c.
tumors. Iba1 expression is typically upregulated in activated
macrophages/microglia, which exhibit a distinct morphology with an
amoeboid shape and short processes (26,27).
Macrophages may be broadly divided into the following two groups:
i) Classically activated M1-type macrophages that typically
participate in the coordinated response to immunogenic antigens,
primarily through the production of proinflammatory mediators,
including interleukin (IL)-1B, IL-12 and tumor necrosis factor-α
(TNF-α), and have an overall enhanced ability to phagocytose
pathogenic material (28,29); and ii) alternatively
activated M2-type macrophages that do not secrete proinflammatory
mediators such as IL-1B and TNF-α (30) and are believed to exert
immunomodulation primarily through the secretion of the potent
immunosuppressive cytokines IL-10 and TGF-β and have a decreased
phagocytic capacity (31,32). In the present study, numerous
Iba1-positive macrophages gathered at the surface of and within the
s.c. tumors following 5-ALA administration of multi-dose ionizing
irradiation. In particular, these Iba1-positive macrophages with
phagocytic features primarily gathered in the boundary zones
between the coagulation necrosis and the surviving tumor cells. By
contrast, although coagulative necrotic changes were revealed
within the s.c. tumors in all the groups, Iba1-positive macrophages
scarcely gathered at the surface of the s.c. tumors in the control
group. Thus, these Iba1-positive macrophages did not gather at the
surface of the s.c. tumors merely for the removal of the
coagulative necrotic tissues within the tumors. Therefore, it is
hypothesized that 5-ALA-induced PpIX with multi-dose ionizing
irradiation treatment induced not only a direct cytotoxic effect,
but additionally a long-lasting immunological effect following the
ionizing irradiation, including the induction of tumor cytotoxic
M1-type macrophages, and consequently caused a strong inhibition of
tumor growth.
A previous study reported that photodynamic therapy
with 5-ALA led to an increase in tumor-infiltrating mononuclear
cells and the activation of intraperitoneal macrophages in Lewis
lung carcinoma (33). Of note, the
present study found that repeated administration of 5-ALA alone
inhibited tumor growth. While it was not possible to completely
exclude all photodynamic effects, the results indicated that even
when exposure to room light was limited and indirect, 5-ALA alone
enhanced the host antitumor immune response. It has been suggested
that the mechanisms of these immunological effects with response to
5-ALA in cancer therapy shall be investigated in greater detail in
future studies (34). A recent
clinical study showed that 5-ALA administered orally once a day
over a 12-week period reduced both fasting and postprandial glucose
levels in type-2 diabetes mellitus without adverse effects
(35). Patients with malignant
brain tumors frequently receive fractionated radiotherapy following
surgical resection. Various radiotherapy modalities, including
stereotactic radiotherapy (SRT), stereotactic radiosurgery (SRS)
and intensity-modulated radiotherapy, precisely control the
intensity of ionizing irradiation, thereby avoiding or reducing the
exposure of healthy tissues and limiting the side-effects of the
treatment. Thus, SRT and SRS provide high doses of irradiation to
small, precise areas of intracranial lesions (36,37).
5-ALA has a high affinity for malignant brain tumors and has a
number of advantages, including reduced skin phototoxicity compared
with that to other photosensitizers. The present study presents
preliminary data of the radiosensitizing effects of 5-ALA-induced
PpIX in experimental glioma. Multi-dose ionizing irradiation with
5-ALA-induced PpIX may be a novel therapeutic strategy for
malignant gliomas with possible clinical applications.
The present study demonstrates the radiosensitizing
effect of 5-ALA in experimental glioma in vivo. Multi-dose
ionizing irradiation alongside 5-ALA administration induces strong
inhibition of tumor growth and enhances the antitumor immune
response in experimental glioma. Although investigation of the
mechanisms underlying the combined effects of multi-dose
irradiation and 5-ALA-induced PpIX is essential to clearly define
the interactions between these components, multi-dose ionizing
irradiation with 5-ALA-induced PpIX may be an appropriate treatment
for patients with malignant brain tumors and should be assessed in
clinical trials.
