Introduction
Post-traumatic stress disorder (PTSD) is a mental
and behavioral disorder that occurs as a result of exposure to
traumatic events. Patients exhibit a number of characteristic
symptoms, including avoiding stimuli that they associate with the
trauma, continuously re-experiencing the traumatic event, a general
numbing of responsiveness and hyperarousal (1,2).
Following exposure to the danger of injury or death PTSD may
develop and seriously affect the patient’s quality of life and
social stability (3). The
pathophysiology of PTSD has been widely studied in neuroscience.
However, the underlying mechanisms behind PTSD are yet to be
elucidated.
Numerous studies have revealed that the amygdala,
hippocampus and medial prefrontal cortex (mPFC) are closely
associated with the occurrence of PTSD (4). The mPFC is a higher-order structure
that controls the stress and fear responses of the amygdala and the
hippocampus (5). Computed
tomography and functional magnetic resonance imaging have confirmed
that the mPFC in the brains of patients with PTSD is significantly
smaller than that of healthy individuals, and that their emotional
adjustment function is weakened (6,7). A
previous study have also confirmed that the aerobic function of the
prefrontal cortex in a rat model of PTSD was reduced, the neuronal
mitochondria were destroyed, cytochrome oxidase release was
enhanced and the neurons were damaged (8). In addition, the study indicated that
the mPFC neurons of PTSD rats underwent apoptosis, leading to
changes in the structure of the mPFC and a decrease in its volume,
which may cause a functional decline. It has been reported that
neuronal apoptosis of the amygdala, hippocampus and mPFC are
associated with the pathogenesis of PTSD (9,10).
Apoptosis is a genetically programmed,
morphologically distinct type of cell death, which is triggered by
a range of pathological and physiological stimuli. Integrins are
transmembrane proteins that are expressed in neurons and are
involved in numerous physiological and pathological processes
within the central nervous system (11). A previous study found that in
neurodegenerative diseases, integrin αv was closely associated with
synaptic function disorders, changes in plasticity, long-term
potentiation inhibition and the death and regeneration of neurons
(12). Focal adhesion proteins
activate the integrin-mediated signal transduction pathway between
the extracellular matrix (ECM) and cytoskeletal proteins, thus
regulating the physiological functions of a number of cells,
including neurons (13). Changes
in cytoskeletal proteins affect cell function, and may activate the
suicide program of neurons, causing apoptosis. In addition, these
changes may affect apoptosis through gap junctional communication
among cells. Under this effect, signals initiating apoptosis are
diffused through gap junction transfer, thus accelerating the
distribution of apoptosis.
Considering the complexity of conducting studies of
PTSD in humans, a number of animal models have been developed to
replicate the various kinds of trauma that may cause PTSD.
Single-prolonged stress (SPS) is a reliable animal model of PTSD
based on the time-dependent dysregulation of the
hypothalamic-pituitary-adrenal axis, and as such it has been
developed and employed for use in PTSD studies (14,15).
In effort to elucidate the mechanisms underlying the
PTSD-associated reduction in function of the mPFC, the current
study employed the rat SPS model to investigate the changes in
neuronal apoptosis and the expression levels of integrin αv,
vinculin and connexin43 in the mPFC in order to ascertain the
correlation between three proteins and neuronal apoptosis. In
addition, these observations may provide an experimental basis for
further investigation of the mechanism of PTSD.
Materials and methods
SPS model and experimental groups
The Wistar rats were supplied by and were
conventionally housed in the Experimental Animal Center of China
Medical University (Shenyang, China). The rats were housed
individually in clear polycarbonate cages (46×24×20 cm) for one
week prior to the experiments. All rats were habituated to their
cage and given standard food pellets and water. They were housed
under a reversed 12 h:12 h light/dark cycle (lights off at 10.00
am), at an ambient temperature (23±2°C) with a humidity of 55±5%. A
total of 120 male Wistar rats (7–8 weeks, 150–180 g), were randomly
divided into a control and four SPS groups (1, 4, 7 and 14 days
post-SPS). The models of PTSD were established using SPS as
determined by international PTSD scientific meetings (2). Briefly, the rats were detained for 2
h and immediately underwent a 20-min forced swim in 25°C water in a
40-cm deep tub. Following a 15 min rest period, the rats were
anesthetized with ethyl ether (Suzhou Jin Pure Chemical, Co., Ltd.,
Suzhou, China) until loss of consciousness. All animal experiments
were performed in accordance with the guidance suggestions for the
Care and Use of Laboratory Animals were supplied by the Ministry of
Science and Technology of the People’s Republic of China and
approved by the welfare and ethics committee of experimentation,
China Medical University. The present study was approved by the
ethics committee of the National Natural Science Foundation of
China (no. 81171282).
