Introduction
Histamine is a well-known biogenic and cationic
amine, which is synthesized, stored and released by professional
histamine-synthesizing cells. Mast cells, basophils and
enterochromaffin cells contain the endoplasmic 54 kDa histidine
decarboxylase (HDC), which converts L-histidine to histamine
(1). Histamine is released into
and stored within storage granules, prior to regulated release
(1). Following activation of
professional histamine-producing cells, a burst release results in
a transient high histamine concentration in the extracellular
space. These transient histamine concentrations are sufficient to
stimulate the conventional histamine receptors, histamine receptor
type 1 [H1R; binding affinity (pKi) = 4.2]
and histamine receptor type 2 (H2R; pKi =
4.3) (2). Smooth muscle cells
(3,4), cardiomyocytes (5,6) and
skeletal muscle tissue (7) express
these conventional histamine receptors, which regulate cellular
proliferation and the contraction state of the cells stimulated via
the histamine/H1R or H2R axes (3–6).
A previous study identified that the cytoplasmic 73
kDa ‘pro-form’ of HDC produced histamine, however, at a
100–1,000-fold lower rate compared with the typical enzyme isoform
of the professional histamine-synthesizing cells (1). In non-professional
histamine-producing cells, histamine is released into the cellular
cytoplasm rather than being stored, and is therefore not subjected
to regulated burst release (8).
These cells contain organic cation transporters, which are
equilibrative uniporters and transport the intracellularly
synthesized histamine from the non-professional histamine
synthesizing cells along the histamine concentration gradient to
the extracellular space (8).
Histamine concentrations achieved in this manner are not sufficient
to stimulate conventional histamine receptors. Therefore, this
mechanism was hypothesized to represent an ancestral vestigium of a
function that had become obsolete during phylogenesis. However,
studies conducted within the last decade that focus on G-protein
coupled receptors have revealed novel members of the histamine
receptor family (2). These novel
histamine receptors, histamine receptor type 3 (H3R;
pKi = 8.0) and histamine receptor type 4
(H4R; pKi = 8.2), have >10,000-fold
greater affinity for histamine compared with the conventional
receptors (2). In addition, the
low basal levels of histamine produced by non-professional
histamine-producing cells, including dendritic cells (9) and lymphocytes (10,11),
have been demonstrated to be sufficient in order to bind to and
regulate cells equipped with these novel, high-affinity histamine
receptors. The role of high histamine concentration in the
regulation of muscle cell tone was investigated in previous studies
(3–6). Studies using histamine receptor
agonists and/or antagonists have suggested that novel histamine
receptors may also be present and functional in the bronchial
smooth muscle cells at least (3).
However, to date, no studies indicating the presence of histamine
receptors at the messenger RNA (mRNA) and protein level in
myoblasts, myocytes or myotubes during skeletal myogenesis have
been reported. Due to the presence and role of H1R,
H2R and H3R in the function of other muscle
cell types, the present study aimed to assess whether striated
muscle cells synthesize and express the histamine receptors,
H3R and H4R. In addition, the current study
investigated whether these receptors are developmentally regulated
during myogenesis in association with various markers of myogenic
maturation.
Materials and methods
Cell culture
The present study was approved by the institutional
Medical Ethics Committee of the Institue of Clinical Medicine,
University of Helsinki (Helsinki, Finland) and was performed in
accordance with the 1983 Declaration of Helsinki. Mouse C2C12
myoblasts were obtained from the Turku Center for Biotechnology,
University of Turku (Turku, Finland) (12), and maintained in growth medium
comprising Dulbecco’s modified Eagle’s medium (DMEM;
Lonza/BioWhittaker, Walkersville, MD, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone, GE Healthcare Life Sciences,
Little Chalfont, UK), antibiotics (100 U/ml penicillin and 100
μg/ml streptomycin; Lonza) and 200 mM L-glutamine (Lonza) at 37°C
in a humidified 5% CO2 atmosphere. The composition of
the differentiation medium was similar to the growth medium, with
the exception of FBS, which was reduced from 10% to 1%. The cells
were passaged using trypsinization (0.5% trypsin in 0.5 mM EDTA;
Gibco-BRL Life Technologies, Carlsbad, CA, USA) from the culture
plate at 80% confluence.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To investigate the expression of histamine receptors
in C2C12 myogenesis, 50,000 cells/well were seeded in 12-well
plates (CellStar; Greiner Bio-One, Frickenhausen, Germany). The
cells were initially grown in growth medium for two days to reach
80% confluence. Next, the medium was exchanged with differentiation
medium to induce myogenesis. Total RNA was isolated from the cells
at days 0, 2, 4 and 6 using an RNeasy Mini kit (Qiagen, Düsseldorf,
Germany) according to the manufacturer’s instructions. Total RNA (1
μg) was reverse transcribed using iScript cDNA Synthesis kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). RT-qPCR was
performed with 100 ng first-strand cDNA using iQ SYBR®
Green Supermix (Bio-Rad Laboratories, Inc.) in an iCycler iQ5
Multicolor Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc.). Primers for mouse desmin (Des), myogenin (Myog), myosin
heavy chain IIa (Myh2), H1R, H2R,
H3R, H4R and porphobilinogen deaminase (PBGD)
genes were designed using the National Center for Biotechnology
Information Primer-Blast tool (Table
I; http://www.ncbi.nlm.nih.gov/tools/primer-blast/;
accessed: 01/03/2012). The mRNA copy numbers of the samples
analyzed were determined in triplicate and normalized against the
PBGD gene.
