Introduction
Over the past two decades, esophageal stent
placement has been considered a simple and effective method to
relieve dysphagia in patients with advanced esophageal carcinoma
(1,2). In addition, esophageal stents were
shown to improve patients’ nutritional status and quality of life,
resulting in an increased longevity (3–6).
However, esophageal stents present obvious disadvantages (7); for instance, stent restenosis due to
tissue hyperplasia on the upper edge of the stents constitutes a
common long-term complication and presents major limitation for
interventional therapies (8,9). Of
note, a decrease in benign restenosis was reported, with a 22.2%
incidence rate, following treatment with irradiated stents
(10,11) compared with that of treatment with
conventional stents, where benign restenosis incidence rates ranged
from 30–60% (12,13). These high incidence rates can
seriously affect the long-term efficacy of esophageal stents.
Numerous studies have shown that stricture
generation following esophageal stenting was associated with
fibroblast proliferation (14–16).
Of note, it has been demonstrated that catheter-based intracoronary
β and γ radiotherapy significantly altered the occurrence of
subsequent restenosis, including the neointimal proliferation which
occurs during the restenotic process (17–19).
To the best of our knowledge, there are no existing studies
demonstrating the incidence reduction of benign restenosis
following esophageal stenting through inhibition of esophageal
fibroblast proliferation. It is thought that these esophageal
fibroblasts may deliver growth factors in conjunction with
monocytes as the stimuli for restenosis (20).
However, fibroblasts may be inhibited using
low-energy radiation of γ-rays produced by the widely clinically
used iodine-125 (125I) seeds. The cytotoxicity of
125I seeds has been demonstrated in various cells
derived from lung (21),
colorectal (22) and prostate
carcinomas (23). Therefore,
125I seed irradiation therapy was proposed for the
treatment of brain tumors (24)
and gastric cancer xenografts (25), among others.
In the present study, three clinically used
125I seeds with activities of 11.1, 22.2 and 33.3 MBq
were evaluated for their inhibitory effects on esophageal
fibroblast proliferation and their optimal doses were determined
in vitro. Novel esophageal stents were designed using
125I seeds at the determined optimal inhibitory doses.
125I seed-preloaded stents were implanted in dogs in
order to evaluate their preventive effects on benign restenosis as
well as to perform safety studies. The present study aimed to
provide a basis for the potential development of more effective and
safer novel devices for clinical use in the treatment of benign
restenosis.
Materials and methods
Animals
One-year-old male and female Beagle dogs (body
weight, 11±0.1 kg) were provided by the Experimental Animal Center
of Southeast University (Jiangsu, China). Beagle dogs were kept by
the Experimental Animal Center of Southeast University under as
12-h light/dark cycle at 23°C and fed standard dog chow. All
procedures and animal experiments were approved by the Animal
Ethical Committee of the Southeast University and conducted in
accordance with State and international regulations.
Induction of dog esophageal
fibroblasts
One Beagle dog was intravenously anesthetized with 1
ml/kg 3% sodium amobarbital (Sinopharm Chemical Reagent Co., Ltd,
Shanghai, China) following one day of fasting. The dog then
underwent layer by layer surgery and the esophagus was partly
dissociated. Then, a 8×2 mm nitinol wire (Micro-tech, Jiangsu,
China) was implanted into the esophageal muscle followed by
incision sutures (layer by layer). Two weeks later, the esophagus
was dissociated following the same procedure and the nitinol wire
as well as the surrounding esophageal tissues were extracted.
Primary culture of Beagle dog esophageal
fibroblasts
Esophageal tissues were immerged in
phosphate-buffered saline (PBS; Life Technologies, Grand Island,
NY, USA) with high concentrations of penicillin-streptomycin
(Biosharp, Seoul, Korea) for 10 min and washed 10–15 times in the
same solution. Subsequently, the tissues were minced with
ophthalmic scissors to ~1 mm3 and placed in culture
medium. Minced tissues were then transferred to a centrifuge tube
and digested with excess trypsin (HyClone Laboratories, Inc.,
Logan, UT, USA) at 37°C, with agitation every 5 min. The digestion
was ceased when the medium became cloudy and free cells were
observed under a microscope (BX53; Olympus, Tokyo, Japan).
Throughout this experiment centrifugation was performed at 453 × g
for 5 min and the supernatant was discarded. Low glucose Dulbecco’s
modified Eagle’s medium (DMEM; HyClone Laboratories, Inc.)
supplemented with 15% fetal calf serum (Hyclone Laboratories,
Inc.), 100 U/ml penicillin (Biosharp) and 100 μg/ml streptomycin
(Biosharp), were added to the resulting pellets. Cells were
incubated in a humidified incubator (HERACELL 150i; Thermo Fisher
Scientific, Waltham, MA, USA) with 5% CO2 at 37°C. The
culture medium was removed 24–36 h following incubation and
deformed adherent cells were observed under a microscope. Cells
were washed with PBS and further incubated in 5 ml culture medium
which was replaced every three days. Regular cell subcultures were
performed for preservation at 85% confluency.
Identification of Beagle dog esophageal
fibroblasts
Detection of the relative expression of vimentin in
fibroblasts was performed as previously described (26), using the Vimentin
immunohistochemistry kit (Nanjing KeyGEN Biotech Co., Ltd, Jiangsu,
China) according to the manufacturer’s instructions. Cells of
passage three were seeded at a density of 3.0×105
cells/ml into a six-well plate containing sterilized coverslips.
Following two days of incubation, cells were fixed for 30 min with
4% paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd), washed
three times with PBS and then incubated in 3% hydrogen peroxide
(Sinopharm Chemical Reagent Co., Ltd) at room temperature for 5
min. Following blocking with goat serum (Nanjing KeyGEN Biotech
Co., Ltd) for 30 min, samples were incubated with mouse anti-human
monoclonal anti-vimentin antibodies (1:100; Nanjing KeyGEN Biotech
Co., Ltd) overnight at 4°C followed by incubation with
biotin-labeled goat anti-mouse immunoglobulin G (1:100 in antibody
diluents; Nanjing KeyGEN Biotech Co., Ltd) at 37°C for 30 min.
Samples were then incubated with horseradish peroxidase-labeled
streptavidin solutions (Nanjing KeyGEN Biotech Co., Ltd) at 37°C
for 30 min and the signals were evaluated following the addition of
diaminobenzidine (Nanjing KeyGEN Biotech Co., Ltd) and hematoxylin
staining under an inverted microscope (BX53; Olympus).