Acknowledgements
This study was supported by JSPS KAKENHI (grant no.
25462282).
References
|
1
|
Mimura S, Ito Y, Nagayo T, et al:
Cooperative clinical trial of photodynamic therapy with photofrin
II and excimer dye laser for early gastric cancer. Lasers Surg Med.
19:168–172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mlkvy P, Messmann H, Pauer M, et al:
Distribution and photodynamic effects of
meso-tetrahydroxyphenylchlorin (mTHPC) in the pancreas and adjacent
tissues in the Syrian golden hamster. Br J Cancer. 73:1473–1479.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto J, Hirano T, Li S, et al:
Selective accumulation and strong photodynamic effects of a new
photosensitizer, ATX-S10. Na (II), in experimental malignant
glioma. Int J Oncol. 27:1207–1213. 2005.PubMed/NCBI
|
|
4
|
Stummer W, Pichlmeier U, Meinel T, et al:
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: a randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muller PJ and Wilson BC: Photodynamic
therapy for malignant newly diagnosed supratentorial gliomas. J
Clin Laser Med Surg. 14:263–270. 1996.PubMed/NCBI
|
|
6
|
Yamamoto J, Yamamoto S, Hirano T, et al:
Monitoring of singlet oxygen is useful for predicting the
photodynamic effects in the treatment for experimental glioma. Clin
Cancer Res. 12:7132–7139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schaffer M, Ertl-Wagner B, Schaffer PM, et
al: Feasibility of photofrin II as a radiosensitizing agent in
solid tumors - preliminary results. Onkologie. 29:514–519. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luksiene Z, Juzenas P and Moan J:
Radiosensitization of tumours by porphyrins. Cancer Lett.
235:40–47. 2006. View Article : Google Scholar
|
|
9
|
Berg K, Luksiene Z, Moan J and Ma L:
Combined treatment of ionizing radiation and photosensitization by
5-aminolevulinic acid-induced protoporphyrin IX. Radiat Res.
142:340–346. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schaffer M, Schaffer PM, Jori G, et al:
Radiation therapy combined with photofrin or 5-ALA: effect on Lewis
sarcoma tumor lines implanted in mice. Preliminary results. Tumori.
88:407–410. 2002.PubMed/NCBI
|
|
11
|
Takahashi J, Misawa M, Murakami M, et al:
5-Aminolevulinic acid enhances cancer radiotherapy in a mouse tumor
model. Springerplus. 2:6022013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto J, Ogura S, Tanaka T, et al:
Radiosensitizing effect of 5-aminolevulinic acid-induced
protoporphyrin IX in glioma cells in vitro. Oncol Rep.
27:1748–1752. 2012.PubMed/NCBI
|
|
13
|
Benda P, Someda K, Messer J and Sweet WH:
Morphological and immunochemical studies of rat glial tumors and
clonal strains propagated in culture. J Neurosurg. 34:310–323.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidek HH, Nielsen SL, Schiller AL and
Messer J: Morphological studies of rat brain tumors induced by
N-nitrosomethylurea. J Neurosurg. 34:335–340. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barth RF: Rat brain tumor models in
experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2
and CNS-1 gliomas. J Neurooncol. 36:91–102. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hagiya Y, Fukuhara H, Matsumoto K, et al:
Expression levels of PEPT1 and ABCG2 play key roles in
5-aminolevulinic acid (ALA)-induced tumor-specific protoporphyrin
IX (PpIX) accumulation in bladder cancer. Photodiagnosis Photodyn
Ther. 10:288–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishizuka M, Hagiya Y, Mizokami Y, et al:
Porphyrins in urine after administration of 5-aminolevulinic acid
as a potential tumor marker. Photodiagnosis Photodyn Ther.
