Introduction
Lung cancer is a disease characterized by
uncontrolled cell growth in lung tissue and has the highest
incidence and mortality of any type of cancer (1,2).
Small cell lung carcinoma (SCLC) and non-small cell lung carcinoma
(NSCLC) are the most prevalent types of lung cancer, and 80% of
cases of lung cancer are NSCLC. Following diagnosis with lung
cancer, the 5-year survival rate for patients is 10% (3,4), and
the incidence and rate of mortality of lung cancer in China is
rising. Thus, improvements in clinical diagnosis and management are
required. Previous studies have focused on microRNAs (miRNAs) in
the pathogenesis and progression of lung cancer (5–8).
miRNAs are a family of small noncoding RNAs that are
20~23 nucleotides in length, and act predominantly at the
posttranscriptional level, working as critical regulators of gene
expression (9,10). miRNAs are vital in the regulation
of cell apoptosis and proliferation, in addition to functioning as
either oncogenes or tumor suppressors in the cell cycle. At
present, the number of miRNAs encoded by the human genome is
>1,000, and miRNAs are established to be distributed in various
cells and tissues in different proportions (11,12).
miR-33a has been identified to be important in the lipid metabolism
and the regulation of cholesterol and high-density lipoprotein
formation, via the downregulation of ABCA1 and ABCG1 expression
(13). SREBP is an important gene
that regulates lipid metabolism and the synthesis of fat, while the
SREBP/miR-33 locus has been suggested to be involved in cell
proliferation and cell cycle progression (14). Cirera-Salinas et al
(15) demonstrated that miR-33a
was downregulated in lung cancer cells, however, the mechanism of
miR-33a in the modulation of lung cancer progression remains to be
fully elucidated.
In the present study, miR-33a was overexpressed in
the lung cancer cell lines A549 and NCI-H460, and the progression
and cell cycles were determined in miR-33a-transfected lung cancer
cells. An improved understanding of the progression and mechanisms
of lung cancer would be beneficial to patient prognosis.
Materials and methods
Cells and reagents
The human lung cancer cell lines A549 and NCI-H460
were purchased from the American Type Culture Collection (Manassas,
VA, USA) and maintained in our lab in HyClone Dulbecco’s modified
Eagle’s medium (DMEM; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco Life
Technologies, Carlsbad, CA, USA). MTT was obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The pri-miRNA PCR product was amplified and
constructed into a pGenesil-1.1 vector and the sequence was
measured and aligned using the basic local alignment search tool
(blast.ncbi.nlm.nih.gov/Blast.cgi;
National Center for Biotechnology Information). RT-qPCR was used
for detecting mRNA expression levels of miR33a, β-actin and
β-catenin. Total RNA was extracted and reverse transcribed into
cDNA using M-MLV reverse transcriptase and the
oligo(deoxythymine)15 primer, and the resulting cDNA was used as a
template for PCR amplification. The primer sequences were as
follows: miR-33a, F 5′-GGTTAGATCTTGCTCCAGCGGTTTG-3′ and R
5′-GTAAAGCTTGCCCTCCTGTTTCCTG-3′; β-actin, F
5′-AGAGCTACGAGCTGCCTGAC-3′ and R 5′-AGCACTGTGTTGGCGTACAG-3′.
MTT assay
The proliferation of lung cancer cells was measured
using an MTT assay as previously described (16–18).
Briefly, the A549 and NCI-H460 lung cancer cells were plated into
48-well plates. Following culture for 8 h, the cells were
transfected with pGenesil-1.1-miR-33a or the negative control
pGenesil-1.1 and cultured for another 6 h. Next, the medium was
changed to DMEM with 10% FBS and the cells were cultured for 24,
48, 72 or 96 h. The proliferation of the lung cancer cells was
determined by measuring the optical density of the samples at 490
nm.