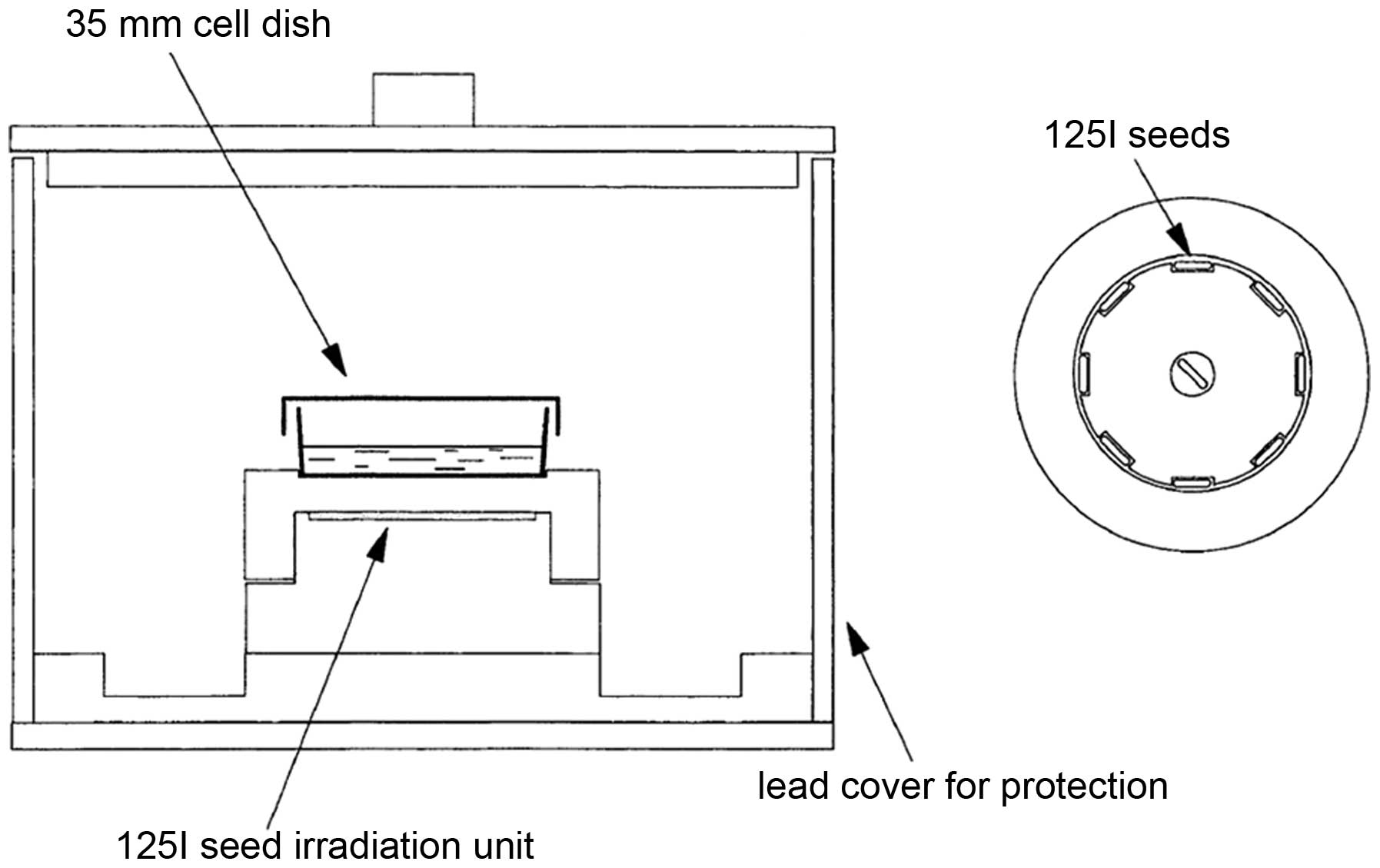
125I seed types and
development of an in vitro 125I seed irradiation
model
In the present study, 125I seeds of
clinical doses with 11.1, 22.2 and 33.3 MBq were purchased from GMS
Pharmaceutical Co., Ltd. (Shanghai, China). The in vitro
125I seed irradiation model developed in the present
study, as previously described (27), is shown in Fig. 1. One irradiation unit consisted of
a 35-mm diameter polystyrene panel with eight recesses (4.5×0.8 mm)
equidistantly spaced at the circumference with one seed in the
center. When irradiated, one petri dish containing cells was placed
6 mm over the panel with a 3-mm lead cover around the outer surface
for irradiation protection.
125I seed irradiation of
cells
Fibroblasts were treated in the presence or absence
of 125I seed irradiation with activities of 11.1, 22.2
and 33.3 MBq. Three replicates were used in the control group
without irradiation, while nine replicates were evaluated in each
experimental group. At passage three, 1.5×105 cells/ml
were seeded into 2 ml culture media, cultured overnight and
subsequently incubated in irradiation units for 72 h. The average
absorbed doses in cells within 72 h were 0.75, 1.50 and 2.25 Gy,
respectively. These data were determined using a software provided
by Beijing Tianhangkelin Technology Development Co, Ltd
(Radioactive seed source implantation treatment planning system;
China State Food and Drug Administration, approval no. 3700398 of
2009).
MTT assay
Fibroblasts at a density of 1.5×105
cells/ml in 200 μl were seeded into a 96-well plate. Following
addition of 20 μl MTT solution (5 mg/ml; Beyotime Institute of
Biotechnology, Jiangsu, China), plates were incubated in a
humidified environment with 5% CO2 at 37°C for 4 h.
Dimethyl sulfoxide (200 μl; Biosharp) was added to each well
following supernatant removal to dissolve the purple crystals.
Absorbance was then read at 490 nm on a microplate reader (Thermo
Fisher Scientific) and the inhibition rate (IR) in each
experimental group was calculated as follows: IR
(%)=[(ODcontrol group-ODexperimental
group)/ODcontrol group]x100%.
Assessment of cell apoptosis using
Annexin V and propidium iodide (PI) double staining
Apoptosis was quantified using the Annexin
V-Enhanced Green Fluorescent Protein Apoptosis Detection kit
(KeyGEN Biotech Co., Ltd) according to the manufacturer’s
instructions. In brief, 1 ml cells (1.5×105/ml) were
collected in flow cytometry tubes and resuspended in 500 μl binding
buffer. Then 5 μl Annexin V and 5 μl PI were added to cell
suspensions and the samples were incubated in the dark for 15 min
at room temperature. Flow cytometry was performed using a
FACSCalibur (BD Biosciences, San Jose, CA, USA). Data were acquired
and analyzed using CellQuestPro software (BD Biosciences).
Experiments were performed in triplicate. Annexin V and PI were set
on the horizontal and vertical axes, respectively, and flow
cytogram quadrants were divided as follows: Upper left,
mechanically damaged cells; upper right, late apoptotic or necrotic
cells; lower left, normal cells; and lower right, early apoptotic
cells. Total apoptotic rate including early and late apoptotic rate
were calculated.
Assessment of cell cycle distribution
using PI staining
Cells (1 ml; 1.5×105/ml) were collected
into flow cytometry tubes and fixed overnight in 1 ml ethanol
(−20°C, 70%; Sinopharm Chemical Reagent Co., Ltd) at 4°C. Following
centrifugation, supernatants were discarded and cells were
resuspended in 0.5 ml PBS. Following addition of 1 ml DNA
extraction buffer (Beyotime Institute of Biotechnology), the
samples were incubated for 5 min at room temperature and subjected
to centrifugation. The resulting pellets were resuspended and mixed
thoroughly in 400 μl DNA dye solution (PI and RNAase; Beyotime
Institute of Biotechnology) and stained in dark for 30 min. Samples
were analyzed using flow cytometry as described above and data were
acquired and analyzed using ModFitLT V2.0 software (Verity Software
House, Topsham, ME, USA).