8:328–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cerniglia GJ, Wilson DF, Pawlowski M,
Vinogradov S and Biaglow J: Intravascular oxygen distribution in
subcutaneous 9L tumors and radiation sensitivity. J Appl Physiol.
82:1939–1945. 1997.PubMed/NCBI
|
|
19
|
Abels C, Heil P, Dellian M, et al: In vivo
kinetics and spectra of 5-aminolaevulinic acid-induced fluorescence
in an amelanotic melanoma of the hamster. Br J Cancer. 70:826–833.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abels C, Fritsch C, Bolsen K, et al:
Photodynamic therapy with 5-aminolaevulinic acid-induced porphyrins
of an amelanotic melanoma in vivo. J Photochem Photobiol B.
40:76–83. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bozzini G, Colin P, Betrouni N, et al:
Efficiency of 5-ALA mediated photodynamic therapy on hypoxic
prostate cancer: a preclinical study on the Dunning R3327-AT2 rat
tumor model. Photodiagnosis Photodyn Ther. 10:296–303. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niclou SP, Danzeisen C, Eikesdal HP, et
al: A novel eGFP-expressing immunodeficient mouse model to study
tumor-host interactions. FASEB J. 22:3120–3128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prall F, Maletzki C and Linnebacher M:
Microdensitometry of osteopontin as an immunohistochemical
prognostic biomarker in colorectal carcinoma tissue microarrays:
potential and limitations of the method in ‘biomarker pathology’.
Histopathology. 61:823–832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kostron H, Swartz MR, Miller DC and
Martuza RL: The interaction of hematoporphyrin derivative, light,
and ionizing radiation in a rat glioma model. Cancer. 57:964–970.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito E, Yue S, Moriyama EH, et al:
Uroporphyrinogen decarboxylase is a radiosensitizing target for
head and neck cancer. Sci Transl Med. 3:67ra72011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
David S and Kroner A: Repertoire of
microglial and macrophage responses after spinal cord injury. Nat
Rev Neurosci. 12:388–399. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lynch MA: The multifaceted profile of
activated microglia. Mol Neurobiol. 40:139–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boehm U, Klamp T, Groot M and Howard JC:
Cellular responses to interferon-gamma. Annu Rev Immunol.
15:749–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hussain SF, Yang D, Suki D, Grimm E and
Heimberger AB: Innate immune functions of microglia isolated from
human glioma patients. J Transl Med. 4:152006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W and Graeber MB: The molecular profile
of microglia under the influence of glioma. Neuro Oncol.
14:958–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Filipazzi P, Huber V and Rivoltini L:
Phenotype, function and clinical implications of myeloid-derived
suppressor cells in cancer patients. Cancer Immunol Immunother.
61:255–263. 2012. View Article : Google Scholar
|
|
33
|
Skivka LM, Gorobets OB, Kutsenok VV, et
al: 5-aminolevulinic acid mediated photodynamic therapy of Lewis
lung carcinoma: a role of tumor infiltration with different cells
of immune system. Exp Oncol. 26:312–315. 2004.
|
|
34
|
Ishizuka M, Abe F, Sano Y, et al: Novel
development of 5-aminolevurinic acid (ALA) in cancer diagnoses and
therapy. Int Immunopharmacol. 11:358–365. 2011. View Article : Google Scholar
|
|
35
|
Higashikawa F, Noda M, Awaya T, Tanaka T
and Sugiyama M: 5-aminolevulinic acid, a precursor of heme, reduces
both fasting and postprandial glucose levels in mildly
hyperglycemic subjects. Nutrition. 29:1030–1036. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Starke RM, Williams BJ, Hiles C, et al:
Gamma knife surgery for skull base meningiomas. J Neurosurg.
116:588–597. 2012. View Article : Google Scholar
|
|
37
|
Torres RC, Frighetto L, De Salles AA, et
al: Radiosurgery and stereotactic radiotherapy for intracranial
meningiomas. Neurosurg Focus. 14:e52003. View Article : Google Scholar
|