 | Table IPrimer sequences used in reverse
transcription-quantitative polymerase chain reaction and the
corresponding amplicon lengths. |
Table I
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction and the
corresponding amplicon lengths.
| Gene | Forward primer | Reverse primer | Length (bp) |
|---|
| Des |
5′-GCCCTCAAGGGCACCAACGA-3′ |
5′-TTGCTCGGGGCTGGTTTCTCG-3′ | 297 |
| Myog |
5′-CCCAACCAGCGGCTGCCTAA-3′ |
5′-GTAGGGTCAGCCGCGAGCAA-3′ | 245 |
| Myh2 |
5′-AGCTGCACCTTCTCGTTTGCCA-3′ |
5′-CGGTCAGGGTCGCTCCTGCT-3′ | 261 |
| H1R |
5′-CACTGGAGGCTGCCCTTGTGC-3′ |
5′-CACCAGCAGGTTGAGGCCCAC-3′ | 167 |
| H2R |
5′-TCCTAAGCGACCCGGTACAGC-3′ |
5′-ATGGAGACTGAGGCACTGCTGG-3′ | 208 |
| H3R |
5′-TTCGAGCCTCCGCACCCAGAA-3′ |
5′-GGTCCAACGGCCGGTCAGC-3′ | 118 |
| H4R |
5′-TGCTCAGGTCCCCTTGGCATTT-3′ |
5′-ACGTGAGGGATGTACAGAGGAATGG-3′ | 189 |
| PBGD |
5′-AAAGTGCCGTGGGAACCAGC-3′ |
5′-CAGCCACAGCCAGGACGATG-3′ | 156 |
Immunofluorescence staining
The C2C12 cells were seeded at 2×104
cells/well in 24-well plates (CellStar) on coverslips and grown in
growth medium for two days to reach 80% confluence, followed by
culturing in differentiation medium to induce myogenesis.
Differentiated cells from days 0, 2, 4 and 6 were fixed for 15–20
min in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) with
phosphate-buffered saline (PBS; 10mM phosphate buffer, 140 mM
saline; pH 7.4), washed three times in PBS (5 min each time) and in
0.5% Triton X-100 (Thermo Fisher Scientific, Fair Lawn, NJ,
USA)/PBS for 15 min to permeabilize the cells. Subsequently, the
cells were cultured under the following conditions sequentially: i)
10% normal donkey serum (Jackson Immunoresearch Laboratories, Inc.,
West Grove, PA, USA) for 1 h; ii) 1 μg/ml polyclonal peptide
affinity purified rabbit anti-human desmin (1:200), myogenin
(1:400) or myosin heavy chain (Myh) immunoglobulin G (IgG; 1:400)
antibodies (obtained from Dr John E. Erikson, University of Turku,
Turku, Finland) (12), or rabbit
anti-human H3R polyclonal antibodies (1:1,000; LS-A476;
MBL International, Woburn, MA, USA) at 4°C overnight and washed
three times in PBS (5 min each time). Non-immune rabbit IgG
(1:1,000; 1 μg/ml; R&D Systems, Minneapolis, MN, USA), was used