Safety and efficacy evaluation of novel
esophageal stents loaded with 125I seeds for prevention
of benign restenosis in dogs
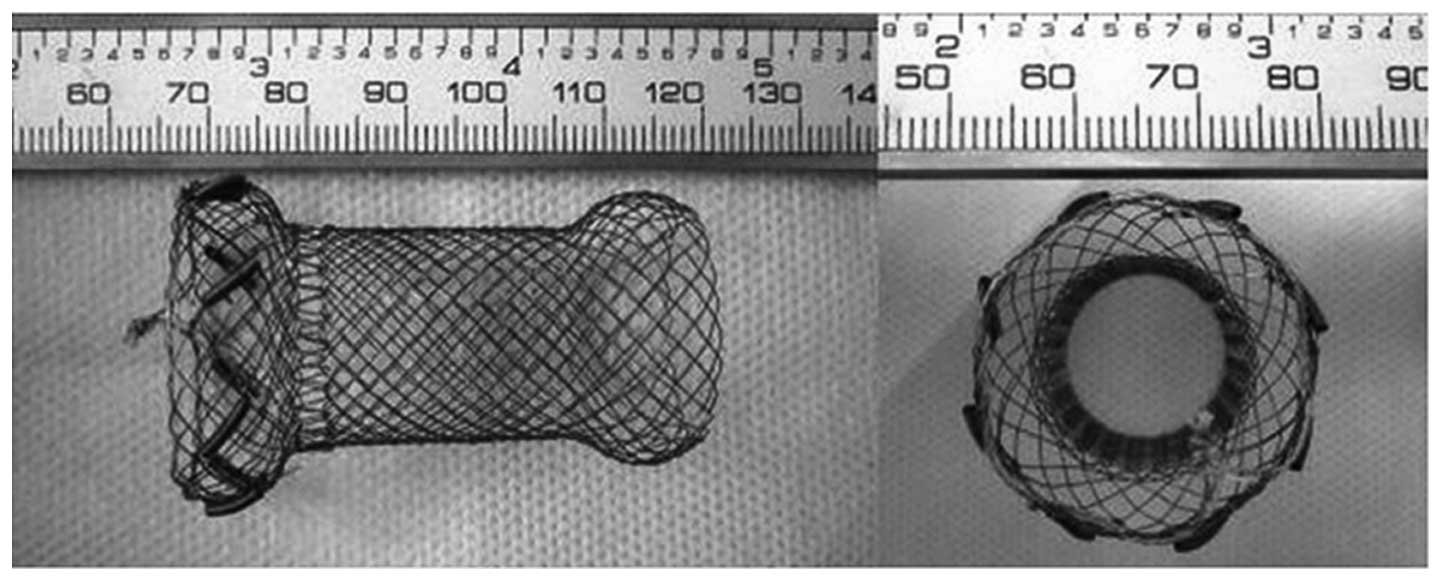
The esophageal stents used in the present study were
based on bare stents of 50 mm in length and 20 mm in diameter
nickel-titanium alloy wires (Micro-tech) and eight sheathes made of
alloy wires with a memory effect as holders for each
125I seed, equidistantly and symmetrically placed at the
circumference of the upper edge of the stents (Fig. 2).
A total of 32 Beagle dogs were randomly divided into
two groups of 16. In the experimental group, stents were loaded
with eight 125I seeds in eight sheathes on the upper
edge with the same activity of 33.3 MBq, as verified to be the
optimum dose, and implanted into the esophagi of the dogs. In the
control group, stents with empty seeds were used as implants. All
dogs fasted for 12 h prior to surgery. Anesthesia was administered
into forelimbs with intravenous injections of 1 ml/kg 3% sodium
amobarbital. Dogs were then immobilized and neck incisions were
made on the upper thorax. Dog esophageal walls were freed during
the surgery and surrounded by polytetrafluoroethene (PTFE)
membranes (MULTI-X9; Steriking, Bomlitz, Germany) with a
predetermined size of 2×6 cm, which were used to fix the stents.
The edges of the membranes were then sutured using no. 3 medical
suture needles and surgical sites were sutured layer by layer.
Esophageal stents were monitored using a digital subtracted
angiography (DSA; Innova 3100; GE Healthcare, Little Chalfont, UK).
Two days following stent implantation, dogs were intravenously
rehydrated and one day later were fed with semi-fluid diets. The
animals were regularly observed for general health conditions and
dietary intake.
DSA
At 1, 2, 4 and 8 weeks following implantation, four
dogs per group were anaesthetized and esophageal radiography under
DSA was performed using a Stenoscop 9000 small C-arm (Parameters:
70 kV, 150mA, 750×750 field; GE Healthcare) in order to detect loss
and disclosure of 125I seeds as well as the displacement
and patency of stents.
Esophageal inner diameters
Animals were sacrificed using an injection of 10%
KCl (Sinopharm Chemical Reagent Co., Ltd) into the forelimb vein.
The stented esophageal segments were harvested along with adjacent
tissues 20 mm above the stents, which were used for further
evaluation. Morphological changes in the esophagus were assessed by
measuring esophageal inner diameters which reflected the degree of
restenosis.
Hematoxylin-eosin (HE) staining and
immunohistochemistry
The stented esophageal segments, along with adjacent
tissues 20 mm above the stents, were harvested, fixed and paraffin
(Specimen Model Factory, Shanghai, China) embedded. Sections were
then stained with HE (Nanjing KeyGEN Biotech Co., Ltd) and
immunohistochemistry (IHC) was performed in order to detect
α-smooth muscle actin (SMA) and proliferating cell nuclear antigen
(PCNA) expression, using mouse anti-human monoclonal anti-α-SMA
antibodies (1:100 Beijing Biosynthesis Biotechnology Co., Ltd,
Beijing, China), mouse anti-human monoclonal anti-PCNA antibodies
(1:100; Beijing Biosynthesis Biotechnology Co., Ltd) and the PCNA
IHC detection kit (Maxin, Fujian, China). Sections were incubated
with the antibodies for 1 h at 37°C. Assays were performed
following routine IHC procedures and the specimens were observed
under a BX53 microscope (Olympus). Optical density values were
determined using Image-Pro Plus 6.0 (MediaCybernetics, Rockville,
MD, USA).
Amino acid composition
Proteins were digested by acid hydrolysis as
previously described (28). In
brief, 80 mg tissue samples were placed into a 10-ml ampoule
followed by the addition of 3 ml HCl (6 M; Sinopharm Chemical
Reagent Co., Ltd). The ampoules were filled with nitrogen
(Sinopharm Chemical Reagent Co., Ltd) and sealed. Following
incubation at 110°C for 12 h, samples were cooled down and 0.28 M
NaOH (Sinopharm Chemical Reagent Co., Ltd) was used for
neutralization prior to analysis using an 8900 Automaticamino acid
analyzer (Hitachi, Tokyo, Japan).
Statistical analysis
All statistical analyses were conducted using SPSS
version 18.0 (International Business Machines, Armonk, NY, USA).
Values are presented as the mean ± standard deviation of three
independent experiments performed in duplicate, unless otherwise
stated. Statistical significance was evaluated using a Student’s
t-test or one-way analysis of variance with a Dunnett’s test for
post hoc analysis. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Identification of Beagle dog esophageal
fibroblasts
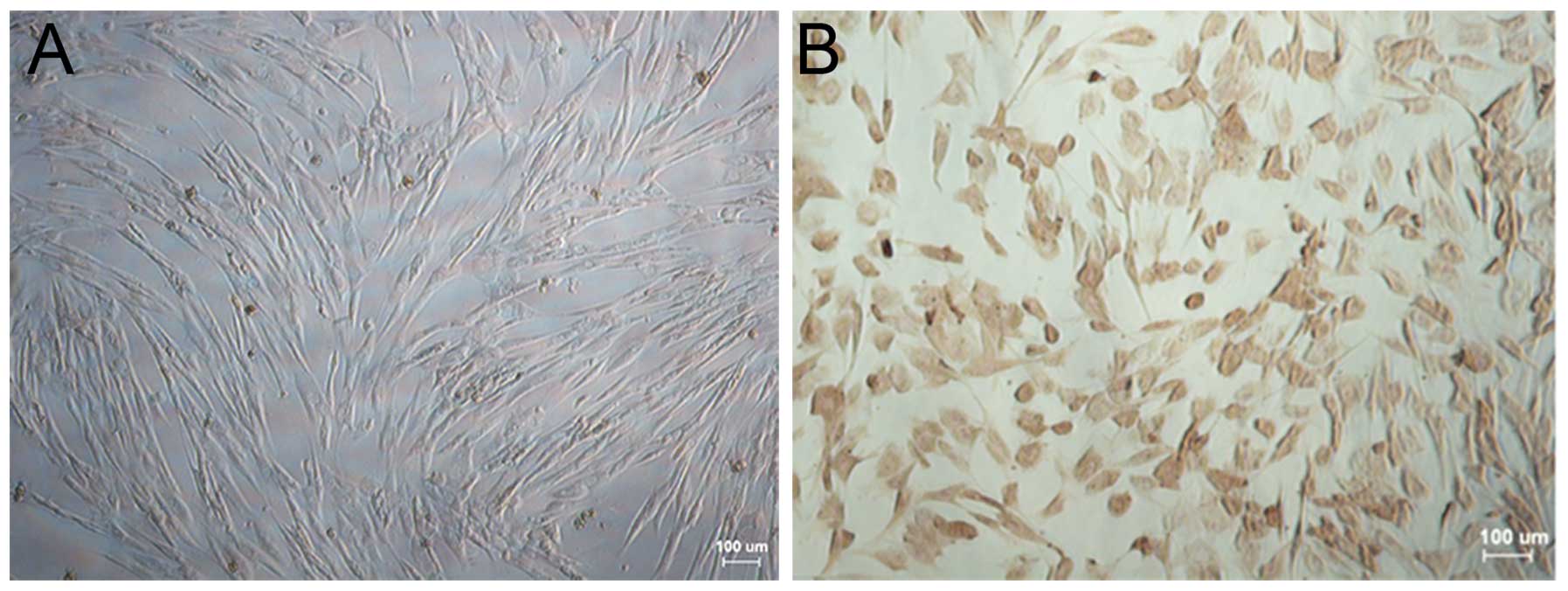
The morphological and immunohistochemical
characteristics of esophageal fibroblasts from Beagle dogs are
shown in Fig. 3. Fibroblasts were
translucent, spindle-shaped, elongated and radial or spiral-shaped
following overgrowth (Fig. 3A). In
addition, the relative specific expression of vimentin in
fibroblasts was determined using immunohistochemistry, which
revealed a large number of brown-stained granules in the cytoplasm
(Fig. 3B).