Transmission electron microscope
The rats of each group were anesthetized with 10 %
chloral hydrate (Suzhou Jin Pure Chemical Co., Ltd.). The
hearts were exposed, and the left ventricles were perfused with
200–300 ml of 0.9% saline via a catheter through the ascending
aorta until a colorless infusion was achieved, followed by
perfusion with 300 ml of 4% paraformaldehyde (Suzhou Jin Pure
Chemical Co., Ltd.). The whole brains were rapidly removed and
dissected on ice, followed by 6–10 h of post-fixation in 4%
paraformaldehyde at 4°C. Following being immersed in 20% sucrose
solution, glutaraldehyde-fixed (Alfa Aesar China Chemical Co.,
Ltd., Shanghai, China) mPFC samples were dehydrated with gradient
ethanol and acetone (Suzhou Jin Pure Chemical Co., Ltd.) and were
embedded with EPON 812 (SPI Supplies, West Chester, PA, USA) epoxy
resin. The 70 nm-thick ultrathin sections were stained with uranyl
acetate (Shanghai Resonance Biological Science and Technology Co.,
Ltd., Shanghai China) and lead citrate (Suzhou Jin Pure Chemical
Co., Ltd.) and were observed with JEOL1200EX at 100KV.
Light microscopy
Formaldehyde (Suzhou Jin Pure Chemical Co.,
Ltd.)-fixed mPFC samples were embedded in paraffin (Shanghai Hua
Ling rehabilitation Machinery Factory, Shanghai China) and sliced
into 10 μm thick slices for light microscopy.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) method
The TUNEL staining was performed according to the
manufacturer’s instructions (KeyGen Biotech Co., Ltd., Nanjing,
China). The apoptosis positive cells were counted under a
high-magnification microscope (860; Olympus, Tokyo, Japan).
Immunohistochemistry for integrin αv,
vinculin and connexin43
Immunohistochemical staining was performed using PV
two-step immunohistochemical detection kit (Beijing Shan Jinqiao
Biological Technology Co., Ltd., Beijing China), integrin αv,
vinculin and connexin43 positive cells were detected using mouse
anti-rat integrin αv monoclonal antibody (1:50), mouse anti-rat
vinculin monoclonal antibody (1:300) and rabbit anti-connexin43
polyclonal antibody (1:200) (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) respectively. Briefly, sections were incubated with
3% H2O2 at 37°C for 30 min, repaired by
microwave, blocked with dripped 10% goat serum for 30 min,
incubated with primary antibody at 4°C overnight, visualized with a
PV two-step immunohistochemical detection kit, and re-stained with
hematoxylin (Suzhou Jin Pure Chemical Co., Ltd.). The Image-Pro
Plus image analysis system (Media Cybernetics, Inc., Rockville, MD,
USA) was used to analyze the average optical density (OD).
Western blot analysis for integrin αv,
vinculin and connexin43
Briefly, the mPFC homogenates were prepared and the
protein concentration was determined using the Coomassie Brilliant
Blue method. The homogenates (20 μg) were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a
condensate gel and the protein was transferred to a PVDF membrane
(Millipore, Bedford, MA, USA). Integrin αv (1:300), vinculin
(1:1,500) and connexin43 (1:1,200) antibodies were used to probe,
respectively. The signals were detected using horseradish
peroxidase-labeled immunoglobulin G (1:200) and enhanced
chemiluminescence methods. Three bands were subjected to
semi-quantitative analysis.
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR) detection for integrin αv and
connexin43
Total RNA was extracted from the mPFC cells using
TRIzol® (Life Technologies, Carlsbad, CA, USA). The
purity and concentration of the RNA was detected using the
analyzer. Reverse transcription was conducted using the RNA PCR kit
(AMV) Ver 3.0 according to the manufacturer’s instructions (Takara
Bio, Inc., Otsu, Japan). The specific primers were synthesized by
Shenggong Biological Engineering Technology and Services Co., Ltd.
(Shanghai, China). The primer sequences used for PCR amplification
are presented in Table I. PCR
products were semi-quantified on 1% agarose gel using
electrophoresis, and the density of each band was analyzed on the
Gel Image Analysis system (Tanon 2500R; Tanon, Shanghai, China).
The levels of integrin αv and connexin43 mRNA were normalized to
β-actin.
 | Table IPrimers for integrin αv, connexin43
and β-actin. |
Table I
Primers for integrin αv, connexin43
and β-actin.