at the same concentration as the primary antibodies as a negative
staining control; iii) secondary antibody
AlexaFluor®488-conjugated monoclonal donkey anti-rabbit
IgG (1:400; Invitrogen Life Technologies, Carlsbad, CA, USA) in
0.1% bovine serum albumin (Sigma-Aldrich)-PBS for 1 h and washed
three times in PBS (5 min each time); iv) DAPI dye (Sigma-Aldrich;
1:2,000 in distilled water) for 5 min. The coverslips were washed
twice in PBS and distilled water for 10 min, prior to mounting with
Vectashield medium (Vector Laboratories, Inc., Burlingame, CA,
USA). Labeled slides were analyzed and photographed using a Leica
DM 6000 B/M fluorescence microscope, with a motorized Leica
XY-stage connected to a Leica DFC 420 digital camera, and analyzed
using the Leica Application Suite Advanced Fluorescence 2.5.0.6735
software (Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
SPSS software, version 17.0 (SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analyses in addition to
Matlab (MathWorks, Inc., Natick, MA, USA), which was used to
perform the Mann-Whitney U test. All values are presented as the
mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Myogenesis of C2C12 cells
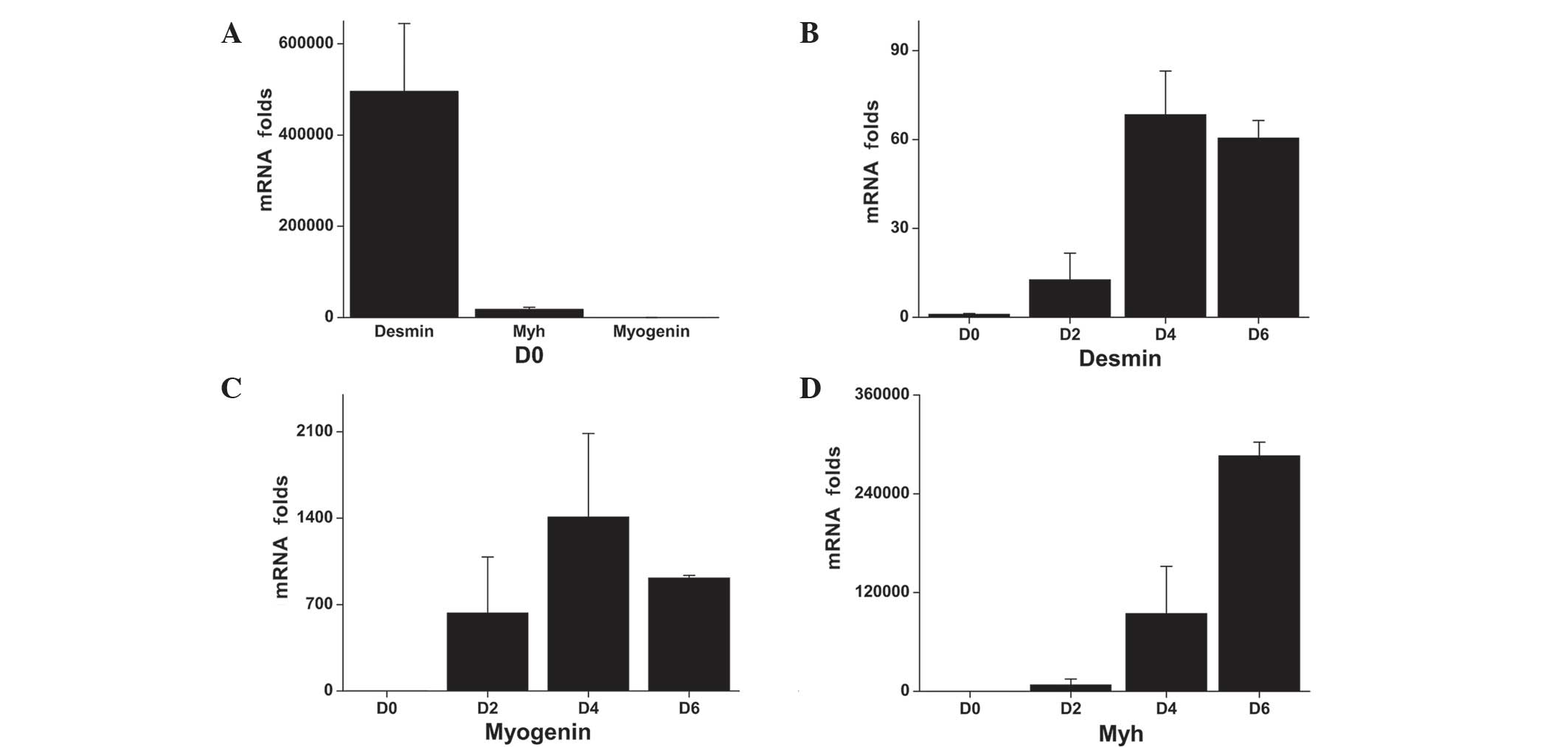
RT-qPCR was used to detect the mRNA expression
levels of the early, intermediate and late myogenesis markers,
desmin, myogenin and Myh2, respectively, during differentiation. On
day 0, desmin was expressed in myoblasts at significantly higher
levels compared with the myogenin or Myh (Fig. 1A). The desmin expression levels
increased during myogenesis, reaching 12-, 68- and 60-fold over the
baseline level (day 0), on days 2, 4 and 6, respectively (Fig. 1B). On day 0, the myogenin mRNA
exxpression levels were low; however, the mRNA expression levels
increased by 631-, 1,408- and 914-fold at days 2, 4 and 6,
respectively (Fig. 1C). Desmin and
myogenin expression levels peaked on day 4, whereas the expression
of Myh, a late myogenesis marker, continued to increase over the
entire study period, reaching 7,718-, 94,487- and 286,288-fold
higher expression levels at days 2, 4 and 6, respectively, compared
with the baseline level (Fig.
1D).