Effect of 125I seed
irradiation on proliferation, cell cycle distribution and apoptosis
in esophageal fibroblasts
For cell viability assays, absorbance was measured
at 490 nm following exposure of primary fibroblasts to
125I seed irradiation in vitro. The IR in each
experimental group was calculated as described above (Table I). The results demonstrated a
significant dose-dependent inhibition of fibroblast proliferation
(P<0.05), of which the most potent 125I seed activity
was at 33.3 MBq with an IR of 45.33±2.59%.
 | Table IEffects of 125I seed
irradiation on esophageal fibroblast proliferation. |
Table I
Effects of 125I seed
irradiation on esophageal fibroblast proliferation.
| Group |
OD490 | IR (%) |
|---|
| 0 MBq | 0.964±0.052 | 0 |
| 11.1 MBq | 0.706±0.019a | 26.81±1.96 |
| 22.2 MBq | 0.632±0.031a | 34.52±3.21 |
| 33.3 MBq | 0.527±0.025a | 45.33±2.59 |
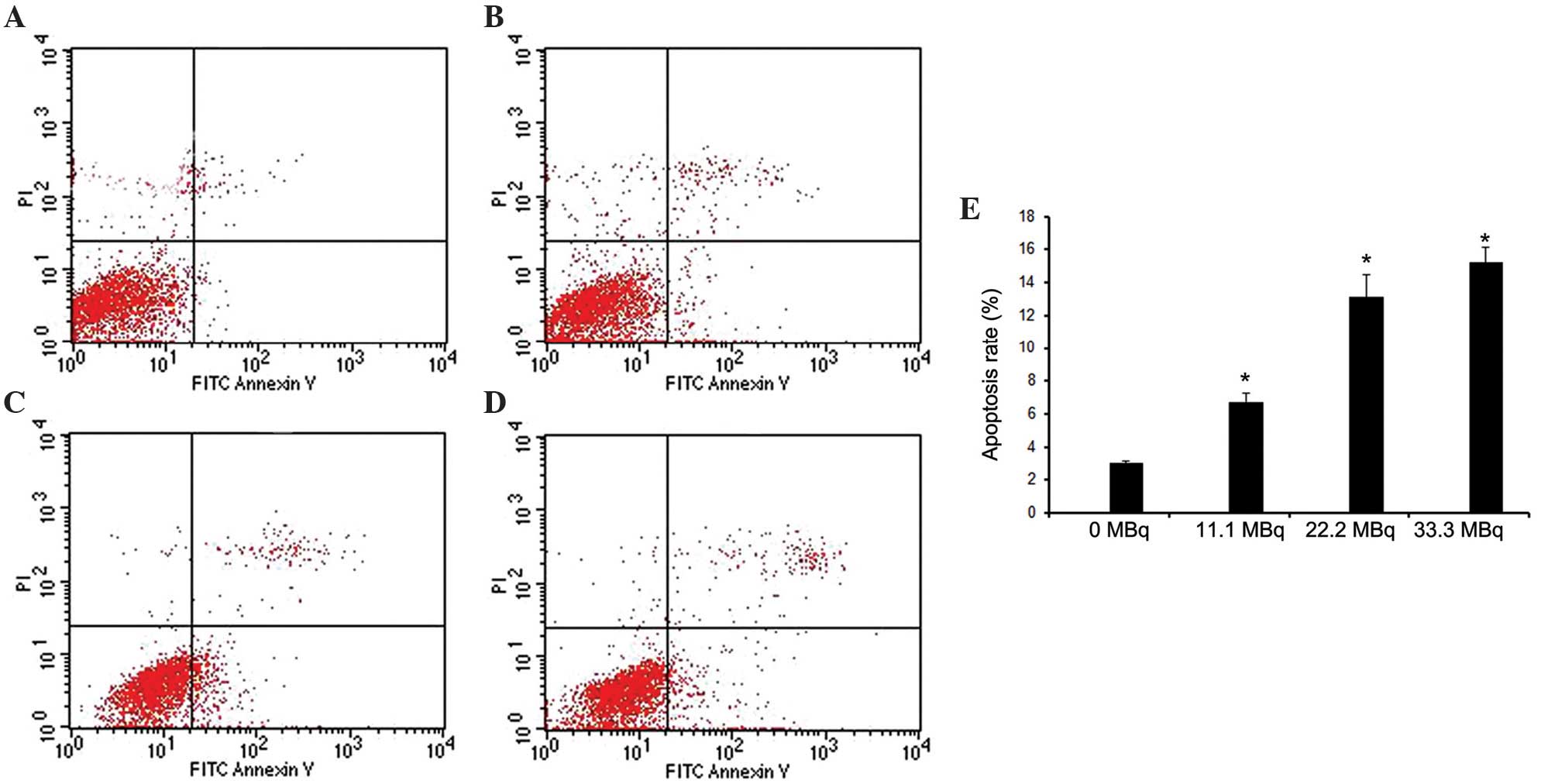
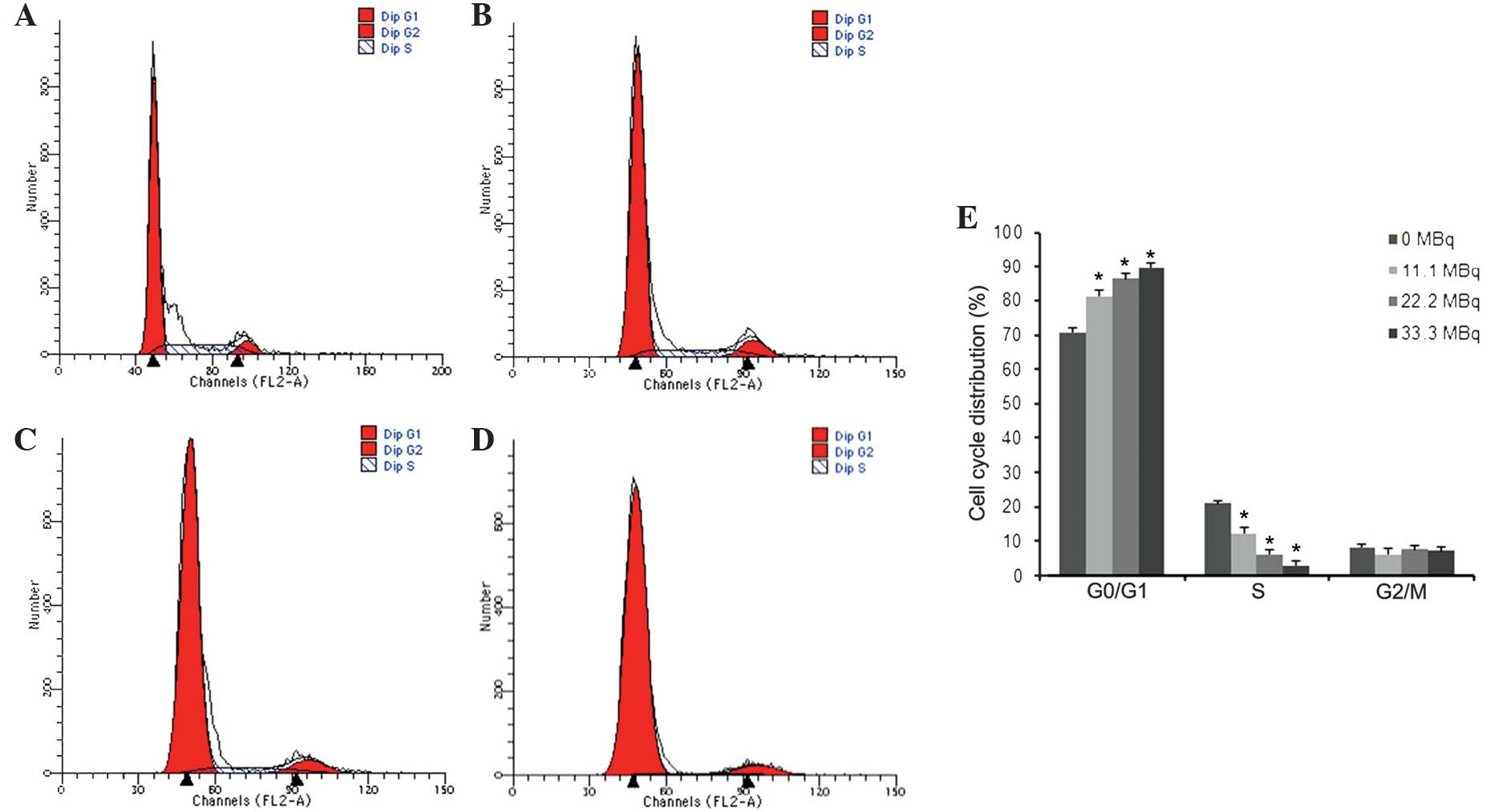
Apoptosis and cell cycle distribution in fibroblasts
were evaluated using flow cytometry following exposure to
125I seed irradiation. As shown in Fig. 4, the apoptotic rate gradually
increased in a dose-dependent manner with the activity of
125I seeds, with apoptotic rates of 6.73±0.57,
13.11±1.39 and 15.23±0.90% at 11.1, 22.2, and 33.3 MBq,
respectively (P<0.05 vs. 0MBq). PI staining was used to
determine cell cycle distribution, the results of which showed that
increased 125I seed activity resulted in decreased
S-phase populations and increased G1/G0-phase populations. However,
G2/M populations were not significantly different among groups
(Fig. 5).
Safety and efficacy of a novel esophageal
