Introduction
Necrosis and apoptosis are two common and important
patterns of cell death in ischemia-reperfusion injury. The process
of nerve cell death cannot be stopped, unlike apoptosis, which is a
programmed process (1,2); however, apoptosis caused by ischemic
brain injury can be prevented by interfering with the apoptotic
process. Glycogen synthase kinase-3β (GSK-3β) regulates the
β-catenin/Wnt signaling pathway and functions as a downstream
molecule of the phosphoinositide 3-kinase (PI3K)/Akt signaling
pathway (3,4). GSK-3β, an active
serine/threonine-protein kinase in cells, is widely involved in
regulating basic cellular functions including cell cycle, gene
expression and cytoskeleton stabilization via protein
phosphorylation (5,6). Abundant GSK-3 in the brain is also a
negative regulatory factor of signaling cascades, including
PI3K/Akt (7). GSK-3β can be
deactivated through Akt-mediated phosphorylation at serine 9, which
produces antiapoptotic effects (8,9).
During GSK-3β phosphorylation, β-catenin dissociates from the
anaphase-promoting complex, enters the nucleus and potentiates the
transcription of Wnt target genes, thereby promoting cell
proliferation and inhibiting apoptosis (10–12).
Previous studies on the regulation of β-catenin by Akt have
focussed on tumor prevention; however, the applicability of this
regulation in ischemic brain injury remains to be elucidated.
In the present study, basic fibroblast growth factor
(bFGF) and the PI3K inhibitor LY294002 were administered in a rat
model in vivo to activate and inhibit Akt activity,
respectively. The intracellular β-catenin content was also examined
to investigate the regulatory effects of Akt on β-catenin and to
further elucidate the mechanism of β-catenin in ischemic brain
injury.
Materials and methods
Experimental animals
A total of 96 healthy male Sprague Dawley rats aged
between 10 and 12 weeks (280–320 g) were provided by the Laboratory
Animal Center of Shenyang Medical College [Shenyang, China; animal
certification no. SCXK (liao) 2003-0016]. Animals were kept at
25–30°C in 60–70% relative humidity, with a 12 hour light:dark
cycle and ad libitum access to food and water. The rat
models of focal cerebral ischemia-reperfusion were established
according to the modified Longa’s (13) method and were randomly divided into
four groups with 24 rats in each group. These groups were labeled
as: The sham-operated (S), cerebral ischemia-reperfusion injury
(I), cerebral ischemia-reperfusion + bFGF post-processing (bFGF)
and cerebral ischemia-reperfusion + bFGF post-processing + LY294002
(LY) groups. Each group was further divided into four subgroups,
according to different reperfusion times of 12, 24, 48 and 72 h.
The present study was performed in accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
procedures were reviewed and approved by the Institutional Animal
Care and Use Committee of the First Affiliated Hospital, China
Medical University (Shenyang, China).
Animal models
The focal cerebral ischemia-reperfusion injury was
induced in the rats according to a modification of Longa’s method
(14). Anesthesia was induced via
intraperitoneal injection of 10% chloral hydrate (350 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA). A median cervical incision was
then performed to expose the right common carotid artery (CCA),
external carotid artery (ECA) and internal carotid artery (ICA).
Following ligation of the CCA, ECA and ICA, a 4-0 nylon suture with
a rounded end was inserted into the ICA 2 mm above the bifurcation
of the CCA until a slight resistance was felt. The insertion depth
of the suture was between 18 and 20 mm. For the sham-operated rats,
the insertion depth was 10 mm. After 2 h occlusion, reperfusion was
permitted by drawing the nylon suture. The room temperature was
maintained between 25 and 28°C during and following the surgery.
The rectal temperature of the rats was maintained at 37.0±0.5°C by
using incandescent lamps. The focal cerebral ischemia-reperfusion
injury was successfully induced when the conscious rats exhibited
hemiplegia on the left side of their bodies, specifically on the
left forelimb. Subsequently, 30 min after the induction of
ischemia-reperfusion injury, 10 μl physiological saline containing
1.2 μg bFGF (Sigma-Aldrich), was administered via the right lateral
ventricle. At each indicated time-point, 20 μl physiological saline
was injected into the right lateral ventricle of the rats in the S
and I groups. At 15 min prior to reperfusion, 0.3 mg/kg LY294002
(Cell Signaling Technology, Inc., Danvers, MA, USA) was injected
via the femoral vein. The rats from each group were then
re-anesthetized and administered with 4% paraformaldehyde via
cardiac perfusion at different reperfusion time-points (12, 24, 48
and 72 h). The whole brain was harvested and fixed for the
preparation of 5 μm paraffin (Sigma-Aldrich) sections, which were
stained with hematoxylin and eosin (Sigma-Aldrich).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)
An in situ end-labeling apoptosis detection
kit (Haoyang Biological Product Science and Technology Co. Ltd.,
Tianjin, China) was used according to the manufacturer’s
instructions. The paraffin sections were routinely dehydrated in a
series of graded ethanol solutions and treated with 3%
H2O2 (Zhongshan Gold Bridge Biotechnology
Co., Ltd., Beijing, China), for 10 min to deactivate endogenous
peroxidase. The sections were then subjected to microwave antigen
retrieval (Zhongshan Gold Bridge Biotechnology Co., Ltd.) and
incubated at 37°C for 1 h following the addition of TUNEL solution
and at 37°C for 30 min following the addition of propylene oxide
(Zhongshan Gold Bridge Biotechnology Co., Ltd.). Finally, the
sections were developed using diaminobenzidine (Zhongshan Gold
Bridge Biotechnology Co., Ltd.), dehydrated, cleared and mounted.
Sections were viewed using an Olympus DSX500 (Wuxi Lianfa
Technology Co., Ltd., Wuxi, China).