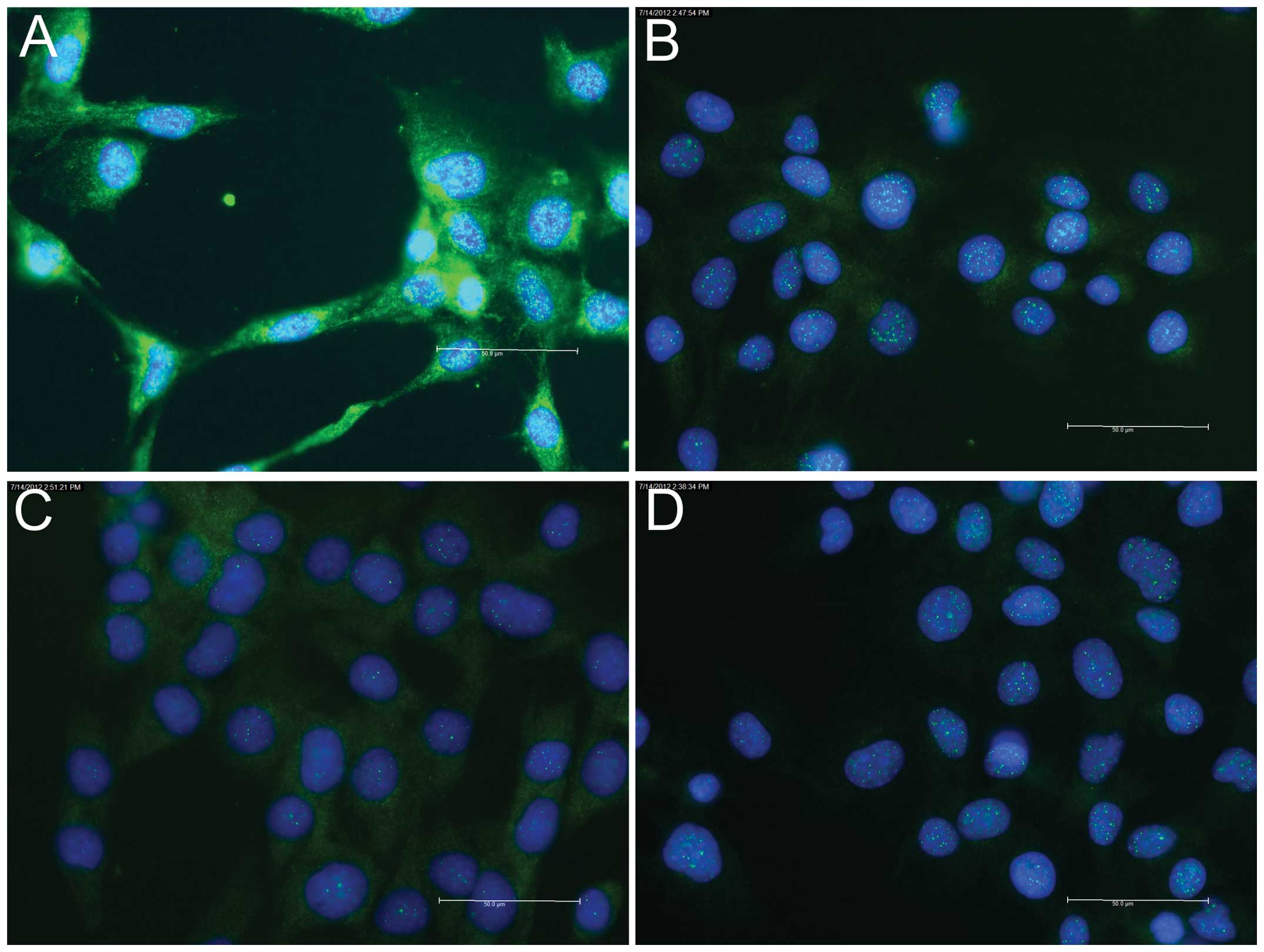
Indirect immunofluorescence staining of the
myogenesis marker proteins revealed positive staining of the early
marker, desmin, at day 0 (Fig.
2A); however, no staining was observed for the intermediate
marker, myogenin (Fig. 2B), or the
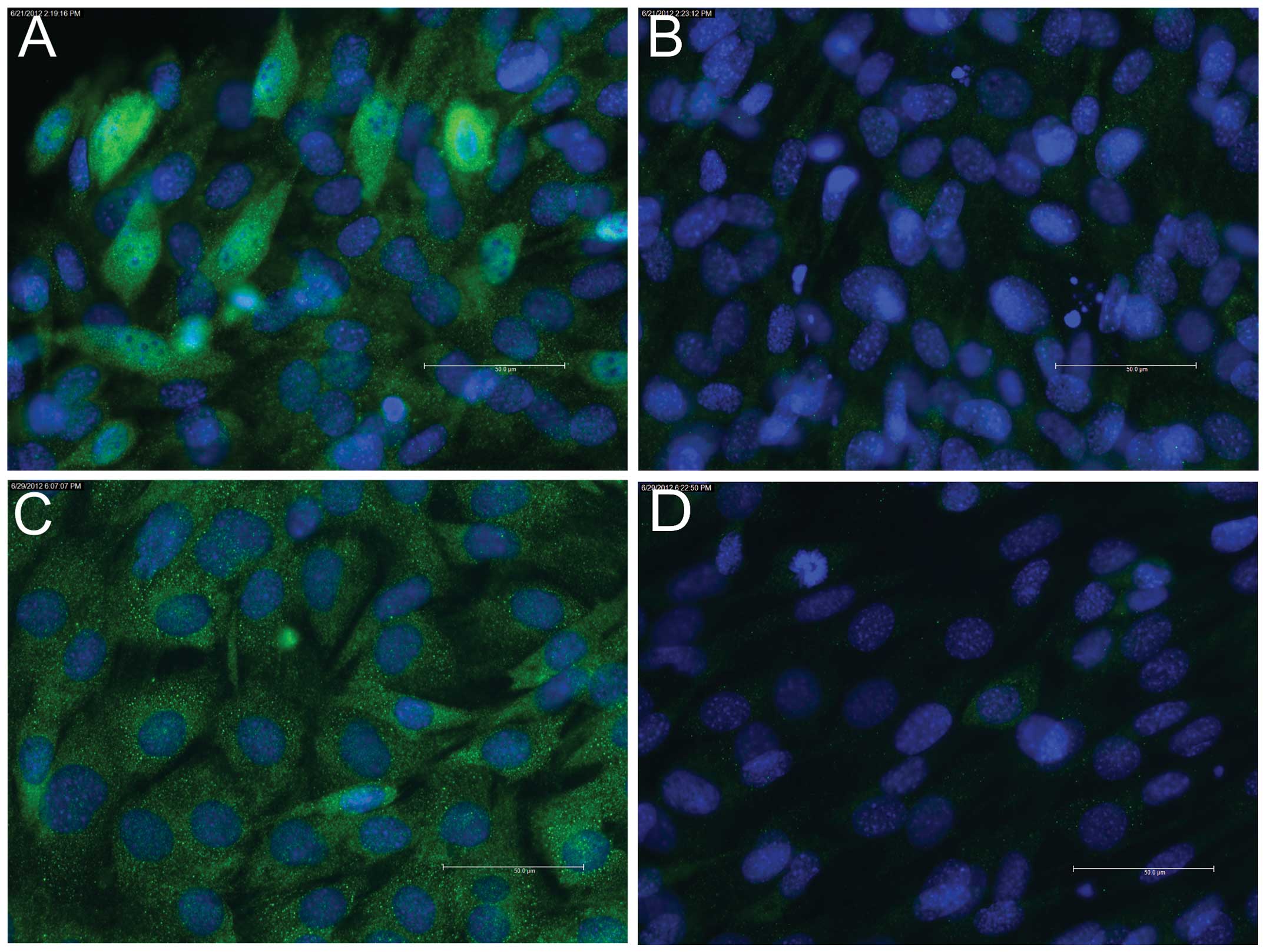