stent loaded with 125I seeds for prevention of benign
restenosis in dogs
All stents were successfully released at the
targeted sites. Following stent implantation, no stent migration,
seed shedding or displacement were observed using DSA monitoring.
At 1 and 2 weeks following implantation, a contrast agent was
passed through the stents in the experimental and control groups;
the agent passed smoothly with no significant stricture observed in
the upper edge of stents. At four weeks, the contrast agent passed
less smoothly and different levels of stricture were observed in
the upper edge of the stents between experimental and control
groups (data not shown). At eight weeks, the contrast agent passed
with great difficulty due to significant stricture in both groups
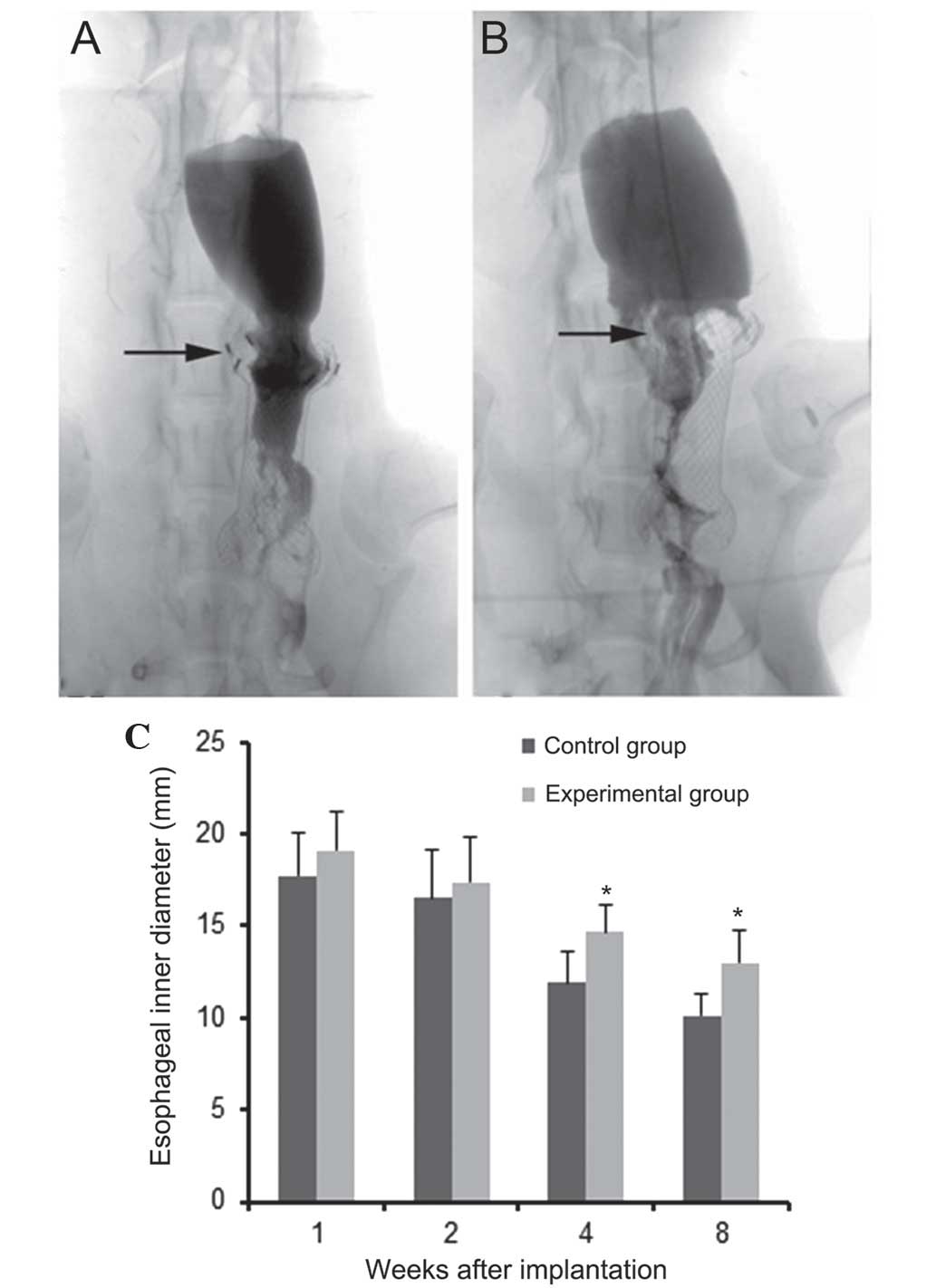
(Fig. 6). During DSA monitoring,
no signs of contrast extravasation or esophageal perforation were
observed in the experimental animals (Fig. 6A). Stricture was more pronounced in
the control animals (Fig. 6B). In
addition, no esophageal ulcerations or perforations were observed
within the range of 125I seed irradiations on the upper
edge following sacrification. Comparisons of intra-esophageal
diameters at the upper edge of the stents in each group were
measured at 1, 2, 4 and 8 weeks following implantation (Fig. 6C). These results showed that the
intra-esophageal diameters gradually decreased in a time-dependent
manner in each group. However, intra-esophageal diameters in the
experimental group were significantly higher at 4 and 8 weeks
compared with those in the control group (P<0.05), whereas at 1
and 2 weeks, there was no significant difference between groups
(P>0.05).