| Gene | Primer | Product size
(bp) |
|---|
| Integrin αv | Sense:
5′-TCGTTTCTATCCCACCGC-3′
Antisense: 5′-GGCTTTCCTTGTGCTCCC-3′ | 366 |
| Connexin43 | Sense:
5′-AAAGGCGTTAAGGATCGCGTG-3′
Antisense: 5′-GTCATCAGGCCGAGGCCT-3′ | 438 |
| β-actin | Sense:
5′-GTCACCCACACTGTGCCCATCT-3′
Antisense: 5′-ACAGAGTACTTGCGCTCAGGAG-3′ | 542 |
Statistical analysis
The experimental results were analyzed using the
SPSS statistical package version 18.0 (SPSS, Inc., Chicago, IL,
USA). Data obtained were expressed as the means ± standard
deviation. Data analysis among groups was performed using one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
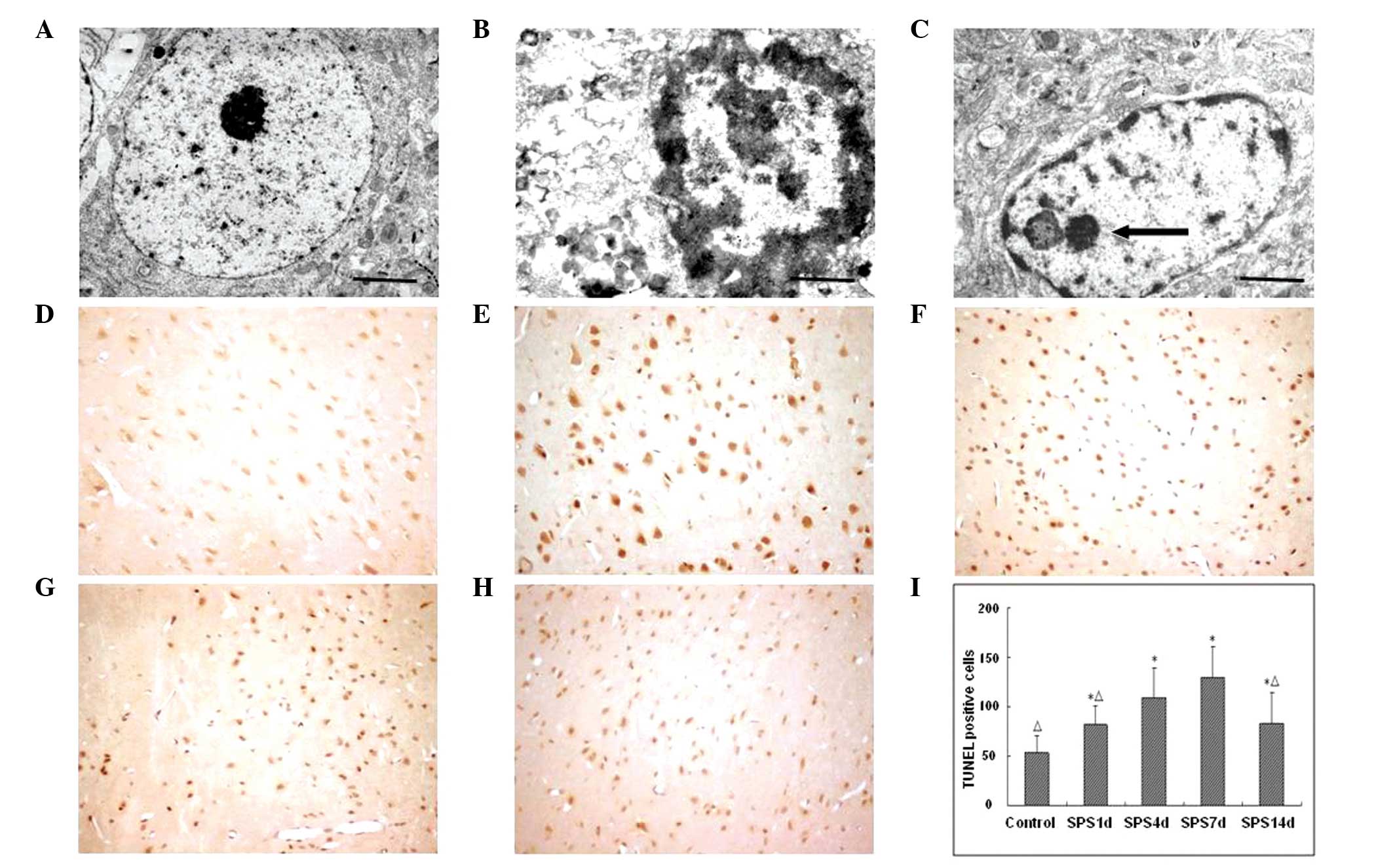
Morphological changes observed in neurons
using transmission electron microscopy (TEM)
Under TEM, normal mPFC neurons had a clear neuronal
shape, an intact nuclear membrane and nucleolus, evenly distributed
chromatin and normal organelle structure. Following SPS
stimulation, the ultrastructures of the neurons of mPFC rats had
varying degrees of changes. These included early morphological
changes such as cell shrinkage, condensed cytoplasm and shrunken
chromatin in the form of a set of edges in the nuclear membrane
under the crescent-shaped bodies. With the passage of time, the
nucleus degenerated and the nuclear membrane subsided, followed by
generation of obvious folds of nuclear cleavage fragments. The
majority of the vesicles within the cytoplasm and cell membrane
disappeared, and in their place apoptotic bodies appeared (Fig. 1A–C).
Apoptotic index detected using TUNEL
staining
Brown or tan particles present in the nucleus under
microscopy were cells that stained positive for apoptosis. The
control group showed a small number of apoptotic cells and a
lighter color. The number of apoptotic cells in the mPFC of PTSD
rats was significantly greater than that of the control group. The
apoptotic cells had an irregular shape, uneven size and a darker
nucleus color. At ~7 days following SPS stimulation, the number of
apoptotic cells reached a peak and then gradually reduced. The
number of apoptotic positive cells in each group was compared with
that of the control group and the difference was estimated to be
statistically significant (Fig.
1).