late marker, Myh (data not shown). On day 2, staining for myogenin
was found to be positive (Fig.
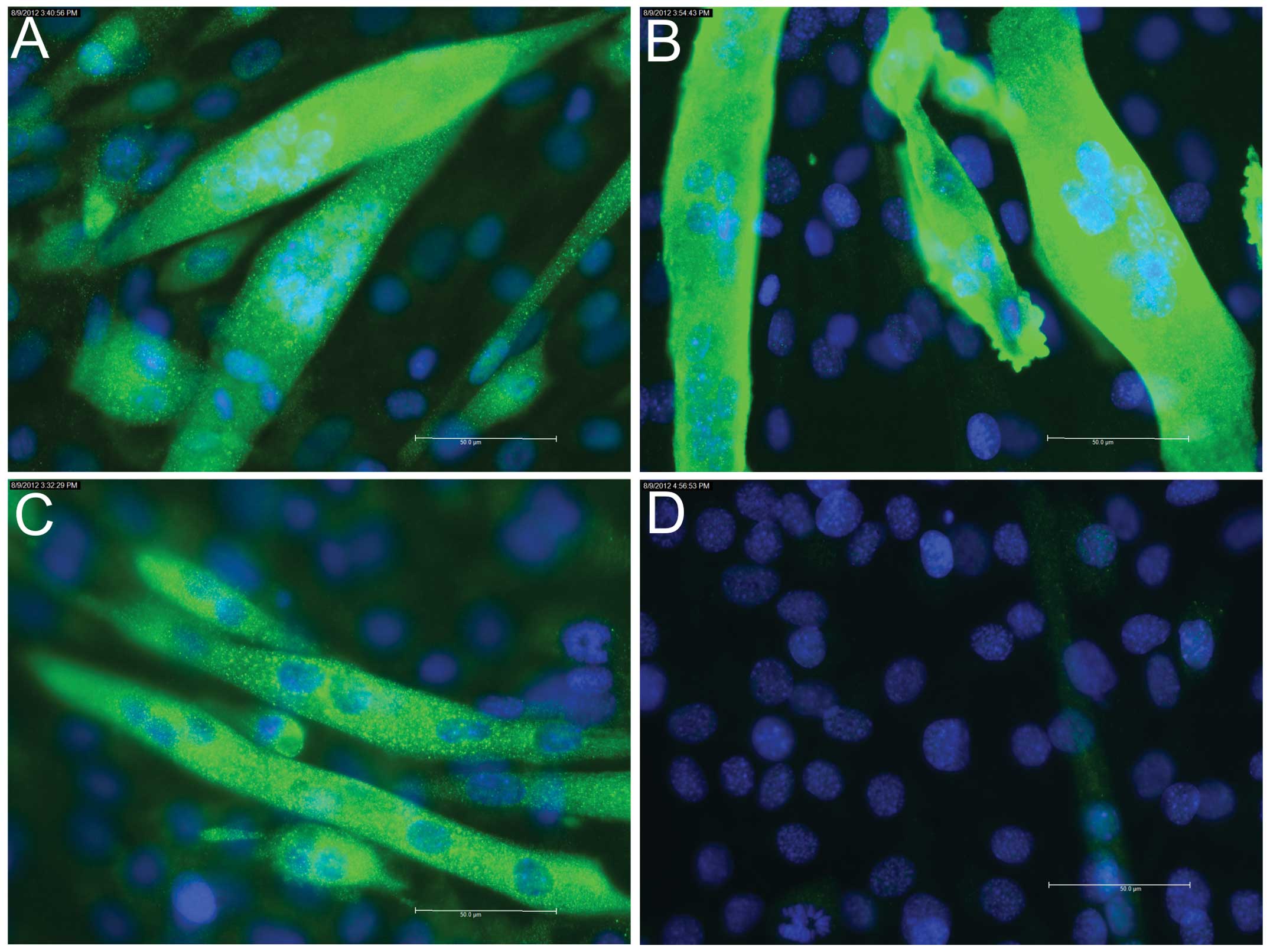
3A), whereas staining for Myh remained negative (Fig. 3B). On days 4 (data not shown) and
6, positive staining for myogenin (Fig. 4A) and Myh (Fig. 4B) was detected.