Immunohistochemical staining
The streptavidin-peroxidase method was used for
immunohistochemical analysis. The paraffin sections were dehydrated
in a series of graded ethanol (Zhongshan Gold Bridge Biotechnology
Co., Ltd.) solutions, treated with 3% H2O2 at
room temperature for 10 min, subjected to microwave antigen
retrieval, inhibited using 50 μl goat serum (Zhongshan Gold Bridge
Biotechnology Co., Ltd.) and incubated at room temperature for 20
min. Following the addition of phosphorylated (p-)Akt primary
antibody (1:200; Cell Signaling Technology, Inc.), the sections
were incubated at 4°C overnight. Biotinylated secondary working
solution (Zhongshan Gold Bridge Biotechnology Co. Ltd., Beijing,
China) was then added and incubated at 37°C for 30 min, followed by
addition of horseradish peroxidase (HRP)-conjugated streptavidin
(Zhongshan Gold Bridge Biotechnology Co., Ltd.) working solution
and incubation at 37°C for 30 min. The sections were developed
using diaminobenzidine and were then routinely dehydrated, cleared
and mounted. Phosphate-buffered saline (PBS; Zhongshan Gold Bridge
Biotechnology Co., Ltd.) was used instead of the primary antibody
in the negative control.
In situ hybridization
The present study used digoxin-labeled multiphase
oligonucleotide probes (Zhongshan Gold Bridge Biotechnology Co.,
Ltd.) and the sequence of GSK-3β was
5′-CTCCTCGGACCAGCTGCTTTGCACTTCCAA-3′. The sections were treated
with freshly prepared 0.5% H2O2 in methanol
at room temperature for 30 min and were digested with pepsin
(Zhongshan Gold Bridge Biotechnology Co., Ltd.), which was freshly
diluted with 3% citric acid. Subsequently, The sections were
pre-hybridized for 2 h in a thermostat container at 37°C and,
following addition of the hybridization solution, were incubated
overnight at 37°C. The sections were then treated with blocking
solution, biotinylated rat anti-digoxin, streptavidin-peroxidase
complex and biotinylated peroxidase (Zhongshan Gold Bridge
Biotechnology Co., Ltd.). Finally, the sections were routinely
dehydrated, cleared and mounted. PBS was used as the hybridization
solution in the negative control.
Western blot analysis
At each indicated time-point following
intraperitoneal injection of 10% chloral hydrate (350 mg/kg), the
brain of each rat was harvested and placed on ice; the cortical
tissues were stored at −70°C for later use. Following addition of
the homogenate (1:9; NaH2PO4
(H2O), NaH2PO4 ·12 H2O,
NaCl), the samples were lysed and homogenated on ice. The
supernatant was collected and protein levels were determined using
the Coomassie Brilliant blue G-250 method (15). Equal quantities of lysate protein
were run on 10% SDS-PAGE gels and electrophoretically transferred
onto a polyvinylidene difluoride membrane (Zhongshan Gold Bridge
Biotechnology Co., Ltd.). Following inhibition with 5% defatted
milk powder (Sigma-Aldrich), the blots were incubated overnight at
4°C. They were then incubated for 2 h at room temperature with the
β-catenin primary antibody (1:200) and for 1 h at room temperature
with the HRP-conjugated secondary antibody (1:200). Enhanced
chemiluminescence (ECL; Sigma-Aldrich) was used for the
visualization of the protein bands.
Statistical analysis
The average number of apoptotic cells was determined
using a MetaMorph/Evolution MP5.0 micro-imaging analysis system
(Alpha, USA). The average optical densities of the cortical p-Akt
and cortical GSK-3β mRNA and the integrated density value of the
cortical β-catenin band were determined by immunohistochemical
staining, in situ hybridization and western blot analysis,
respectively. All data were statistically analyzed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA) and are expressed as the
mean ± standard deviation. Analysis of variance was used to assess
the difference between the means. P<0.05 was considered to
indicate a statistically significant difference.
Results
bFGF improves survival of neuronal cells
following ischemia-reperfusion via activating the PI3K/Akt
signaling pathway
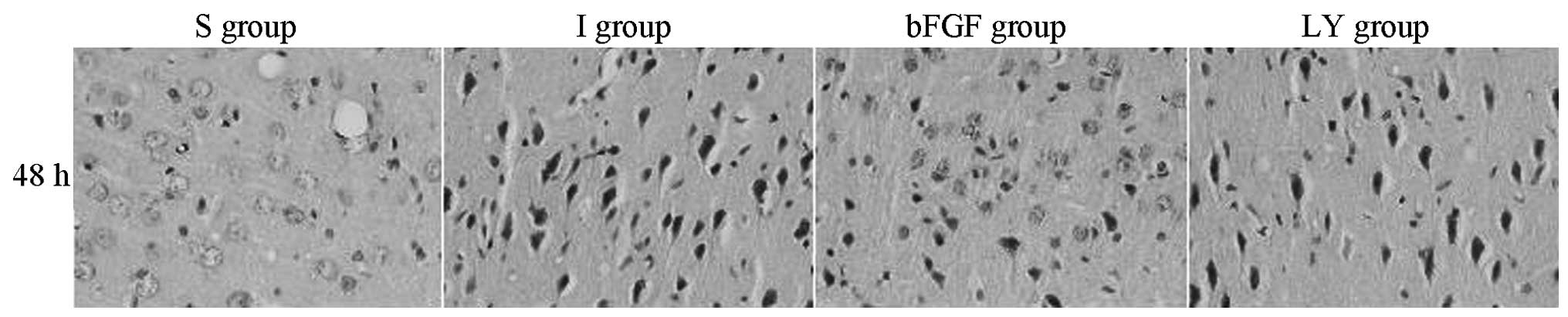
The histological changes in the right cortex, caused
by the cerebral ischemia-reperfusion injury, were evaluated under
an optical microscope (Fig. 1).