Clone formation assay
The cells were plated into 6-well plates
(5×105 cells/well) and transfected with pGenesil-1.1 and
pGenesil-1.1-miR-33a cultured for 10 days. The medium was refreshed
every 4 days. The surviving colonies (≥50 cells/colony) were fixed
with methanol. The colonies were stained with 1.25% crystal violet
staining solution (#C0121; Beyotime Institute of Biotechnology,
Jiangsu, China) and counted under a light microscope.
Western blot analysis
The cell extracts were lysed in SDS lysis buffer
(#P0013G; Beyotime Institute of Biotechnology) and separated using
SDS-PAGE as previously described (19–21)
on 10% polyacrylamide gel. The SDS buffer consisted of the
following reagents: 250 mM Tris-HCl (pH 6.8); 10% (w/v) SDS; 0.5%
(w/v) bromophenol blue; 50% (v/v) glycerin; and 5% (w/v)
β-mercaptoethanol. The antibodies used were as follows: β-Catenin
monoclonal mouse anti-human IgG1 (12F7; sc-59737);
β-actin monoclonal mouse anti-gizzard IgG1 (C4;
sc-47778); goat anti-mouse horseradish peroxidase-conjugated
secondary antibody (sc-2005; 1:10,000). All antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Flow cytometric analysis
Cell cycle progression was determined using
propidium iodide (PI) staining as previously described (22,23).
A total of 1×106 A549 lung cancer cells were washed
twice in cold HyClone phosphate-buffered saline (PBS; GE Healthcare
Life Sciences) and fixed in 4% paraformaldehyde (Sigma-Aldrich).
Following a 30-min resting period, the cells were washed twice
again in PBS, PI (Sigma-Aldrich) and RNase A (Sigma-Aldrich) and
were added at a final concentration of 100 ng/ml. Subsequent to
incubation for 10 min at room temperature, the cells were detected
and analyzed using flow cytometry (Beckman Coulter, Brea, CA,
USA).
Statistical analysis
SPSS, version 11.5 (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis and all results are presented as
the mean ± standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
The expression of miR-33a in A549 lung
cancer cells
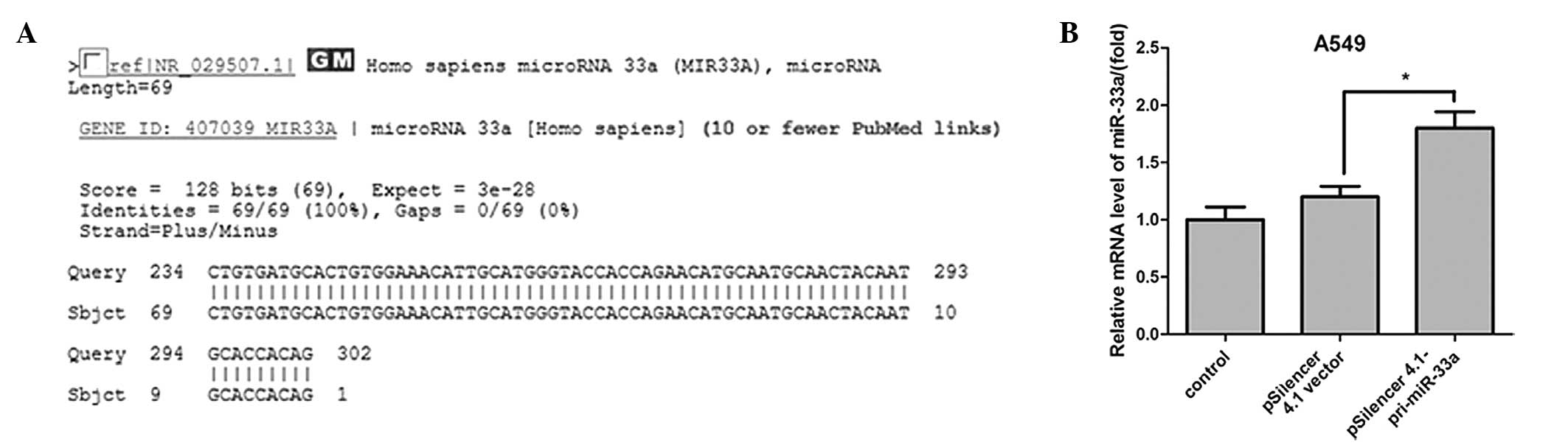
The pri-miRNA PCR product sequence was measured and
aligned using the basic local alignment search tool (Fig. 1A). The expression level of miR-33a
was detected in A549 cells using qPCR, and the results demonstrated
that miR-33a expression was significantly upregulated when A549
cells were transfected with miR-33a for 24 h (median ratio of
1.8-fold; P<0.05; Fig. 1B)
compared with the control vector.