Expression of histamine receptors
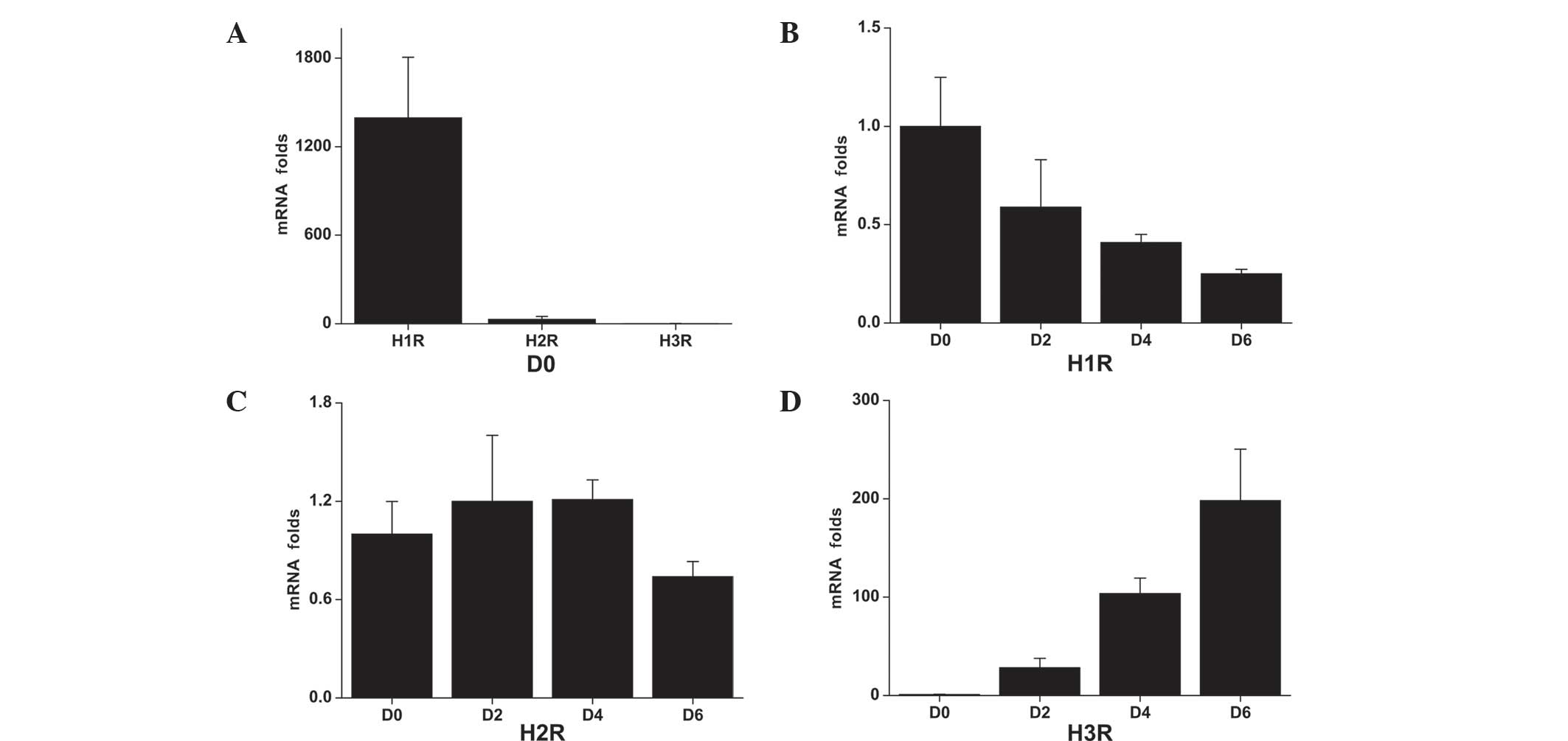
RT-qPCR was used to detect the mRNA expression
levels of histamine receptors associated with the differentiation
stages (Fig. 5). H1R
mRNA was found to be highly expressed in C2C12 myoblasts (day 0),
whereas expression was decreased during the differentiation process
(Fig. 5A and B). By day 6, the
expression level decreased to ~25% of the baseline level (day 0).