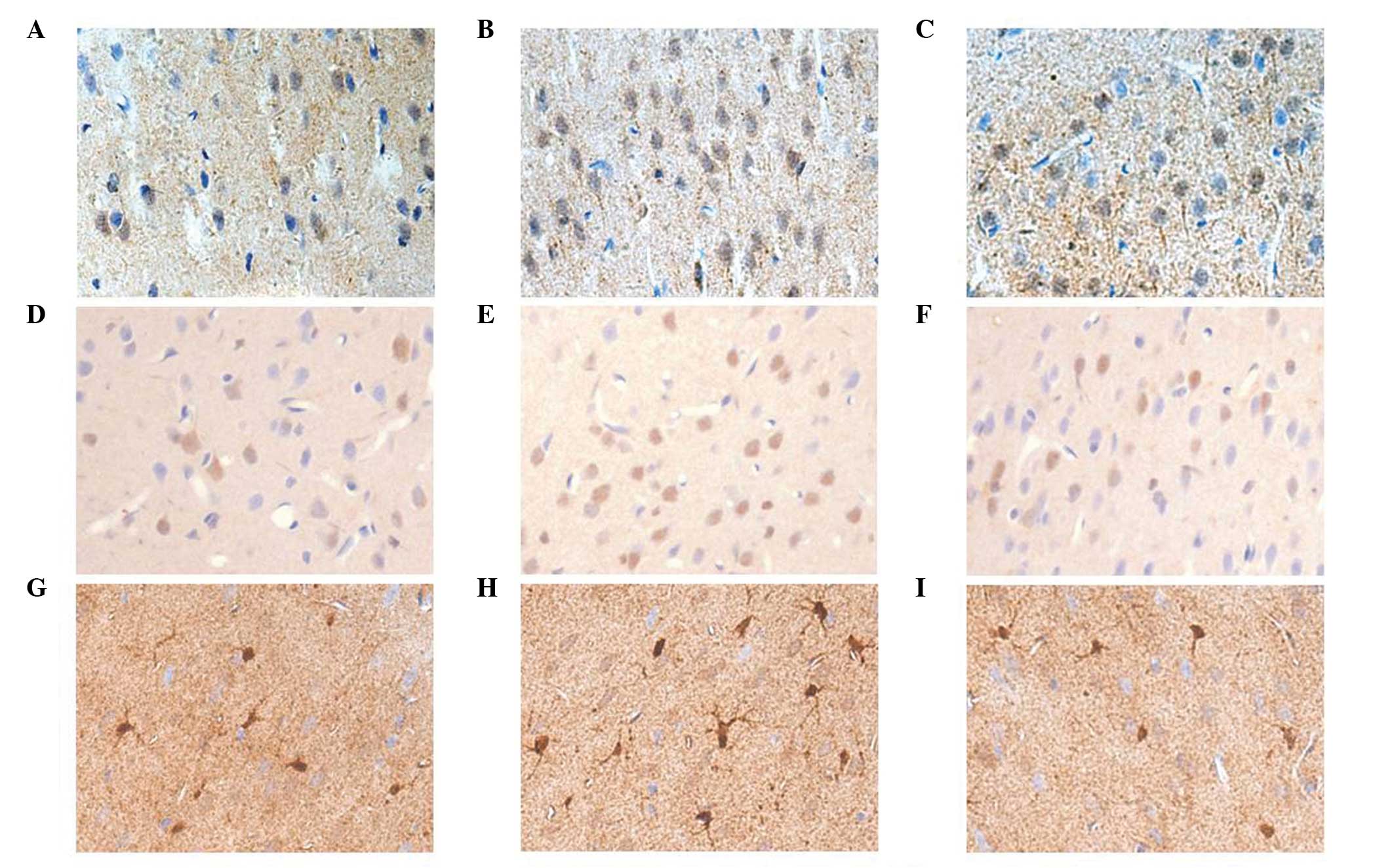
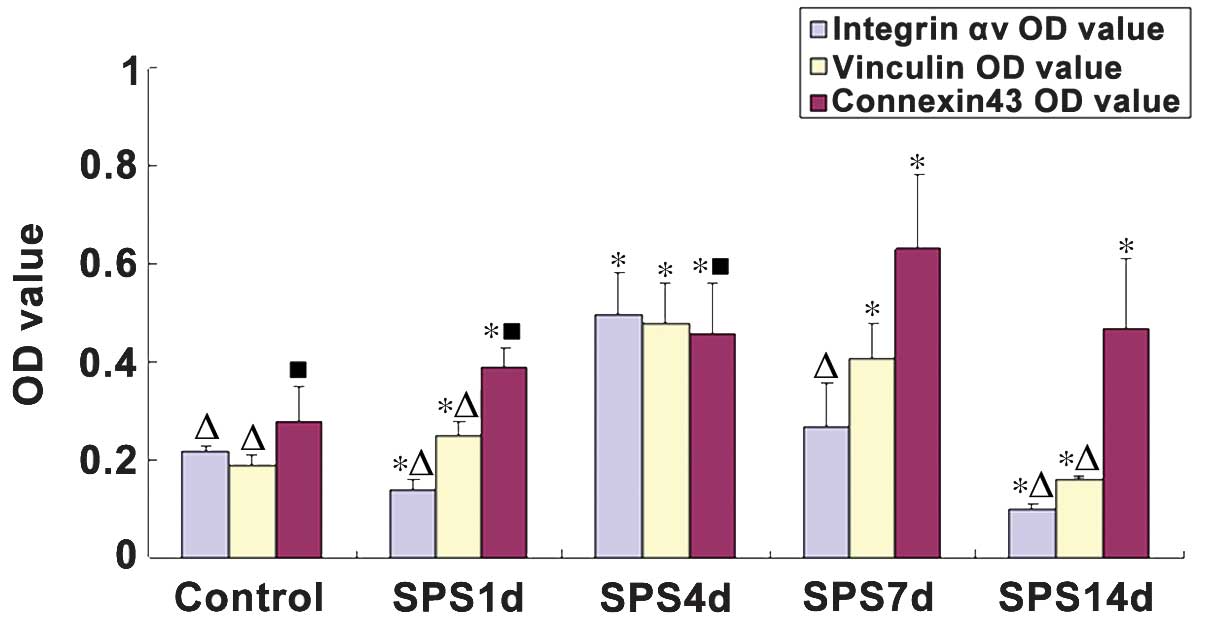
Integrin αv, vinculin and connexin43
detection using immunohistochemical staining
Integrin αv and vinculin demonstrated a weak
positive reaction in normal rat mPFC neurons with a relatively
light staining, which were primarily distributed in the
cytomembrane and the cytoplasm of mPFC neurons. Following SPS
stimulation, the expression levels of integrin αv markedly
decreased on day 1, following which it began to increase, reaching
a peak on day 7. In the SPS rats, increased vinculin levels were
observed compared with those of the controls, with the highest
expression levels were identified 4 day after SPS stimulation.