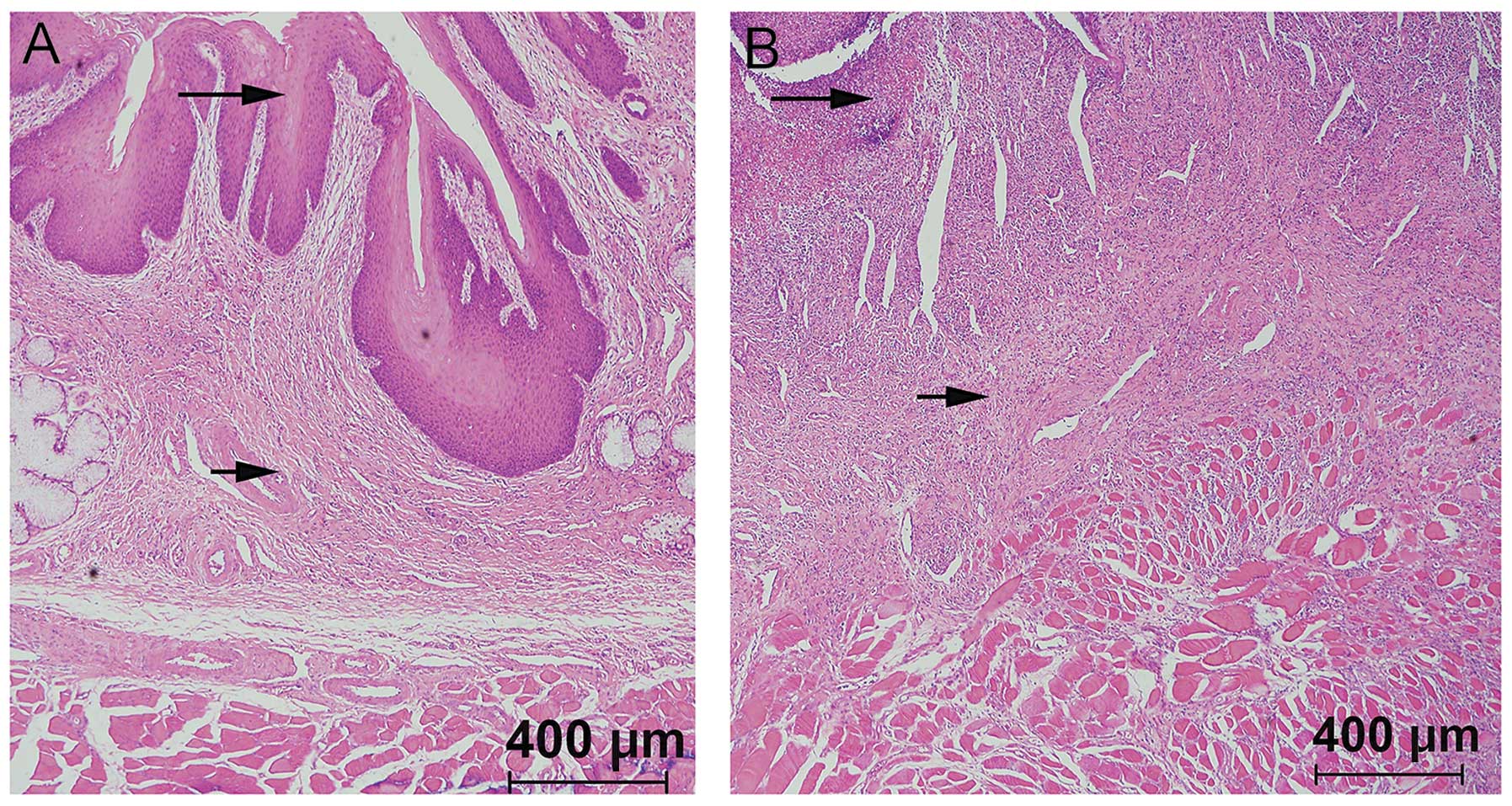
Following sacrification, the stented esophageal
segments were harvested along with the adjacent tissues for optical
microscopy and immunohistochemical analyses (Fig. 7A and B). Using HE staining and
microscopy, significant proliferation and thickening of the
esophageal squamous tissue was observed in each group. Submucosal
congestion, edema, vascular proliferation, infiltration of
inflammatory cells as well as the formation of fibrous connective
tissues and thickened esophageal muscle layers were also
detected.
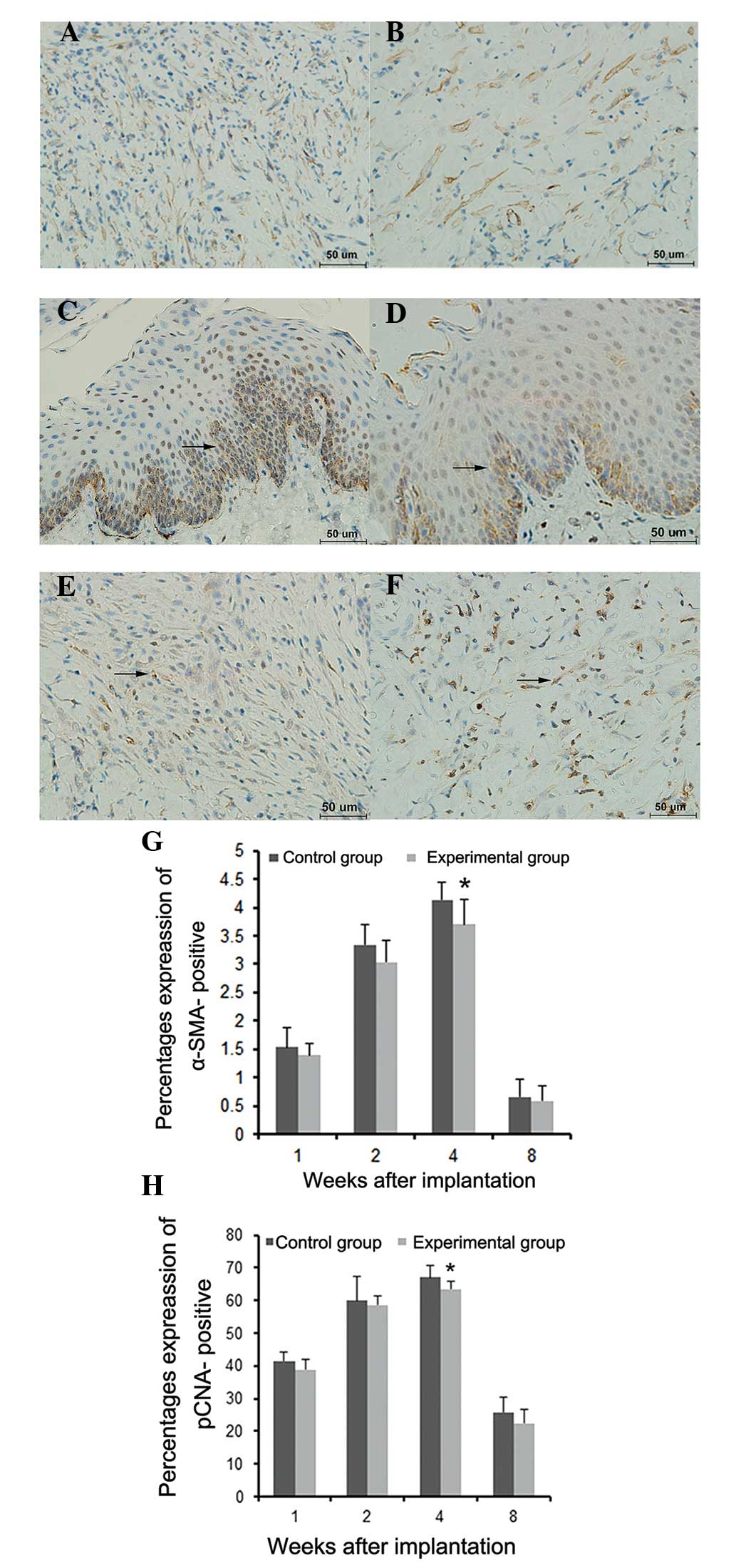
Immunohistochemical data showed no obvious
expression of α-SMA at either 1 or 8 weeks following implantation;
however, α-SMA was detected in the submucosal layer at 2 weeks
following implantation, which mainly consisted of fibroblast
cytoplasmic, nuclear and neovascular staining. In addition, α-SMA
expression was significantly lower in the experimental group at
four weeks after implantation compared with that in the control
group (P<0.05) (Fig. 8A, B and
G).