Transfection with miR-33a inhibits the
proliferation of the lung cancer cell lines A549 and NCI-H460
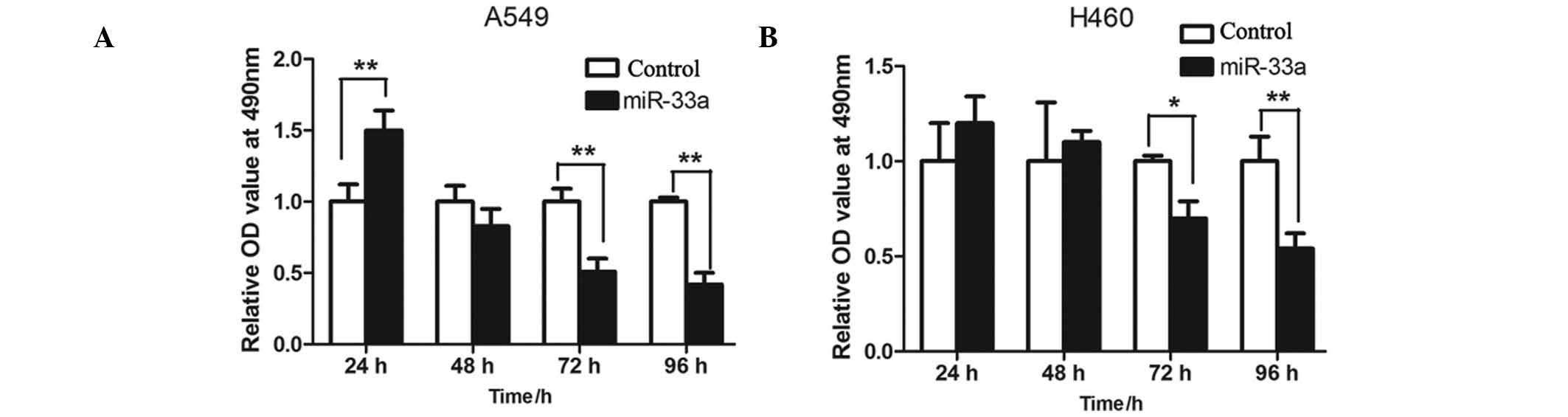
In order to detect the effects of miR-33a on
proliferation of lung cancer cells, MTT assay was used to measure
proliferation levels in the A549 and NCI-H460 cells. The two cell
lines were transfected with miR-33a and cultured for 24, 48, 72 and
96 h, and the relative optical density values measured at a
wavelength of 490 nm were determined. The results indicated that
the rate of proliferation of miR-33a-transfected A549 cells was
significantly lower than the controls at 72 and 96 h (P<001;
Fig. 2A). This was consistent with
levels detected in the NCI-H460 cells (P<0.05 and P<0.01,
respectively; Fig. 2B).