The S group exhibited a large number of orderly arranged neurons
with complete morphology. In the I and LY groups, the neurons
became plump and were distributed unevenly, with widened cellular
interspaces. Several cytons became small and the nuclei were
pyknotic and darkly stained. All of these characteristics were most
marked after 48 h. In the bFGF group, increased neuron survival was
observed in the right cortex compared with that in the I and LY
groups, cyton swelling was alleviated and cell morphology was
significantly improved. bFGF promoted the survival of the nerve
cells in the ischemic cortex inhibited by LY294002, a PI3K/Akt
signaling pathway-specific inhibitor. These findings suggested that
bFGF activated the PI3K/Akt signaling pathway.
bFGF inhibits apoptosis of neuronal cells
following ischemia-reperfusion via activating the PI3K/Akt
signaling pathway
The apoptosis of nerve cells in the right cortex,
induced by cerebral ischemia-reperfusion injury, was evaluated
under an optical microscope. The TUNEL-positive cells exhibited
yellow-brown granules in the nuclei. In the I and LY groups, the
number of apoptotic cells gradually increased with reperfusion
time, reaching a peak level at 48 h, followed by a gradual decrease
after 72 h. At the same indicated time-points, the numbers of
apoptotic cells in the I and LY groups were increased significantly
compared with those in the S group (P<0.05), whereas the numbers
of apoptotic cells in the bFGF group were significantly lower
compared with those in the I and LY groups (P<0.05; Fig. 2). bFGF inhibited the apoptosis of
nerve cells in the ischemic cortex and the effect of bFGF was
inhibited by LY294002. This observation suggested that bFGF
inhibited cellular apoptosis by activating the PI3K/Akt signaling
pathway.
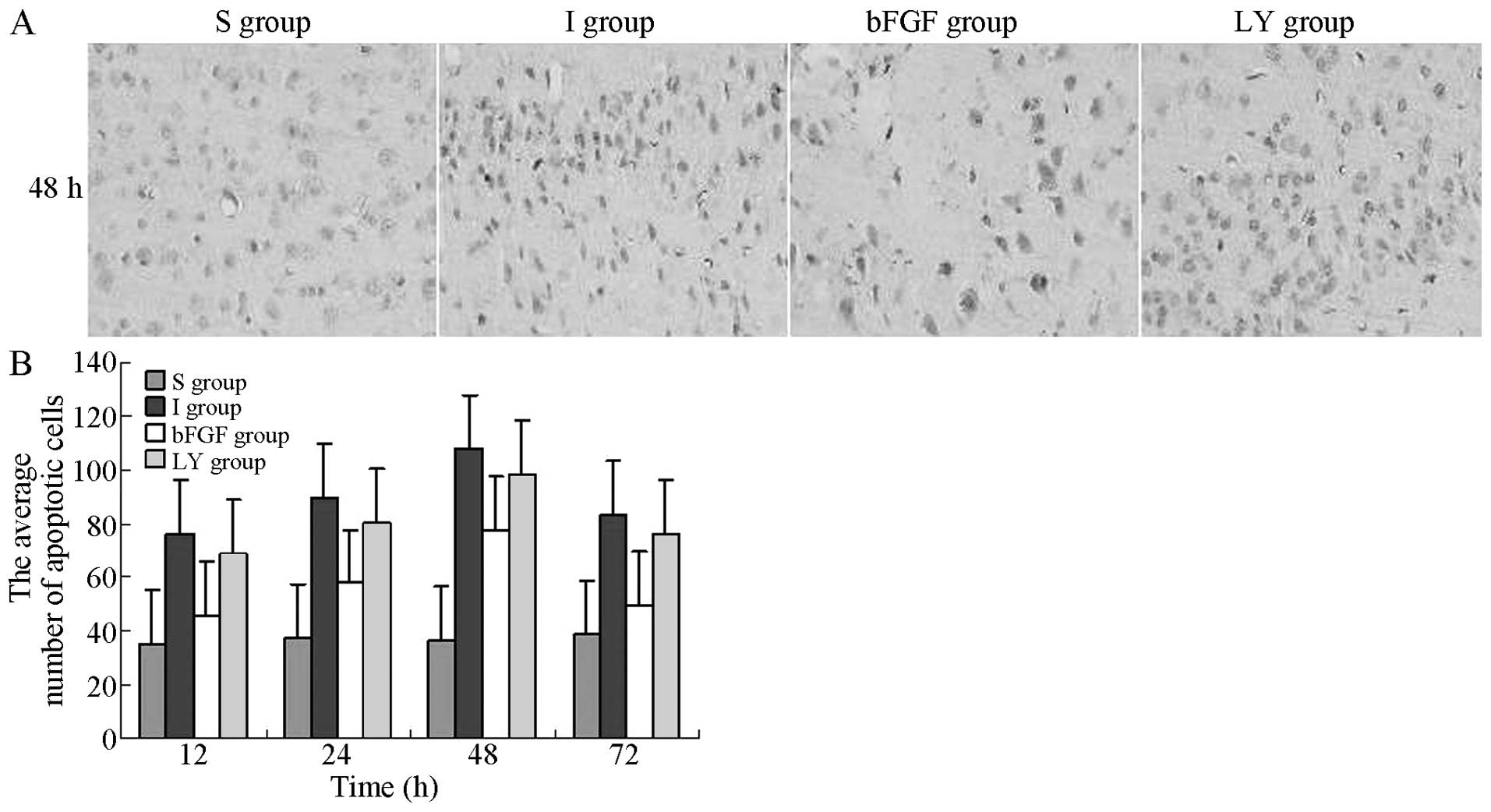 | Figure 2TUNEL detection of apoptotic cells in
the right cortex following ischemia-reperfusion injury in each
group. TUNEL-positive cells exhibited brown granules in the nuclei.
Magnification, ×400. (A) Number of apoptotic cells in the right
cortex 12, 24, 48 and 72 h after ischemia-reperfusion injury. (B)
Average number of apoptotic cells 48 h after ischemia-reperfusion
injury. The average number of apoptotic cells in the right cortex
at each indicated time-point is expressed as the mean ± standard
deviation (n=6 for each time-point). P<0.05, I group or LY
group, vs. S group; P<0.05, bFGF group, vs. I group or LY group.