Furthermore, immunohistochemical analysis showed
PCNA expression 1 week following implantation. At 2 weeks post
implantation, PCNA was primarily expressed in the basement membrane
of the squamous epithelium and partially in the submucosal layer.
In addition, PCNA expression in the experimental group was
significantly lower at 4 weeks following implantation compared with
that of the control group (P<0.05) (Fig. 8C–F and H).
As shown in Table
II, the hydroxyproline contents of esophageal tissues at the
upper edge of stents significantly increased in each group in a
time-dependent manner. However, the hydroxyproline content in the
experimental group was significantly lower compared with that in
the control group at 4 and 8 weeks following implantation
(P<0.05).
 | Table IIHydroxyproline content in esophageal
tissues in upper edge of stents following 125I seed
esophageal stent implantation. |
Table II
Hydroxyproline content in esophageal
tissues in upper edge of stents following 125I seed
esophageal stent implantation.
| Hydroxyproline
(mg/l) |
|---|
|
|
|---|
| Time period | Control group | Experimental
group |
|---|
| 1 week post
surgery | 55.21±2.36 | 51.85±1.53 |
| 2 weeks post
surgery | 67.91±1.94 | 64.88±2.29 |
| 4 weeks post
surgery | 90.59±1.98 | 80.56±1.97a |
| 8 weeks post
surgery | 93.19±1.55 | 84.50±2.53a |
Discussion
The in vitro irradiation model used in the
present study has been widely used for 226Ra short
distance irradiation treatment and radiation dosimetry, which was
first described by Meredith (29). This model was improved by
Aird et al (27) for
application in the study of 125I seed irradiation. An
important concept, D/h, was implemented in this work, where D was
the diameter of the irradiated circumference and h was the height
between the irradiated circumference and the Petri dish. When
D/h≤3, radiation sources only require to be placed at the
circumference; however, when 3<D/h≤6, an additional 5% radiation
source is required at the circumference, and when D/h>6, the
radiation source requires to be placed in the coaxial
circumferences as well as their centers. The purpose of this design
was to obtain the best homogeneous dose distribution throughout
Petri dishes. In the present study, 35-mm diameter petri dishes
were used with small doses of 125I seeds, which had a
very small effective radius; therefore, h=6 mm was selected.
D/h≈5.8 was designed with eight 125I seeds equidistantly
placed around the circumferences and one seed in the center to
obtain a homogeneous ray; therefore, the variation of rays passing
through the dishes was <10%. The average absorbed doses in the
three experimental groups of 125I seed activity of 11.1,
22.2 and 33.3 MBq were 1.04, 2.08 and 3.12 cGy/h, respectively. The
average absorbed doses in cells within 72 h were 0.75, 1.50 and
2.25 Gy, respectively. These data were determined using a software
provided by Beijing Tianhangkelin Technology Development Co, Ltd
(Radioactive seed source implantation treatment planning system;
China State Food and Drug Administration, approval no. 3700398 of
2009).
The in vitro effects of 125I seed
irradiation on fibroblasts were determined through the evaluation
of cell proliferation, apoptosis and cell cycle distribution. The
results of the present study demonstrated the significant
inhibitory effects of 125I seeds on fibroblast
proliferation in a dose-dependent manner. The highest
125I seed activity of 33.3 MBq showed the most potent IR
of 45.33±2.59%. The effects of 125I seeds on apoptosis
and cell cycle distribution were detected using Annexin V/PI double
staining and PI staining, respectively; the results showed that
apoptosis increased with 125I seed activity. In gastric
cancer MKN45 cell lines, apoptosis rates of 13.67±1.58% and
14.00±1.87% were observed following irradiation with
125I seeds at 33.3 and 22.2 MBq, respectively, showing
no statistically significant differences among groups (30). In addition, CL187 cells were
treated with a similar in vitro 125I seed
irradiation model and an apoptotic rate of 13.74±1.63% was reported
in the group with an absorbed dose of 2 Gy (22). In the present study, cell cycle
experiments demonstrated that 125I seed irradiation
resulted in a dose-dependent increase of cells in G0/G1 phase,
while S-phase populations decreased. G2 phase cell populations were
not significantly different among groups, and no sub-G1 peaks were
observed in any experimental group; by contrast, previous studies
have reported a gradual dose-dependent elevation and the presence
of sub-G1 peaks (22,23,31–33).
The discrepancies between these data and the present findings may
be due to the different doses as well as cell types that were
studied; the previous studies mentioned the use of 125I
seeds with higher activities. The low doses of 125I
seeds used in the present study reflect those used clinically. It
is likely that low doses of 125I seeds may result in
blocking G1 to S-phase transition in fibroblasts, which results in
the breakdown of cellular DNA into small fragments; therefore, no
sub-G1 peaks were observed. In summary, all three activities of
125I seeds showed significant inhibitory effects on cell
proliferation and certain pro-apoptotic effects in Beagle dog
esophageal fibroblasts, with 33.3 MBp displaying the most potent
effects. G1/S cell cycle arrest was induced in fibroblasts via
irradiation of 125I seeds, resulting in the inhibition
of fibroblast proliferation.
In the present study, a novel stent was developed
through loading 8 125I seeds with the same activity of
33.3 MBq onto the upper edge of a normal esophageal stent, which
was then successfully implanted into the esophagus of Beagle dogs.
No esophageal bleeding or perforation was observed using DSA
monitoring and macroscopic observation 8 weeks following stent
implantation, indicating the safety and feasibility of this
technology. α-SMA is a known indicator of fibroblast proliferation
and excessive extracellular matrix (ECM) secretion (34). During restricture, fibroblasts
secrete large amounts of ECM, including different types of
collagen, proteoglycans, elastin and certain adhesion proteins.
Collagen proteins contain large amounts of hydroxyproline while
most non-collagen proteins are hydroxyproline free (35); therefore, the level of fibroblast
proliferation may be evaluated using hydroxyproline content and
α-SMA expression assays. In the present study, α-SMA expression and
hydroxyprolin content were found to be significantly decreased in
the experimental group at four weeks following implantation
compared with those of the control group. This therefore indicated
that the proliferation of fibroblasts was effectively inhibited by
125I seeds. These findings were in accordance with the
results of the intra-esophagus diameter experiments in the present
study, which demonstrated that at 4 weeks following implantation,
there were significant differences between the intra-esophagus
diameters of the experimental and control groups. In addition, at 8
weeks following implantation, hyperplasia in the upper edge of the
stent was significantly reduced in the experimental group compared
with that in the control group.
In conclusion, the in vitro experiments
showed that 125I seeds significantly inhibited cell
proliferation via cell cycle arrest and apoptosis in Beagle dog
esophageal fibroblasts. In addition, animal studies demonstrated
that the application of a novel esophageal stent loaded with
125I seeds inhibited benign hyperplasia in the upper
edge of the stent and, to a certain extent, relieving benign
restenosis; this stent was also found to safely prevent benign
restenosis following stent implantation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81071238).