However, the expression levels of integrin αv and vinculin markedly
reduced on day 11 in the SPS group rats. Positive
immunohistochemical cells stained with the antibody against
connexin43 were brown. The immunoreactivity against connexin43 was
primarily observed in the cytoplasm and neurite of astrocytes.
There were statistically significant differences in the connexin43
expression levels between the control and SPS groups (P<0.05).
In the SPS groups, the expression of connexin43 gradually increased
from day 1, reaching a peak on day 7 (Figs. 2 and 3).
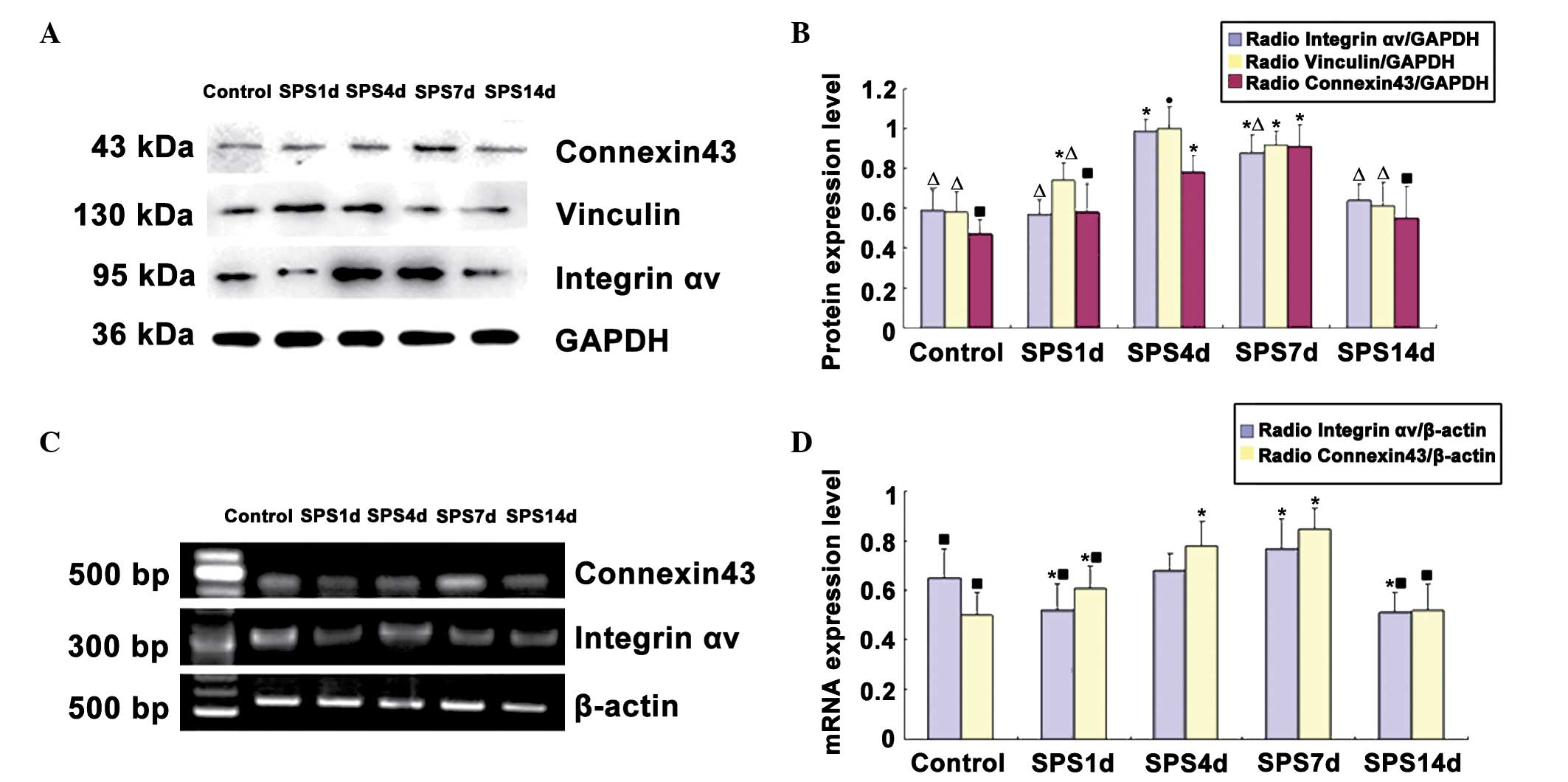
Western blot analysis of integrin αv,
vinculin and connexin43
The integrin αv, vinculin, connexin43 and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) immunoreactive
signals appeared at 95, 130, 43 and 36 kDa, respectively, and the
mean values of the band densities of the control group were set as
100%. Data were expressed with normalized OD. Following SPS
stimulation, the protein levels of integrin αv were downregulated
at 1 day and then recovered gradually compared with those of the
control group. Analysis of the expression levels of vinculin and
connexin43 protein revealed a significant increase in the SPS
groups compared with the levels in the control group (P<0.05).