S, sham-operated; I, cerebral ischemia-reperfusion injury; bEGF,
cerebral ischemia-reperfusion + bFGF post-processing; LY, cerebral
ischemia-reperfusion + bFGF post-processing + LY294002; TUNEL,
terminal deoxynucleotidyl transferase dUTP nick end labeling; bFGF,
basic fibroblast growth factor. |
bFGF activates cortical Akt following
ischemia-reperfusion injury
Following ischemia-reperfusion injury, the
expression of cortical p-Akt was investigated at each time-point by
immunohistochemical staining in each group to determine whether
ischemia-reperfusion injury and bFGF activated Akt. In the S group,
a marginal expression of p-Akt was observed in the cortex. In the I
and LY groups, the expression of p-Akt appeared in the right cortex
at 12 h, peaked at 24 h and gradually decreased following this. Of
note, the expression of p-Akt in the groups that underwent
ischemia-reperfusion remained significantly higher compared with
that in the S group (P<0.05; Fig.
3). In the bFGF group, the expression of p-Akt exhibited a
similar trend; however, the expression levels were significantly
higher compared with those in the I and LY groups (P<0.05;
Fig. 3). These findings suggested
that bFGF activated Akt in ischemic brain injury and that the
effect of bFGF was inhibited by LY294002, an inhibitor of the
PI3K/Akt signaling pathway.
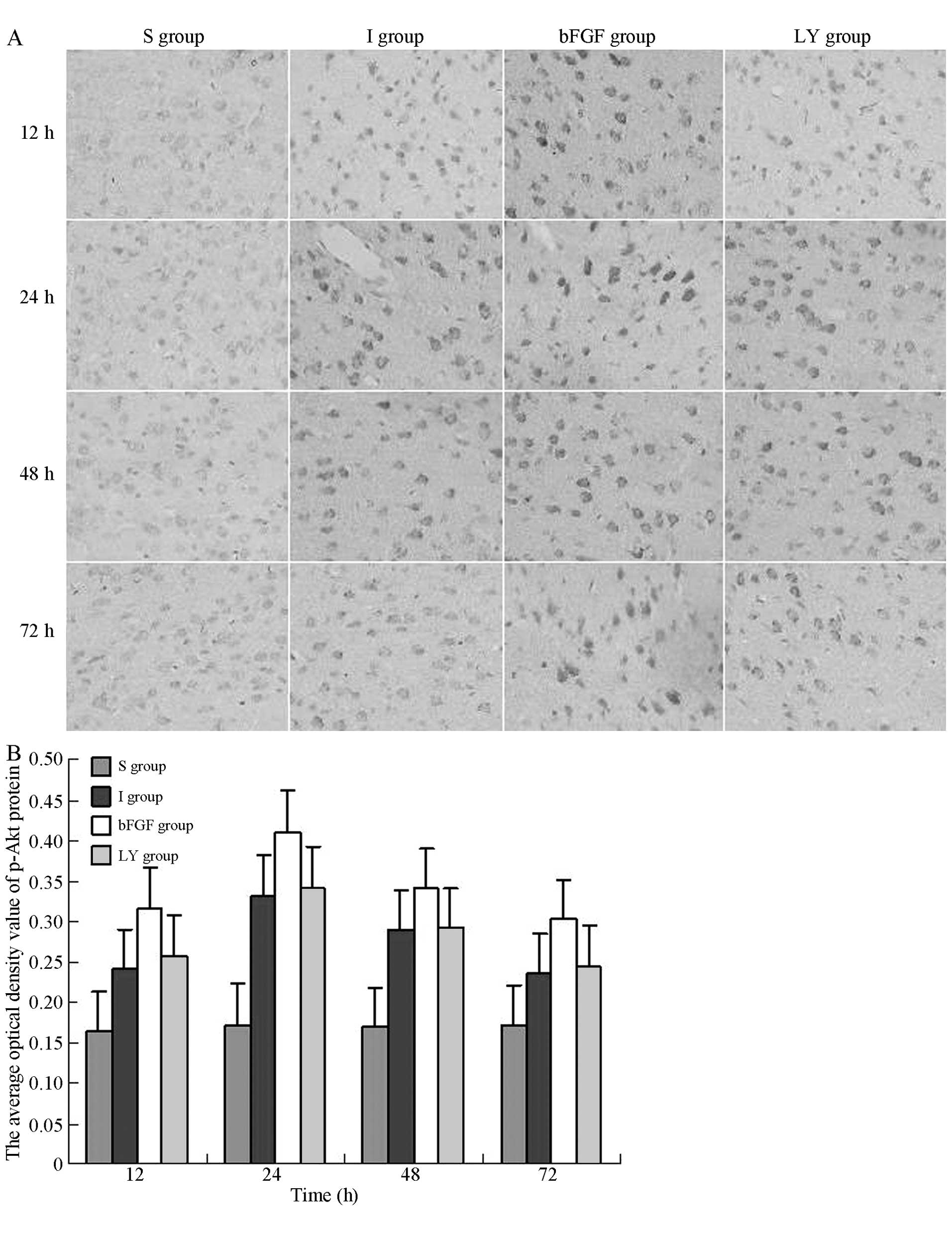 | Figure 3Immunohistochemical assessment of the
cortical expression of p-Akt following ischemia-reperfusion injury.
p-Akt immunoreactive cells exhibited brown granules in the
cytoplasm and nuclei. Magnification, ×400. (A) p-Akt-positive
protein in the right cortex 12, 24, 48 and 72 h after
ischemia-reperfusion injury. (B) Average optical density values of
p-Akt-positive protein in each group. All data are expressed as the
mean ± standard deviation (n=6 rats for each time-point).