Clone formation rate is reduced in lung
cancer cells transfected with miR-33a
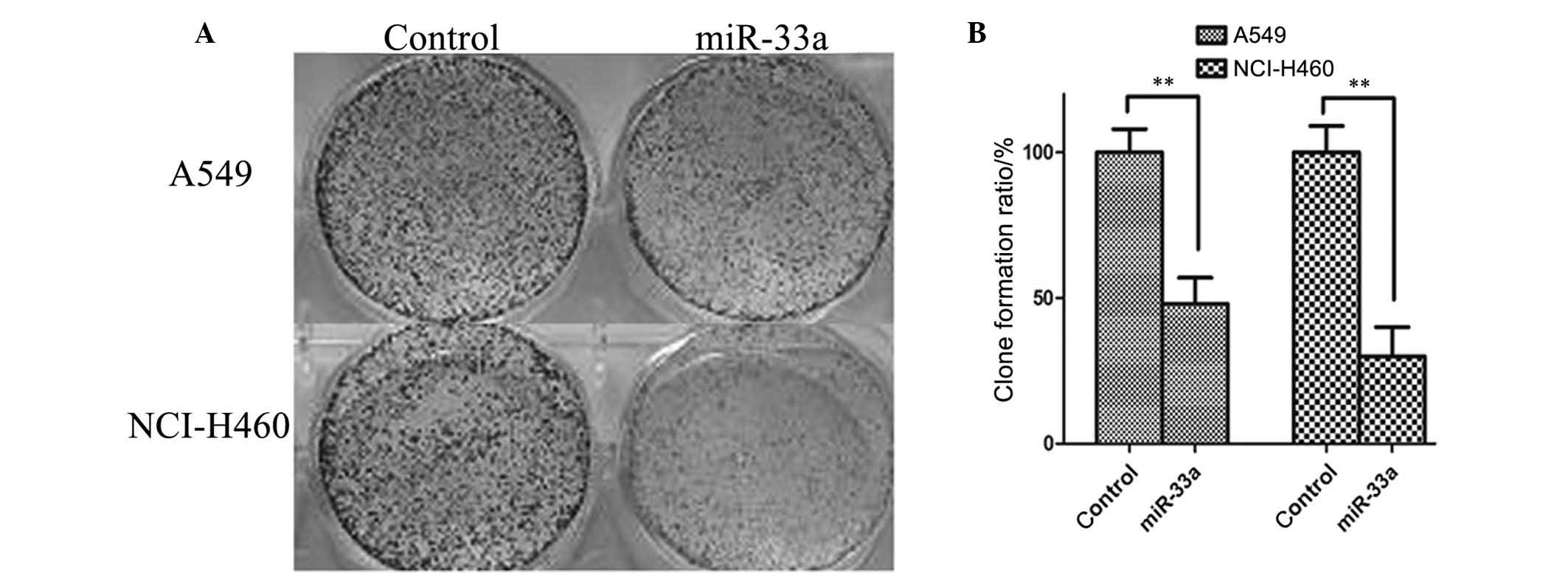
The colony formation assay was used to confirm the
inhibitory effect of miR-33a on the lung cancer cell lines A549 and
NCI-H460. As illustrated in Fig.
3, the number of colonies formed was significantly reduced in
A549 and NCI-H460 cells transfected with miR-33a for 48 h compared
with controls (P<0.01). Thus, upregulation of miR-33a may
inhibit the proliferation of A549 and NCI-H460 lung cancer
cells.