The intensity of vinculin reached a peak on day 4. The expression
of connexin43 protein reached its highest level on day 7. The time
course of the western blot analysis results was consistent with the
findings obtained by immunohistochemical analysis (Fig. 4).
mRNA expression of integrin αv and
connexin43
The mRNA levels of integrin αv and connexin43 were
normalized to the β-actin mRNA level. The integrin αv mRNA
expression levels in the mPFC were significantly reduced on day 1
of the SPS group compared with the control group (P<0.05). The
levels increased gradually and peaked on SPS day 7. The integrin αv
mRNA expression changed over time, which was consistent with the
results of immunohistochemistry and western blot analysis. However,
the mRNA expression levels of connexin43 markedly increased in the
SPS group rats compared with the control group, reaching a peak on
day 7 (Fig. 4).
Discussion
PTSD is a psychiatric disorder, which may occur as a
result of experiencing or witnessing life-threatening events,
including military combat, natural disasters, terrorist incidents
or violent personal assaults (16). A study has shown that patients with
PTSD exhibit a dietary status change, enhanced negative feedback
inhibition, enhancement of the acoustic startle response and
impaired spatial memory (17).
Further studies have reported that patients with PTSD have a
smaller amygdala and hippocampus (18,19).
In addition, our previous studies have confirmed that the amygdala
and hippocampal neurons of PTSD rats displayed signs of apoptosis
(20,21). The varying degrees of PTSD mental
symptoms may be caused by inadequate descending inhibition of
reduced mPFC function to the amygdala (22).
In the current study, transmission electron
microscopy revealed that the mPFC neurons in PTSD model rats
underwent apoptosis. In addition to the extension of SPS
stimulation time, the number of decrescent neuronal cell bodies
increased. Through TUNEL staining, the apoptotic index was found to
be significantly increased following SPS stimulation compared with
that of the controls. In addition, TUNEL indicated that the mPFC
neurons of PTSD rats underwent apoptosis, leading to changes in the
structure of the mPFC and a reduction in its volume causing
functional decline. This exhibits a certain association with the
pathogenesis of PTSD.
Apoptosis, also known as programmed cell death, is a
programmed cell death process that is regulated by genes (23,24).
The results of the current study have confirmed that factors that
trigger apoptosis transfer certain stimulus signals to cells by the
way of transmembrane information transfer to connect the entire
death program, causing apoptosis (25). The integrin family is a class of
transmembrane heterodimeric glycoprotein adhesion molecules. They
are involved in cell signaling, and regulating cell survival and
apoptosis (26). Integrin αv is an
important member of the integrin family. It can regulate neuronal
function and mediate astrocyte adhesion and migration. A previous
study found that the subunit ectodomain of integrin αv could
identify arginine-glycine-aspartic acid sequences of the ECM, while
the intracellular domain integrated intracellular and extracellular
signals via the interaction with cytoskeletal cross-linker
proteins, including the signaling pathway-related proteins
vinculin, α-actin and talin, which have an important role in the
regulation of cell adhesion, proliferation, differentiation,
invasion, migration and apoptosis (27,28).
Vinculin is a cytoskeletal protein and a focal adhesion protein,
mainly located in cell-cell junctions and cell-ECM focal adhesion
sites (29,30). Vinculin is joined to the
microfilament cytoskeleton of cells, anchoring the microfilament to
the cell membrane, and having an important role in the maintenance
of cell morphology and the regulation of cell adhesion, motility,
proliferation and survival (31).
In addition, vinculin, as an important component of focal
adhesions, is involved in the cytomechanics and chemistry of the
integrin-mediated signal transduction. Integrins can promote
vinculin activation when in contact with the ECM. Vinculins are
raised to focal adhesions where the integrins are located, causing
the polymerization and reorganization of cytoskeleton actins,
therefore they have an important role in cell connection and
fixation (20). Connexin43 is
primarily expressed in astrocytes in the brain. It can connect a
large number of astrocytes together, allowing for the mutual
circulation of various ions, small molecular substances and
metabolites, forming functional coupling syncytia (32). Therefore, it can rapidly dilute
extracellularly transported neurotransmitters and metabolites,
remove metabolites gathered around neurons in time, weakening the
damage caused by metabolites to the glial cells themselves, and
having an important role in maintaining the stability of the brain
tissue microenvironment and the coordination of neuronal functions
(33).