H2R mRNA was also expressed in C2C12 cells and the
expression levels remained relatively constant throughout the
differentiation process (Fig. 5A and
C). The expression of H3R was found to be low in
C2C12 myoblasts; however, following differentiation, the expression
levels increased by 28-, 103- and 198-fold over the baseline level
on days 2, 4 and 6, respectively (Fig.
5A and D). H4R mRNA expression was not detected at
any time-point.
Indirect immunofluorescence staining for
H3R protein during the myogenesis of C2C12 cells
revealed almost negative staining at day 0 (Fig. 2C), weakly positive staining on day
2 (Fig 3C) and strongly positive
staining on days 4 (data not shown) and 6 (Fig. 4C).
Discussion
To the best of our knowledge, the present study
demonstrated for the first time that striated muscle cells
expressed H1R, H2R and H3R-coding
mRNA and corresponding receptor proteins, but lacked receptor,
H4R. The lack of H4R in striated muscle cells
may be due to the fact that H4R(+) cells have been
previously been identified in the bone marrow, thymus and spleen,
as well as at the cellular level in bone marrow-derived cells,
including mast cells, basophils, eosinophils, neutrophils,
dendritic cells and lymphocytes (13).
Investigation of the early, intermediate and late
phases of myogenesis was performed using desmin, myogenin and Myh
as markers, respectively. The results indicated that histamine
receptors were dynamically regulated during differentiation,
suggesting that they may have distinct regulatory functions.
H1R presented the highest expression in myoblasts on day
0, compared with the other receptors; however, the expression
levels of H1R were subsequently decreased during
myogenesis. H2R expression was found to be low on day 0
and remained relatively constant throughout all the phases of
myogenesis. By contrast, H3R showed the lowest
expression in myoblasts on day 0; however, the H3R
expression levels were subsequently increased, and continued to
increase throughout myogenesis.
The low affinity of H1Rs for histamine
requires burst release from professional histamine-synthesizing
cells in order to induce target cell effects. Notably, in
cardiomyocyte precursor cells, H1Rs are abundant and
regulate Ca2+ oscillation and frequency. In such
progenitor cells, this process is coupled with the entry of cells
into the cell cycle and bromodeoxyuridine incorporation (5). The results of the present study,
which revealed high levels of H1R expression during
early myogenesis, along with the aforementioned previous
observations, suggested that high histamine levels may stimulate
myoblast proliferation during the early phases of differentiation.
This hypothesis is further supported by the observations of a
previous study, which demonstrated that mast cell precursors
migrated from bone marrow to skeletal muscle tissue in 17 to
20-day-old rat fetuses, indicating interactions between the
professional histamine-producing mast cells and skeletal muscle
cells in proliferation or differentiation (14).
In the present study, H2R expression
remained constant throughout all the phases of myogenesis, and
thus, may be involved in the maintenance of relaxation following
burst release of histamine (since H2R stimulation
requires high histamine concentrations), with a curare-like effect
(which is a competitive antagonist of the nicotinic acetylcholine
receptor) (15). By contrast,
H2R antagonists have been demonstrated to possess an
anti-cholinesterase activity (16).
Due to the high affinity of H3R for
histamine, the non-professional histamine-producing cells are able
to stimulate H3R-expressing cells. The levels of
histamine released by the non-professional histamine-producing
cells are not sufficient to activate the conventional, low-affinity
receptors (2). Furthermore, in
contrast to the conventional H1R and H2R,
H3R has a relatively high constitutive activity level,
which is ~25% active in the absence of H3R-ligands
(17,18). According to the two-state model of
receptor activation, G-protein coupled receptors exist in
equilibrium between an active and inactive receptor state. Upon
ligand binding, the G-protein becomes activated (R*) and
begins to ‘couple’ and transduce the extracellular stimulus into an
intracellular signal, while ligand-free G-protein coupled receptors
exist in a passive, uncoupled conformation. However, H3R
spontaneously acquires the R* state, which promotes
G-protein-mediated signaling in the absence of an agonist.
Therefore, H3R is hypothesized to have significant
constitutive functions in mature myocytes and myotubes, which are
independent of burst release (cellular emergencies) and driven by
the low histamine concentrations generated by non-professional
histamine-producing cells and by their constitutive activity
(17,18).
High histamine concentrations are known to mediate
the pathological contraction of smooth muscles cells in the
bronchiolar walls, including during acute attacks of asthma and
anaphylactic reactions mediated by H1R. H1R
is coupled to Gαq/11 protein, which cleaves
phosphatidylinositol 4,5-bisphosphate to diacylglycerol and
inositol 1,4,5-trisphosphate, via the activation of phospholipase
C. This results in Ca2+ influx and initiates smooth
muscle contraction (3). Notably,
low histamine concentrations act as potent relaxant agents for
pre-contracted smooth muscle cells via H3Rs (3). In the
present study, the time course of H3R expression during
myogenesis indicated that H3R may have long-term,
constitutive effects on mature skeletal muscles cells, rather than
being activated under exceptional circumstances that results in
burst release of the histamine stores from mast cells and
basophils. Based on the findings of Cardell and Edvinsson (3) and the long-term low histamine
level-induced and constitutive H3R function,
H3R was hypothesized to maintain the relaxed state of
mature skeletal muscle cells.