miR-33a induces cell cycle arrest at
G1/S phase in lung cancer cells
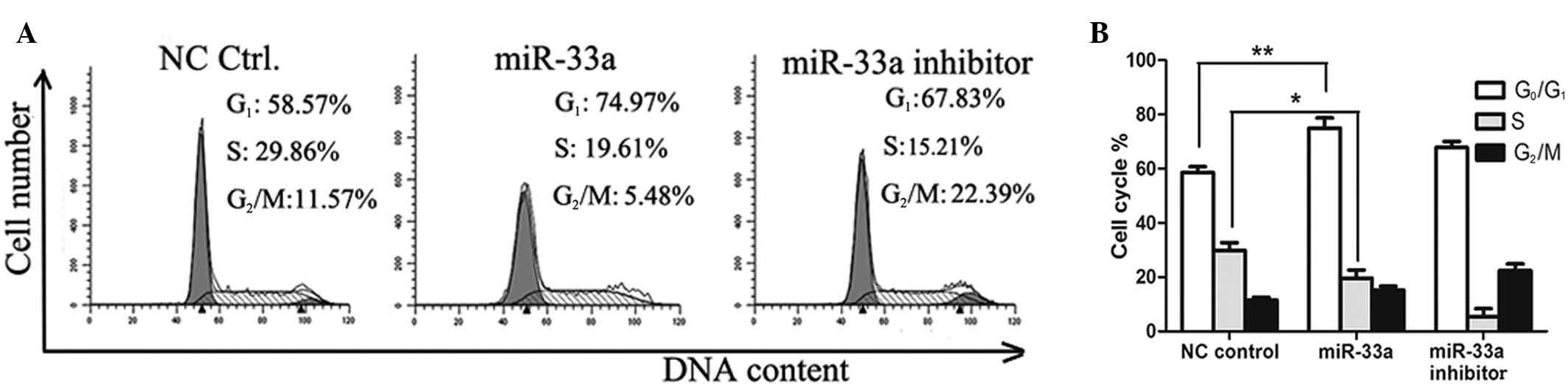
Next, cell distribution was determined by
fluorescence-activated cell sorting (FACS) analysis in a different
group of cells. As demonstrated in Fig. 4, a significantly greater number of
miR-33a-transfected A549 cells were arrested at the G1/S
phase compared with the negative control group (P<0.01), with
the percentage of G1 phase cells increasing from 58.57
to 74.97%.
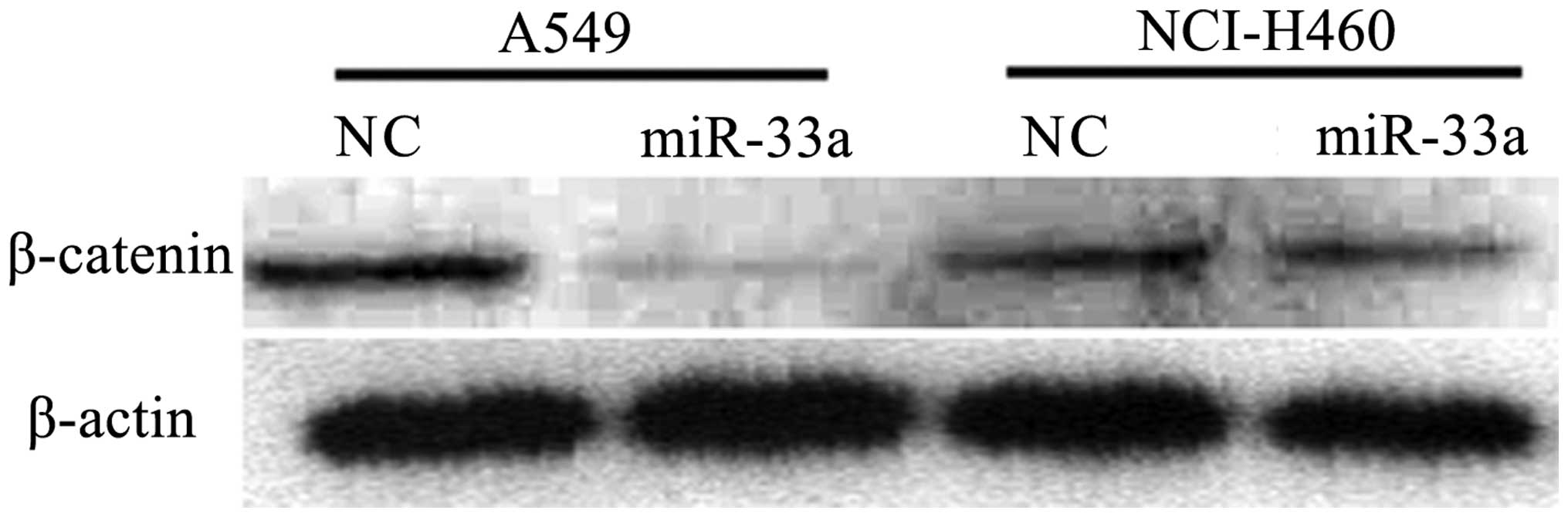
Transfection with miR-33a reduces the
expression of β-catenin
β-catenin is established to be involved in cell
cycle progression, and its abnormal expression is observed in
various tumor cell lines. In order to detect whether the
transfection with miR-33a affects the expression of β-catenin,
western blot analysis was used to detect the expression of
β-catenin in lung cancer cell lines transfected with miR-33a. As
demonstrated in Fig. 5, in
miR-33a-transfected A549 and NCI-H460 cells, the expression of
β-catenin was reduced compared with that in negative control cells.