In the current study, the location and expression of
integrin αv, vinculin and connexin43 in the mPFC of PTSD rats were
observed using immunohistochemical staining, and semi-quantitative
analysis was conducted using western blot analysis and RT-PCR. The
results revealed that integrin αv expression is significantly
decreased following SPS stimulation. The reduction in the integrin
αv expression levels may lead to the loss of the connection between
astrocytes and the ECM, or between astrocytes, damages of focal
adhesion structures, changes in the structures of cytoskeleton
proteins, breakdown of the signal transduction pathways of cell
growth and the proliferation activation of apoptosis signal
transduction, and eventually astrocyte shrinkage, isolation, and
apoptosis (34). Expression levels
of vinculin and connexin43 in the mPFC of PTSD rats demonstrated a
transient increase. This may be due to the fact that following
stress stimulation, the increased cellular stress and enhanced
adhesion increase cell resistance to injury, therefore the number
of astrocytes increases, triggering more abundant intercellular gap
junctions, resulting in a timely removal or buffer of the stress
factors, cytokines and excitatory neurotransmitters produced
following stress, thereby taking a compensatory protective effect
on neurons. In the time period following the SPS stimulation, the
structure of neurons and glial cells was further damaged, cell
adhesion to the ECM was reduced, the number of gap junctions
between the cells gradually reduced and the gene and protein
expression levels of vinculin and connexin43 were significantly
reduced. The reduction in the expression level of vinculin may
reduce the mechanical strength of the integrin-cytoskeleton
connection, increase cell motility and reduce focal adhesions,
leading to reduced cell adhesion. Meanwhile, changes in the
cytoskeleton and the reduction and depolymerization of
microfilaments and microtubules promote the reduction of the force
of cells binding with ECM, leading to apoptosis (34,35).
The reduction in connexin43 expression results in brain tissue
microenvironment and cell communication disorders, ultimately
leading to mPFC dysfunction of PTSD rats.
In conclusion, the pathogenesis of PTSD has not yet
been completely elucidated. The stressor causes structural changes
in the mPFC of the brain and neuronal apoptosis, leading to the
onset of PTSD. However, the detailed pathogenesis requires further
study.
Acknowledgements
The current study was supported by a grant from the
National Natural Science Foudation of China (no. 81171282). The
authors would like to thank the reviewers for their valuable
comments on how to improve the quality of the study.
References
|
1
|
Sherin JE and Nemeroff CB: Post-traumatic
stress disorder: the neurobiological impact of psychological
trauma. Dialogues Clin Neurosci. 13:263–278. 2011.PubMed/NCBI
|
|
2
|
Vermetten E: Stress, trauma, and
post-traumatic stress disorder. Tijdschr Psychiatr. 51:595–602.
2009.(In Dutch).
|
|
3
|
Liberzon I, Krstov M and Young EA:
Stress-restress: effects on ACTH and fast feedback.
Psychoneuroendocrinology. 22:443–453. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roozendaal B, Griffith QK, Buranday J, De
Quervain DJ and McGaugh JL: The hippocampus mediates
glucocorticoid-induced impairment of spatial memory retrieval:
dependence on the basolateral amygdala. Proc Natl Acad Sci USA.
100:1328–1333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen Y, Li B, Han F, Wang E and Shi Y:
Dysfunction of calcium/calmodulin/CaM kinase IIα cascades in the
medial prefrontal cortex in post-traumatic stress disorder. Mol Med
Rep. 6:1140–1144. 2012.PubMed/NCBI
|
|
6
|
Bremner JD: Neuroimaging studies in
post-traumatic stress disorder. Curr Psychiatry Rep. 4:254–263.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rauch SL, Shin LM and Phelps EA:
Neurocircuitry models of posttraumafic stress disorder and
extinction: human neuroimaging research - past, present, and
future. Biol Psychiatry. 60:376–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao B, Yu B, Wang HT, Han F and Shi YX:
Single-prolonged stress induces apoptosis by activating cytochrome
C/caspase-9 pathway in a rat model of post-traumatic stress
disorder. Cell Mol Neurobiol. 31:37–43. 2011. View Article : Google Scholar
|
|
9
|
Fink G: Stress controversies:
post-traumatic stress disorder, hippocampal volume, gastroduodenal
ulceration. J Neuroendocrinol. 23:107–117. 2011. View Article : Google Scholar
|
|
10
|
Wang HT, Han F and Shi YX: Activity of the
5-HT1A receptor is involved in the alteration of glucocorticoid
receptor in hippocampus and corticotropin-releasing factor in
hypothalamus in SPS rats. Int J Mol Med. 24:227–231.
2009.PubMed/NCBI
|
|
11
|
Becchetti A, Pillozzi S, Morini R, Nesti E
and Arcangeli A: New insights into the regulation of ion channels
by integrins. Int Rev Cell Mol Biol. 279:135–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheppard D: Roles of alphav integrins in
vascular biology and pulmonary pathology. Curr Opin Cell Biol.
16:552–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chatzizacharias NA, Kouraklis GP and
Theocharis SE: The role of focal adhesion kinase in early
development. Histol Histopathol. 25:1039–1055. 2010.PubMed/NCBI
|
|
14
|
Khan S and Liberzon I: Topiramate
attenuates exaggerated acoustic startle in an animal model of PTSD.