In conclusion, further studies are required in order
to determine the functions and potential signalling pathways by
which the expression of the three histamine receptor subtypes,
examined in the present study, are regulated during myogenesis in
skeletal muscle cells. Future research may elucidate novel
information regarding the etiology and potential treatment of
skeletal muscle diseases.
Acknowledgements
The work of Drs Chen, Stegaev, Sillat, Kouri and
Konttinen was supported by the Finska Läkaresällskapet,
Orion-Farmos Foundation, Sigrid Jusélius Foundation, ORTON Invalid
Foundation, HUS evo-grants, Academy of Finland, Center for
International Mobility CIMO and the Danish Council for Strategic
Research and Regenerative Medicine RNP of the European Science
Foundation. The work of Dr Stark was supported by the Hesse LOEWE
programs OSF, NeFF, AFA and the TRIP. The work of Dr Chazot was
supported by the Royal College of Anaesthesia, BBSRC (UK). This
study was supported by the EU COST Action BM0806.
The authors would like to thank Professor John E.
Eriksson at the Turku Center for Biotechnology, Department of
Biosciences, University of Turku and Åbo Akademi University (Turku,
Finland) for providing the cells and antibodies.
References
|
1
|
Ichikawa A, Sugimoto Y and Tanaka S:
Molecular biology of histidine decarboxylase and prostaglandin
receptors. Proc Jpn Acad Ser B Phys Biol Sci. 86:848–866. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walter M and Stark H: Histamine receptor
subtypes: a century of rational drug design. Front Biosci (Schol
Ed). 4:461–488. 2012. View
Article : Google Scholar
|
|
3
|
Cardell LO and Edvinsson L:
Characterization of the histamine receptors in the guinea-pig lung:
evidence for relaxant histamine H3 receptors in the trachea. Br J
Pharmacol. 111:445–454. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neuhaus J, Weimann A, Stolzenburg JU, et
al: Histamine receptors in human detrusor smooth muscle cells:
physiological properties and immunohistochemical representation of
subtypes. World J Urol. 24:202–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferreira-Martins J, Rondon-Clavo C, Tugal
D, et al: Spontaneous calcium oscillations regulate human cardiac
progenitor cell growth. Circ Res. 105:764–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wellner-Kienitz MC, Bender K, Meyer T and
Pott L: Coupling to Gs and Gq/11 of histamine
H2 receptors heterologously expressed in adult rat
atrial myocytes. Biochim Biophys Acta. 1642:67–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukui H, Fujimoto K, Mizuguchi H, et al:
Molecular cloning of the human histamine H1 receptor
gene. Biochem Biophys Res Commun. 201:894–901. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emami Riedmaier A, Nies AT, Schaeffeler E,
et al: Organic anion transporters and their implications in
pharmacotherapy. Pharmacol Rev. 64:421–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szeberényi JB, Pállinger E, Zsinkó M, et
al: Inhibition of effects of endogenously synthesized histamine
disturbs in vitro human dendritic cell differentiation. Immunol
Lett. 76:175–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kubo Y and Nakano K: Regulation of
histamine synthesis in mouse CD4+ and CD8+ T
lymphocytes. Inflamm Res. 48:149–153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Radvány Z, Darvas Z, Kerekes K, et al: H1
histamine receptor antagonist inhibits constitutive growth of
Jurkat T cells and antigen-specific proliferation of
ovalbumin-specific murine T cells. Semin Cancer Biol. 10:41–45.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pallari HM, Lindqvist J, Torvaldson E, et
al: Nestin as a regulator of Cdk5 in differentiating myoblasts. Mol
Biol Cell. 22:1539–1549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zampeli E and Tiligada E: The role of
histamine H4 receptor in immune and inflammatory
disorders. Br J Pharmacol. 157:24–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng CX, Li YN, Ohno H, et al: Mast cells
appearing in long-term skeletal muscle cell cultures of rat. Anat
Rec (Hoboken). 290:1424–1430. 2007. View
Article : Google Scholar
|
|
15
|
Ohta Y, Ariyoshi M and Koketsu K:
Histamine as an endogenous antagonist of nicotinic ACh-receptor.
Brain Res. 306:370–373. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheah LS, Lee HS and Gwee MC:
Anticholinesterase activity of and possible ion-channel block by
cimetidine, ranitidine and oxmetidine in the toad isolated rectus
abdominis muscle. Clin Exp Pharmacol Physiol. 12:353–357. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rouleau A, Ligneau X, Tardivel-Lacombe J,
et al: Histamine H3 receptor-mediated
[35S]GTP gamma (S) binding: evidence for constitutive
activity of the recombinant and native rat and human H3
receptors. Br J Pharmacol. 135:383–392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arrang JM, Morisset S and Gbahou F:
Constitutive activity of the histamine H3 receptor. Trends
Pharmacol Sci. 28:350–357. 2007. View Article : Google Scholar : PubMed/NCBI
|