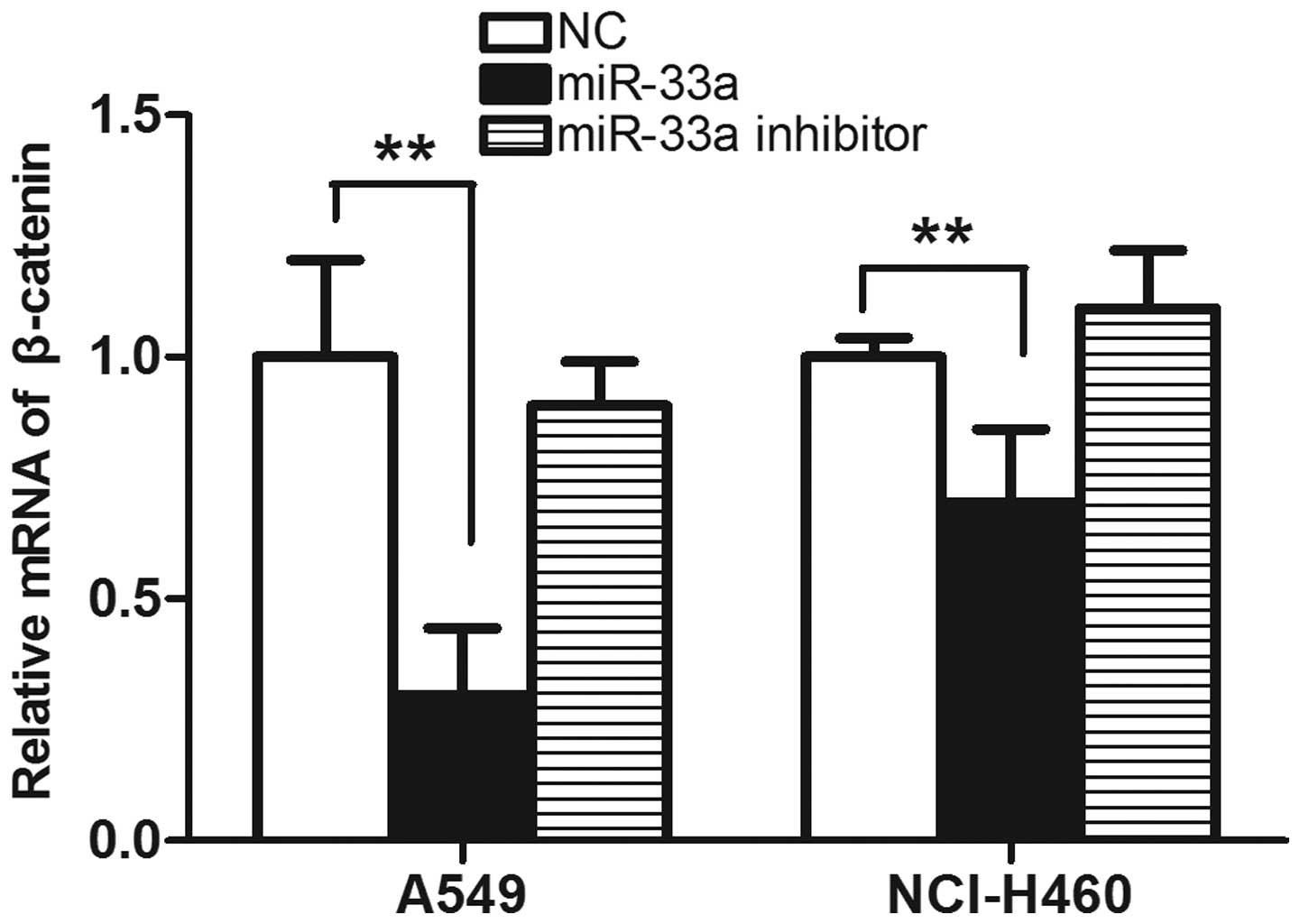
The results from the western blot analysis were consistent with
those determined by qPCR, in which the levels of β-catenin were
observed to be reduced in the miR-33a-transfected group compared
with the control. (P<0.01; Fig.
6) The results suggest that transfection with miR-33a may
significantly reduce the expression of β-catenin, which is likely
to be involved in the suppression of cell cycle progression.
Discussion
miRNAs have been identified to serve an important
role in the proliferation, apoptosis, metastasis and invasion in
lung cancer progression. Abnormal expression of miRNA was first
reported in 2004 (24) and
mutation, misexpression and altered mature miRNA processing have
been suggested to be implicated and involved in tumor progression
(25). miRNAs can function as
oncogenes or tumor supressor genes in order to regulate tumor
progression. For example, miR-494 has been identified to suppress
cell proliferation and induce senescence in A549 lung cancer cells
(26); miR-21 inhibits growth and
promotes apoptosis in the human lung cancer cell line SPC-A1
(27); and miR-378 to
significantly modulate human NSCLC progression and angiogenesis.
miR-378 was thus suggested as a novel therapeutic target (28).
Metastasis and invasion of tumor cells are factors
key to the high mortality rates observed in patients with lung
cancer (29,30). In the present study, whether
miR-33a inhibits the invasion and migration of lung cancer cells
was investigated. The results from the MTT assay demonstrated that
transfection of miR-33a effectively inhibited the proliferation and