Psychopharmacology (Berl). 172:225–229. 2004. View Article : Google Scholar
|
|
15
|
Takahashi T, Morinobu S, Iwamoto Y and
Yamawaki S: Effect of paroxetine on enhanced contextual fear
induced by single prolonged stress in rats. Psychopharmacology
(Berl). 189:165–173. 2006. View Article : Google Scholar
|
|
16
|
Zhu CZ, Situ MJ, Zhang Y, Fang H, Jing LS,
Wang D, Yan J and Huang Y: Influence factors of post-traumatic
stress disorder (PTSD) and depression symptoms in children and
adolescents after Wenchuan earthquake in China. Zhonghua Yu Fang Yi
Xue Za Zhi. 45:531–536. 2011.(In Chinese). PubMed/NCBI
|
|
17
|
Milner R and Campbell IL: The integrin
family of cell adhesion molecules has multiple functions within the
CNS. J Neurosci Res. 69:286–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gold AL, Shin LM, Orr SP, Carson MA, Rauch
SL, Macklin ML, Lasko NB, Metzger LJ, Dougherty DD, Alpert NM,
Fischman AJ and Pitman RK: Decreased regional cerebral blood flow
in medial prefrontal cortex during trauma-unrelated stressful
imagery in Vietnam veterans with post-traumatic stress disorder.
Psychol Med. 13:1–10. 2011.
|
|
19
|
Su TP, Zhang L, Chung MY, Chen YS, Bi YM,
Chou YH, Barker JL, Barrett JE, Maric D, Li XX, Li H, Webster MJ,
Benedek D, Carlton JR and Ursano R: Levels of the potential
biomarker p11 in peripheral blood cells distinguish patients with
PTSD from those with other major psychiatric disorders. J Psychiatr
Res. 43:1078–1085. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mierke CT: The role of vinculin in the
regulation of the mechanical properties of cells. Cell Biochem
Biophys. 53:115–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brohawn KH, Offringa R, Pfaff DL, Hughes
KC and Shin LM: The neural correlates of emotional memory in
posttraumatic stress disorder. Biol Psychiatry. 68:1023–1030. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jordà EG, Verdaguer E, Jimenez A, Arriba
SG, Allgaier C, Pallàs M and Camins A: Evaluation of the neuronal
apoptotic pathways involved in cytoskeletal disruption-induced
apoptosis. Biochem Pharmacol. 70:470–480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gary DS and Mattson MP: Integrin signaling
via the PI3-kinase-Akt pathway increases neuronal resistance to
glutamate-induced apoptosis. J Neurochem. 76:1485–1496. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demir O, Singh S, Klimaschewski L and
Kurnaz IA: From birth till death: neurogenesis, cell cycle, and
neurodegeneration. Anat Rec (Hoboken). 292:1953–1961. 2009.
View Article : Google Scholar
|
|
25
|
Leerberg JM and Yap AS: Vinculin, cadherin
mechanotransduction and homeostasis of cell-cell junctions.
Protoplasma. 250:817–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klee P and Meda P: Connexin signaling: a
new mechanism for protection of insulin-producing cells against
apoptosis. Med Sci (Paris). 28:41–44. 2012.(In French). View Article : Google Scholar
|
|
27
|
Nandrot EF, Silva KE, Scelfo C and
Finnemann SC: Retinal pigment epithelial cells use a
MerTK-dependent mechanism to limit the phagocytic particle binding
activity of αvβ5 integrin. Biol Cell. 104:326–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawaguchi SY and Hirano T: Integrin
alpha3beta1 suppresses long-term potentiation at inhibitory
synapses on the cerebellar Purkinje neuron. Mol Cell Neurosci.
31:416–426. 2006. View Article : Google Scholar
|
|
29
|
Farwell AP, Tranter MP and Leonard JL:
Thyroxine-dependent regulation of integrin-laminin interactions in
astrocytes. Endocrinology. 136:3909–3915. 1995.PubMed/NCBI
|
|
30
|
Mobley AK and McCarty JH: Use of Cre-lox
technology to analyze integrin functions in astrocytes. Methods Mol
Biol. 814:555–570. 2012. View Article : Google Scholar
|
|
31
|
Carisey A and Ballestrem C: Vinculin, an
adapter protein in control of cell adhesion signalling. Eur J Cell
Biol. 90:157–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li WE and Nagy JI: Connexin43
phosphorylation state and intercellular communication in cultured
astrocytes following hypoxia and protein phosphatase inhibition.
Eur J Neurosci. 12:2644–2650. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Solan JL and Lampe PD: Connexin43
phosphorylation: structural changes and biological effects. Biochem
J. 419:261–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Irie A: Integrin family. Nihon Rinsho.
68:163–166. 2010.(In Japanese).
|
|
35
|
Lv X, Su L, Yin D, Sun C, Zhao J, Zhang S
and Miao J: Knockdown of integrin beta4 in primary cultured mouse
neurons blocks survival and induces apoptosis by elevating NADPH
oxidase activity and reactive oxygen species level. Int J Biochem
Cell Biol. 40:689–699. 2008. View Article : Google Scholar
|