P<0.05, I group or LY group, vs S group; P<0.05, bFGF group,
vs. I group or LY group. S, sham-operated; I, cerebral
ischemia-reperfusion injury; bEGF, cerebral ischemia-reperfusion +
bFGF post-processing; LY, cerebral ischemia-reperfusion + bFGF
post-processing + LY294002; p-, phosphorylated; bFGF, basic
fibroblast growth factor. |
bFGF suppresses cortical mRNA expression
of GSK-3β following ischemia-reperfusion injury
To investigate whether ischemia-reperfusion injury
and bFGF activated GSK-3β, the mRNA expression levels of GSK-3β at
each indicated time-point following ischemia-reperfusion injury
were investigated. The in situ hybridization assay indicated
that GSK-3β mRNA was expressed in the right cortex in the I and LY
groups; the mRNA expression of GSK-3β appeared 12 h after
reperfusion, peaked at 48 h and decreased after 72 h. Of note, the
GSK-3β expression levels in the I and LY groups were higher
compared with those in the S group (P<0.05; Fig. 4). The mRNA expression of GSK-3β in
the right cortex was significantly decreased in the bFGF group
compared with those in the I and LY groups (P<0.05; Fig. 4). bFGF inhibited the mRNA
expression of GSK-3β in the cortex subjected to
ischemia-reperfusion injury. LY294002, the PI3 K/Akt pathway
inhibitor, upregulated the mRNA expression of GSK-3β and
effectively inhibited the effect of bFGF.
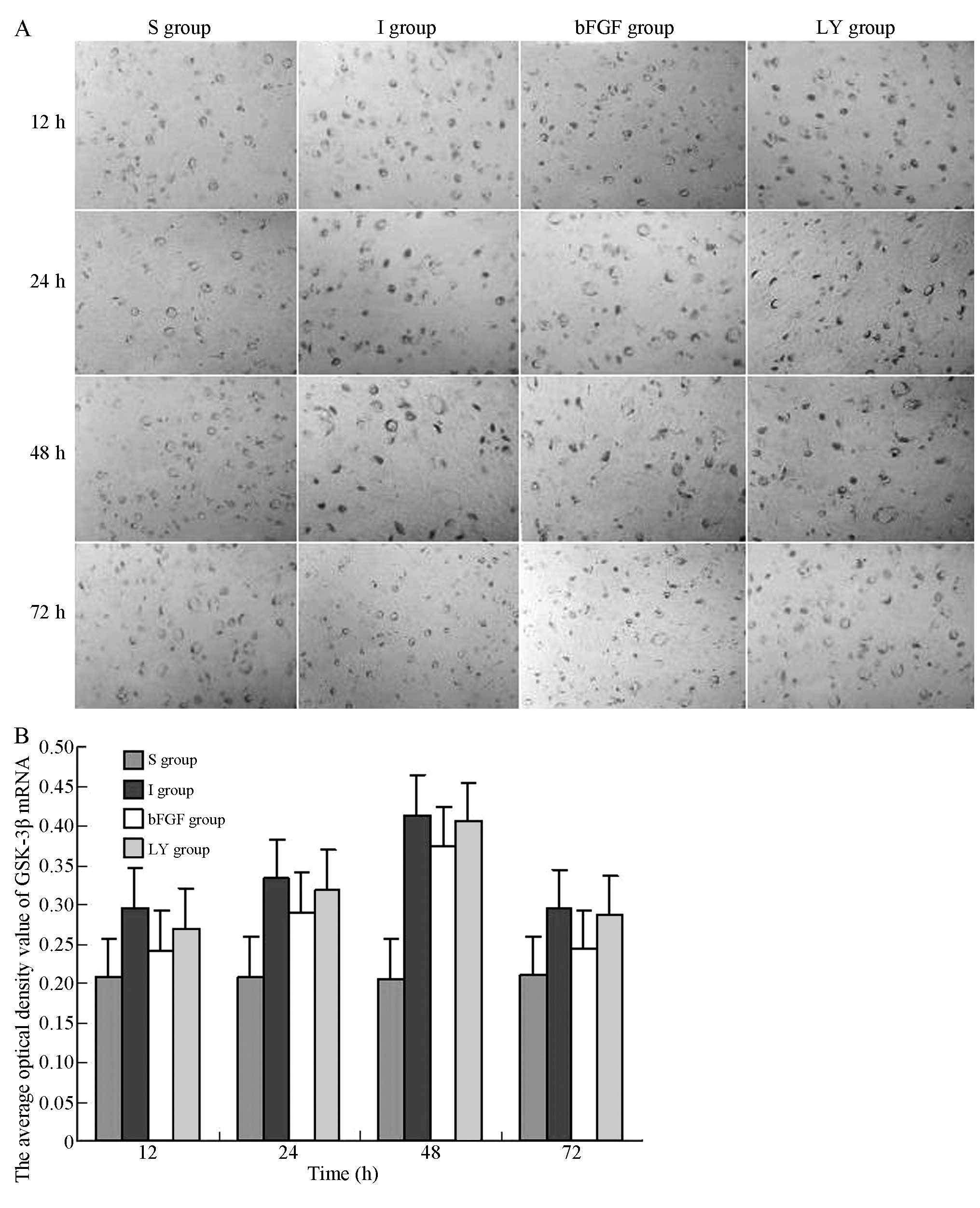 | Figure 4In situ hybridization for the
detection of the mRNA expression of GSK-3β in the right cortex
following ischemia-reperfusion injury. Magnification, ×400. (A)
mRNA expression levels of GSK-3β in the right cortex 12, 24, 48, 72
h after ischemia-reperfusion injury. (B) Average optical density
values of GSK-3β mRNA-positive product in each group. All data are
expressed as the mean ± standard deviation (n=6 rats for each
time-point). P<0.05, I group or LY group, vs. S group;
P<0.05, bFGF group, vs. I group or LY group. S, sham-operated;
I, cerebral ischemia-reperfusion injury; bEGF, cerebral
ischemia-reperfusion + bFGF post-processing; LY, cerebral
ischemia-reperfusion + bFGF post-processing + LY294002; GSK-3β,
glycogen synthase kinase-3β; bFGF, basic fibroblast growth
factor. |
bFGF promotes cortical β-catenin
expression following ischemia-reperfusion injury
Western blot analysis was used to detect the
expression of β-catenin at each time-point following
ischemia-reperfusion injury and to evaluate the effects of bFGF and
LY294002 on the expression of β-catenin. As Fig. 5A shows, the protein levels of
β-catenin in the I and LY groups were reduced compared with those
in the bFGF group, but higher compared with those in the S group.