References
|
1
|
Sharma V, Mahantshetty U, Dinshaw KA,
Deshpande R and Sharma S: Palliation of advanced/recurrent
esophageal carcinoma with high-dose-rate brachytherapy. Int J
Radiat Oncol Biol Phys. 52:310–315. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghosh S, Sau S, Mitra S, Manna A and Ghosh
K: Palliation of dysphagia in advanced, metastatic or recurrent
carcinoma oesophagus with high dose rate intraluminal
brachytherapy-an eastern Indian experience of 35 cases. J Indian
Med Assoc. 110:449–452. 2012.
|
|
3
|
Bhatt L, Tirmazy S and Sothi S:
Intraluminal high-dose-rate brachytherapy for palliation of
dysphagia in cancer of the esophagus: initial experience at a
single UK center. Dis Esophagus. 26:57–60. 2013. View Article : Google Scholar
|
|
4
|
Song HY, Do YS, Han YM, et al: Covered,
expandable esophageal metallic stent tubes: experiences in 119
patients. Radiology. 193:689–695. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dua KS, Vleggaar FP, Santharam R and
Siersema PD: Removable self-expanding plastic esophageal stent as a
continuous, non-permanent dilator in treating refractory benign
esophageal strictures: a prospective two-center study. Am J
Gastroenterol. 103:2988–2994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sreedharan A, Harris K, Crellin A, Forman
D and Everett SM: Interventions for dysphagia in oesophageal
cancer. Cochrane Database Syst Rev. CD0050482009.PubMed/NCBI
|
|
7
|
Ernst A, Feller-Kopman D, Becker HD and
Mehta AC: Central airway obstruction. Am J Respir Crit Care Med.
169:1278–1297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krueger KD, Mitra AK, DelCore MG, Hunter
WJ III and Agrawal DK: A comparison of stent-induced stenosis in
coronary and peripheral arteries. J Clin Pathol. 59:575–579. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauriedel G, Skowasch D, Jabs A, et al:
Insights into vascular pathology after intracoronary brachytherapy.
Z Kardiol. 91(Suppl 3): 1–9. 2002. View Article : Google Scholar
|
|
10
|
Guo JH, Teng GJ, Zhu GY, et al:
Self-expandable esophageal stent loaded with 125I seeds: initial
experience in patients with advanced esophageal cancer. Radiology.
247:574–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo JH, Teng GJ, Zhu GY, He SC, Deng G and
He J: Self-expandable stent loaded with 125I seeds: feasibility and
safety in a rabbit model. Eur J Radiol. 61:356–361. 2007.
View Article : Google Scholar
|
|
12
|
Mayoral W, Fleischer D, Salcedo J, Roy P,
Al-Kawas F and Benjamin S: Nonmalignant obstruction is a common
problem with metal stents in the treatment of esophageal cancer.
Gastrointest Endosc. 51:556–559. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH, Song HY, Choi EK, Kim KR, Shin JH
and Lim JO: Temporary metallic stent placement in the treatment of
refractory benign esophageal strictures: results and factors
associated with outcome in 55 patients. Eur Radiol. 19:384–390.
2009. View Article : Google Scholar
|
|
14
|
Albiero R, Adamian M, Kobayashi N, et al:
Short- and intermediate-term results of (32) P radioactive
beta-emitting stent implantation in patients with coronary artery
disease: The Milan Dose-Response Study. Circulation. 101:18–26.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fareh J, Martel R, Kermani P and Leclerc
G: Cellular effects of beta-particle delivery on vascular smooth
muscle cells and endothelial cells: a dose-response study.
Circulation. 99:1477–1484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
John M, Shroff S, Farb A and Virmani R:
Local arterial responses to 32P beta-emitting stents. Cardiovasc
Radiat Med. 2:143–150. 2001. View Article : Google Scholar
|
|
17
|
Teirstein PS: Prevention of vascular
restenosis with radiation. Tex Heart Inst J. 25:30–33.
1998.PubMed/NCBI
|
|
18
|
Williams DO: Radiation vascular therapy: a
novel approach to preventing restenosis. Am J Cardiol. 81:18E–20E.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishiwata S, Robinson K, Chronos N, Crocker
IR and King SB III: Irradiation and postangioplasty restenosis: a
recent overview. Jpn Heart J. 41:541–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marzocchi A, Marrozzini C, Piovaccari G,
et al: Restenosis after coronary angioplasty: its pathogenesis and
prevention. Cardiologia. 36(12 Suppl 1): 309–320. 1991.(In
Italian). PubMed/NCBI
|
|
21
|
Chen H, Bao Y, Yu L, Jia R, Cheng W and
Shao C: Comparison of cellular damage response to low-dose-rate
125I seed irradiation and high-dose-rate gamma irradiation in human
lung cancer cells. Brachytherapy. 11:149–156. 2012. View Article : Google Scholar
|
|
22
|
Zhuang HQ, Wang JJ, Liao AY, Wang JD and
Zhao Y: The biological effect of 125I seed continuous low dose rate
irradiation in CL187 cells. J Exp Clin Cancer Res. 28:122009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao A, Wang J, Wang J, Zhuang H and Zhao
Y: Relative biological effectiveness and cell-killing efficacy of
continuous low-dose-rate 125I seeds on prostate carcinoma cells in
vitro. Integr Cancer Ther. 9:59–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zamorano L, Yakar D, Dujovny M, Sheehan M
and Kim J: Permanent iodine-125 implant and external beam radiation
therapy for the treatment of malignant brain tumors. Stereotact
Funct Neurosurg. 59:183–192. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma ZH, Yang Y, Zou L and Luo KY: 125I seed
irradiation induces up-regulation of the genes associated with
apoptosis and cell cycle arrest and inhibits growth of gastric
cancer xenografts. J Exp Clin Cancer Res. 31:612012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goodpaster T, Legesse-Miller A, Hameed MR,
Aisner SC, Randolph-Habecker J and Coller HA: An
immunohistochemical method for identifying fibroblasts in
formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem.
56:347–358. 2008. View Article : Google Scholar
|
|
27
|
Aird EG, Folkard M, Mayes CR, Bownes PJ,
Lawson JM and Joiner MC: A purpose-built iodine-125 irradiation
plaque for low dose rate low energy irradiation of cell lines in
vitro. Br J Radiol. 74:56–61. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Macheboeuf M, Basset J, Barbu E, Le Saget
M and Nunez G: Effect of pressure on the hydrolysis of proteins by
acids or alkalis. C R Seances Soc Biol Fil. 144:962–964. 1950.(In
French). PubMed/NCBI
|
|
29
|
Meredith WJ: Radium dosage-The Marchester
system. E&S Livingstone Ltd; Edinburgh: 1947
|
|
30
|
He QM and Wu XT: Experimental study on
apoptosis of gastric cancer cell line MKN45 induced by continuous
irradiation from iodine-125seeds. J Sichuan Univ Med Sci Ed.
40:454–458. 2009.(In Chinese).
|
|
31
|
Wang J, Zhang H, Zhuang H, Zhao Y and Liao
A: Development and validation of radioactive iodine-125 irradiator
in vitro. Chin J Radiol Med Protect. 27:267–271. 2007.
|
|
32
|
Vávrová J, Rezácová M, Vokurková D and
Psutka J: Cell cycle alteration, apoptosis and response of leukemic
cell lines to gamma radiation with high- and low-dose rate. Physiol
Res. 53:335–342. 2004.PubMed/NCBI
|
|
33
|
Mirzaie-Joniani H, Eriksson D, Johansson
A, et al: Apoptosis in HeLa Hep2 cells is induced by low-dose,
low-dose-rate radiation. Radiat Res. 158:634–640. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang YE, Xu ZD and Wang XH: Dynamic
changes in extracellular matrix and related interstitial calls in
experimental organ sclerosis. Chin J Pathol. 23:111–114. 1994.(In
Chinese).
|
|
35
|
Kovacs EJ and DiPietro LA: Fibrogenic
cytokines and connective tissue production. FASEB J. 8:854–861.
1994.PubMed/NCBI
|