progression of the A549 and NCI-H460 lung cancer cells. The colony
formation assay was conducted in order to further confirm the
inhibitory effect of miR-33a on the lung cancer cell lines, and the
results were consistent with those of the MTT assay.
In the current study, miR-33a was identified to have
potential tumor-suppressive activity, as overexpression of miR-33a
was demonstrated to inhibit the growth of lung cancer cells. One of
the mechanisms of this effect was suggested to be via the induction
of G1/S phase cell cycle arrest, which was confirmed by
the FACS assay. Western blot analysis identified that
overexpression of miR-33a in A549 and NCI-H460 cells results in the
downregulation of β-catenin expression, which is established to be
involved in cell cycle progression and is abnormally activated in
various types of tumor cell (31,32).
In conclusion, the results of the current study provide information
that may aid in the development of novel therapeutic strategies for
lung cancer, in addition to aiding in the elucidation of the
antitumor mechanism of miR-33a.
References
|
1
|
Pendharkar D, Ausekar BV and Gupta S:
Molecular biology of lung cancer-a review (Review). Indian J Surg
Oncol. 4:120–124. 2013. View Article : Google Scholar :
|
|
2
|
Xue X, Liu Y, Pan L, et al: Diagnosis of
multiple primary lung cancer: a systematic review (Review). J Int
Med Res. 41:1779–1787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blackhall FH, Shepherd FA and Albain KS:
Improving survival and reducing toxicity with chemotherapy in
advanced non-small cell lung cancer: a realistic goal? (Review).