At 12, 24, 48 and 72 h after reperfusion, the optical densities of
β-catenin in the I and LY groups were significantly higher compared
with those in the S group, but were significantly lower compared
with those in the bFGF group (P<0.05; Fig. 5B). bFGF promoted the expression of
β-catenin in the cortex subjected to ischemia-reperfusion injury;
however, this promoting effect was inhibited by inhibition of the
PI3K/Akt signaling pathway.
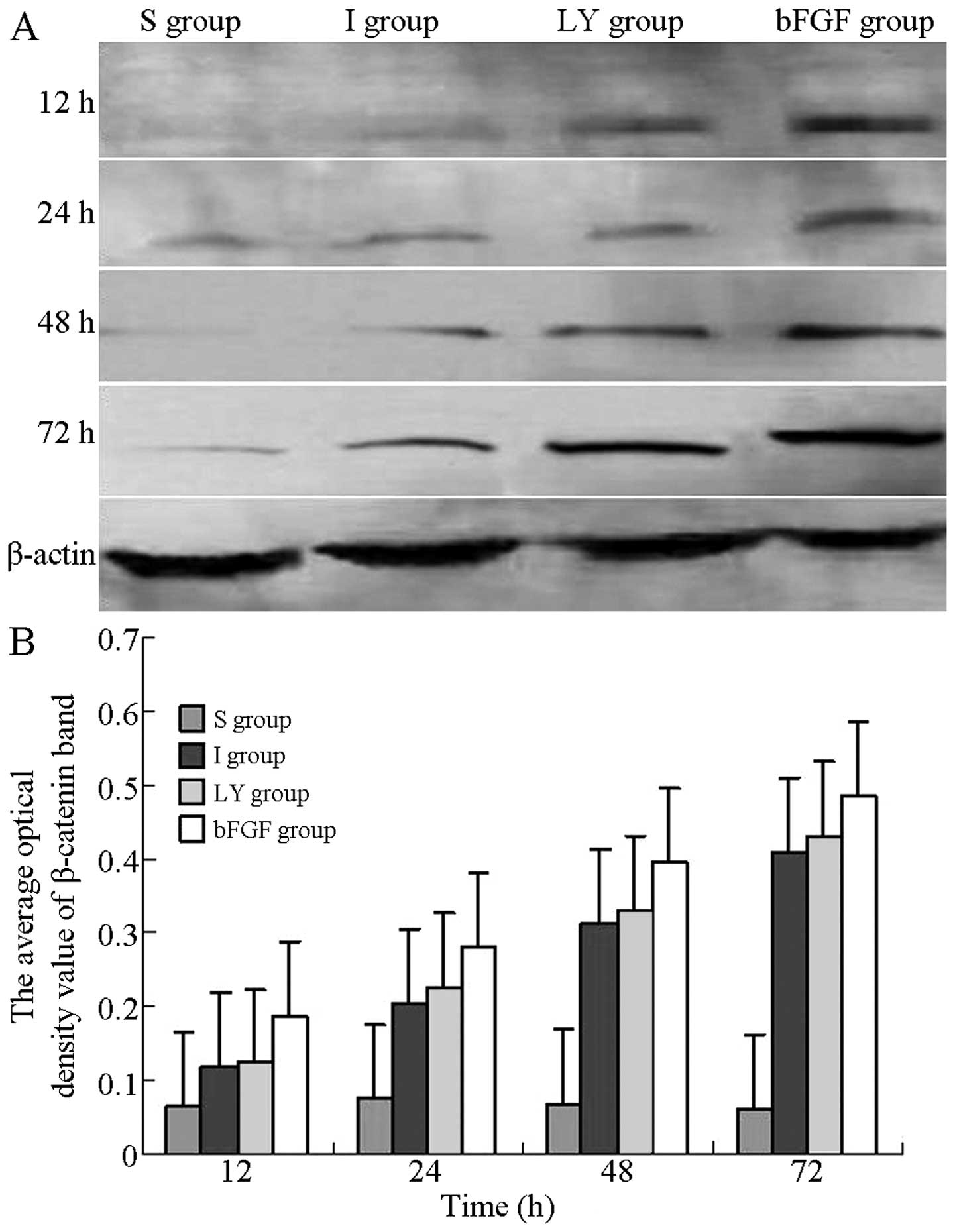 | Figure 5Western blot analysis for detection of
the expression of β-catenin in the right cortex following
ischemia-reperfusion injury. (A) Protein expression of β-catenin in
the right cortex 12, 24, 48 and 72 h after ischemia-reperfusion
injury. (B) Optical density values of the β-catenin band at each
time-point of reperfusion. All data are expressed as the mean ±
standard deviation (n=6 rats for each time-point). P<0.05, I or
LY group, vs. S group; P<0.05, bFGF group, vs. I or LY group. S,
sham-operated; I, cerebral ischemia-reperfusion injury; bEGF,
cerebral ischemia-reperfusion + bFGF post-processing; LY, cerebral
ischemia-reperfusion + bFGF post-processing + LY294002; bFGF, basic
fibroblast growth factor. |
Discussion
β-catenin, a protein molecule with a relative
molecular weight of 92,000 Da, was initially identified to
co-precipitate with the E-cadherin cell-cell adhesive complex and
was subsequently found to link E-cadherin to α-catenin, which
linked the E-cadherin/catenin complex to the cortical cytoskeleton
(16,17). Previous studies have suggested that
β-catenin is also involved in Wnt signaling (18,19).
During Wnt signaling, β-catenin, without binding to E-cadherin in
the cytoplasm, acts as a signal transduction and transcription
factor, enters the nucleus, binds to TCF/LEF and regulates the
transcription of target genes. Thus, β-catenin is important in cell
proliferation, embryonic development and tumor formation (20–22).
Akt contains serine/threonine protein kinase and, following
phosphorylation, Akt phosphorylates numerous intercellular
substrate proteins and regulates their activities (23,24).
Therefore, Akt is involved in regulating a number of intercellular
signal pathways, promoting cell survival and proliferation,
preventing cellular apoptosis and regulating glycometabolism and
protein synthesis (25–27).
bFGF, a polypeptide with various biological
activities, functions in an identical manner as a neurotrophic
factor (28–30) and promotes the survival of nerve
cells via numerous conduction pathways. To the best of our
knowledge, no previous studies have investigated whether bFGF can
activate the Wnt/β-catenin and PI3K/Akt signaling pathways
simultaneously in ischemic brain injury. In the present study,
LY294002, an inhibitor of the PI3K/Akt signaling pathway with high
specificity and efficacy, was used to investigate the effects of
inhibiting the PI3K/Akt signaling pathway and suppressing the
expression of β-catenin, the main member of the Wnt/β-catenin
signaling pathway. Immunohistochemical staining results revealed
that, at each indicated time-point, the protein expression levels
of p-Akt in the I and LY groups were higher compared with those in
the S group and peaked at 24 h. However, at each indicated
time-point, the cortical expression levels of p-Akt in the bFGF
group were higher compared with the levels in the I and LY groups.
These findings suggested that, in ischemic brain injury, bFGF
activated the PI3K/Akt signaling pathway and LY294002, as an
inhibitor of the PI3K/Akt signaling pathway, effectively inhibited
the bFGF-mediated activation of Akt. In situ hybridization
and western blot analysis were also used to investigate the mRNA
expression of GSK-3β and the expression of β-catenin in the
ischemia-reperfusion cortex. The results demonstrated that, at each
indicated time-point, the mRNA expression of GSK-3β and the
expression of β-catenin in the I and LY groups were significantly
higher compared with those in the S group and peaked at 48 and 72
h, respectively. Compared with the I and LY groups, the mRNA
expression of GSK-3β in the bFGF group was significantly decreased,
whereas the protein expression of β-catenin was significantly
increased at each time-point. These findings suggested that bFGF
activated Akt, inhibited GSK-3β activity and antagonized the
β-catenin-degradation complex, composed of GSK-3β, axin and
adenomatosis polyposis coli protein. In this process, β-catenin
cannot be phosphorylated and the dissociated β-catenin accumulates
in the cytoplasm, enters the nucleus, promotes Wnt target gene
transcription and inhibits cellular apoptosis (31–33).
When the PI3K/Akt signaling pathway was inhibited by LY294002,
β-catenin was degraded by adenomatosis polyposis coli polyprotein
and its expression in the ischemic cortex was reduced. The
expression of Akt in the cortex peaked 24 h after
ischemia-reperfusion injury and the expression of β-catenin
increased continuously following ischemia-reperfusion and peaked
after 72 h. These findings suggested that β-catenin was involved in
signal conduction following PI3K/Akt activation and the Akt
regulation of β-catenin affected ischemic brain injury. In
addition, Akt and β-catenin were activated by bFGF via the
bFGF-Akt-GSK-3β-β-catenin signaling cascade.
The present study established a rat model of focal
cerebral ischemia-reperfusion injury, confirmed by
histopathological changes and neuronal apoptosis using hematoxylin
and eosin staining and the TUNEL method. In the bFGF group, few
histological changes or apoptotic cells were observed in the
ischemic cortex, whereas, the protective effect of bFGF was
inhibited in the LY group. These findings suggested that the
PI3K/Akt signaling pathway has a significant function in cell
survival and that, by regulating β-catenin, the PI3K/Akt and Wnt
signaling pathways exhibit protective effects on the brain. This
observation indicates that Akt and β-catenin may be significant
target molecules for the treatment of ischemic brain injury.
Acknowledgements
This study was supported by the Science Research
Plan of Shenyang (no. F11-262-9-17).
References
|
1
|
Guo WP, Fu XG, Jiang SM and Wu JZ:
Neuregulin-1 regulates the expression of Akt, Bcl-2, and Bad
signaling after focal cerebral ischemia in rats. Biochem Cell Biol.
88:649–654. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu J, Shen W, Gao L, et al:
PI3K/Akt-independent negative regulation of JNK signaling by MKP-7
after cerebral ischemia in rat hippocampus. BMC Neurosci. 14:12013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forde JE and Dale TC: Glycogen synthase
kinase 3: a key regulator of cellular fate. Cell Mol Life Sci.
64:1930–1944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye Z, Guo Q, Xia P, Wang N, Wang E and
Yuan Y: Sevoflurane postconditioning involves an up-regulation of
HIF-1α and HO-1 expression via PI3K/Akt pathway in a rat model of
focal cerebral ischemia. Brain Res. 1463:63–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dugo L, Collin M and Thiemermann C:
Glycogen synthase kinase 3beta as a target for the therapy of shock
and inflammation. Shock. 27:113–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishihara M, Miura T, Miki T, et al:
Modulation of the mitochondrial permeability transition pore
complex in GSK-3beta-mediated myocardial protection. J Mol Cell
Cardiol. 43:564–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao H, Sapolsky RM and Steinberg GK:
Phosphoinositide-3-kinase/akt survival signal pathways are
implicated in neuronal survival after stroke. Mol Neurobiol.
34:249–270. 2006. View Article : Google Scholar
|
|
8
|
Gao X, Zhang H, Takahashi T, et al: The
Akt signaling pathway contributes to postconditioning’s protection
against stroke; the protection is associated with the MAPK and PKC
pathways. J Neurochem. 105:943–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng B, Guo QL, He ZJ, et al: Remote
ischemic postconditioning protects the brain from global cerebral
ischemia/reperfusion injury by up-regulating endothelial nitric
oxide synthase through the PI3K/Akt pathway. Brain Res.
1445:92–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chong ZZ, Li F and Maiese K: Cellular
demise and inflammatory microglial activation during beta-amyloid
toxicity are governed by Wnt1 and canonical signaling pathways.
Cell Signal. 19:1150–1162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murase S, Mosser E and Schuman EM:
Depolarization drives beta-catenin into neuronal spines promoting
changes in synaptic structure and function. Neuron. 35:91–105.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Zhang ZG, Liu XS, Hozeska-Solgot
A and Chopp M: The PI3K/Akt pathway mediates the neuroprotective
effect of atorvastatin in extending thrombolytic therapy after
embolic stroke in the rat. Arterioscler Thromb Vasc Biol.
27:2470–2475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carmichael ST: Rodent models of focal
stroke: size, mechanism, and purpose. NeuroRx. 2:396–409. 2005.
View Article : Google Scholar
|
|
14
|
Liu H, Liu X, Wei X, et al: Losartan, an
angiotensin II type 1 receptor blocker, ameliorates cerebral
ischemia-reperfusion injury via PI3K/Akt-mediated enos
phosphorylation. Brain Res Bull. 89:65–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bradford MM: A rapid and sensitive method
for quantitation of microgram quantities of protein utilizing the
principle of protein-dye binding. Anal Biochem. 72:248–254. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu C, Liu L, Chen Y, et al: TLR2 ligand
induces protection against cerebral ischemia/reperfusion injury via
activation of phosphoinositide 3-kinase/Akt signaling. J Immunol.
187:1458–1466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shioda N, Ishigami T, Han F, et al:
Activation of phosphatidylinositol 3-kinase/protein kinase B
pathway by a vanadyl compound mediates its neuroprotective effect
in mouse brain ischemia. Neuroscience. 148:221–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soshnikova N, Zechner D, Huelsken J, et
al: Genetic interaction between Wnt/beta-catenin and BMP receptor
signaling during formation of the AER and the dorsal-ventral axis
in the limb. Genes Dev. 17:1963–1968. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J and Wynshaw-Boris A: The canonical
Wnt pathway in early mammalian embryogenesis and stem cell
maintenance/differentiation. Curr Opin Genet Dev. 14:533–539. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lickert H, Domon C, Huls G, et al:
Wnt/(beta)-catenin signaling regulates the expression of the
homeobox gene Cdx1 in embryonic intestine. Development.
127:3805–3813. 2000.PubMed/NCBI
|
|
21
|
Monga SP, Pediaditakis P, Mule K, Stolz DB
and Michalopoulos GK: Changes in WNT/beta-catenin pathway during
regulated growth in rat liver regeneration. Hepatology.
33:1098–1109. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamada H, Nito C, Endo H and Chan PH: Bad
as a converging signaling molecule between survival PI3-K/Akt and
death JNK in neurons after transient focal cerebral ischemia in
rats. J Cereb Blood Flow Metab. 27:521–533. 2007. View Article : Google Scholar
|
|
24
|
Sun B, Chen L, Wei X, Xiang Y, Liu X and
Zhang X: The Akt/GSK-3β pathway mediates flurbiprofen-induced
neuroprotection against focal cerebral ischemia/reperfusion injury
in rats. Biochem Biophys Res Commun. 409:808–813. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chai YS, Hu J, Lei F, et al: Effect of
berberine on cell cycle arrest and cell survival during cerebral
ischemia and reperfusion and correlations with p53/cyclin D1 and
PI3K/Akt. Eur J Pharmacol. 708:44–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhan L, Li D, Liang D, et al: Activation
of Akt/FoxO and inactivation of MEK/ERK pathways contribute to
induction of neuroprotection against transient global cerebral
ischemia by delayed hypoxic postconditioning in adult rats.
Neuropharmacology. 63:873–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Deng Z, Liao J, et al: Leptin
attenuates cerebral ischemia injury through the promotion of energy
metabolism via the PI3K/Akt pathway. J Cereb Blood Flow Metab.
33:567–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma YP, Ma MM, Cheng SM, et al: Intranasal
bFGF-induced progenitor cell proliferation and neuroprotection
after transient focal cerebral ischemia. Neurosci Lett. 437:93–97.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maric D, Fiorio Pla A, Chang YH and Barker
JL: Self-renewing and differentiating properties of cortical neural
stem cells are selectively regulated by basic fibroblast growth
factor (BFGF) signaling via specific FGF receptors. J Neurosci.
27:1836–1852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing Y, Zhang X, Zhao K, et al: Beneficial
effects of sulindac in focal cerebral ischemia: a positive role in
Wnt/β-catenin pathway. Brain Res. 1482:71–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai L, Ye Z, Zhou BY, Mali P, Zhou C and
Cheng L: Promoting human embryonic stem cell renewal or
differentiation by modulating Wnt signal and culture conditions.
Cell Res. 17:62–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Endo H, Nito C, Kamada H, Nishi T and Chan
PH: Activation of the Akt/GSK3beta signaling pathway mediates
survival of vulnerable hippocampal neurons after transient global
cerebral ischemia in rats. J Cereb Blood Flow Metab. 26:1479–1489.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spaccapelo L, Galantucci M, Neri L, et al:
Up-regulation of the canonical Wnt-3A and sonic hedgehog signaling
underlies melanocortin-induced neurogenesis after cerebral
ischemia. Eur J Pharmacol. 707:78–86. 2013. View Article : Google Scholar : PubMed/NCBI
|