Treat Respir Med. 4:71–84. 2005. View Article : Google Scholar
|
|
4
|
Cortés-Funes H: New treatment approaches
for lung cancer and impact on survival (Review). Semin Oncol.
29(Suppl 8): 26–29. 2002. View Article : Google Scholar
|
|
5
|
Wang XC, Tian LL, Jiang XY, et al: The
expression and function of miRNA-451 in non-small cell lung cancer.
Cancer Lett. 311:203–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Z, Zeng H, Guo Y, et al: miRNA-145
inhibits non-small cell lung cancer cell proliferation by targeting
c-Myc. J Exp Clin Cancer Res. 29:1512010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Yang H, Li Y, et al: microRNA-146
up-regulation predicts the prognosis of non-small cell lung cancer
by miRNA in situ hybridization. Exp Mol Pathol. 96:195–199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salim H, Arvanitis A, de Petris L, et al:
miRNA-214 is related to invasiveness of human non-small cell lung
cancer and directly regulates alpha protein kinase 2 expression.
Genes Chromosomes Cancer. 52:895–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: diagnostics, monitoring and therapeutics.
A comprehensive review (Review). EMBO Mol Med. 4:143–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li YJ, Li ZY and Xu KL: MicroRNA as
potential target for genetherapy of multiple myeloma-review
(Review). Zhongguo Shi Yan Xue Ye Xue Za Zhi. 21:1318–1325.
2013.PubMed/NCBI
|
|
11
|
Piva R, Spandidos DA and Gambari R: From
microRNA functions to microRNA therapeutics: novel targets and
novel drugs in breast cancer research and treatment (Review). Int J
Oncol. 43:985–994. 2013.PubMed/NCBI
|
|
12
|
An J, Zhu X, Wang H and Jin X: A dynamic
interplay between alternative polyadenylation and microRNA
regulation: implications for cancer (Review). Int J Oncol.
43:995–1001. 2013.PubMed/NCBI
|
|
13
|
Wijesekara N, Zhang LH, Kang MH, et al:
miR-33a modulates ABCA1 expression, cholesterol accumulation, and
insulin secretion in pancreatic islets. Diabetes. 61:653–658. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gharipour M and Sadeghi M: Pivotal role of
microRNA-33 in metabolic syndrome: A systematic review (Review).
ARYA Atheroscler. 9:372–376. 2013.
|
|
15
|
Cirera-Salinas D, Pauta M, Allen RM, et
al: Mir-33 regulates cell proliferation and cell cycle progression.
Cell Cycle. 11:922–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernas T and Dobrucki J: Mitochondrial and
nonmitochondrial reduction of MTT: interaction of MTT with TMRE,
JC-1, and NAO mitochondrial fluorescent probes. Cytometry.
47:236–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sylvester PW: Optimization of the
tetrazolium dye (MTT) colorimetric assay for cellular growth and
viability. Methods Mol Biol. 716:157–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campling BG, Pym J, Baker HM, Cole SP and
Lam YM: Chemosensitivity testing of small cell lung cancer using
the MTT assay. Br J Cancer. 63:75–83. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishitani H, Sugimoto N, Roukos V, et al:
Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1
for proteolysis. EMBO J. 25:1126–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng L, Xu Z, Zhou Y, Yang T, Liang ZQ and
Zhang M: Effect of rosiglitazone on cells cycle, apoptosis and
expression of Skp2 and p27Kip1 in hepatocellular carcinoma cell
line. Zhonghua Gan Zang Bing Za Zhi. 18:148–149. 2010.(In Chinese).
PubMed/NCBI
|
|
21
|
Schulman BA, Carrano AC, Jeffrey PD, et
al: Insights into SCF ubiquitin ligases from the structure of the
Skp1-Skp2 complex. Nature. 408:381–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krishan A: Rapid flow cytofluorometric
analysis of mammalian cell cycle by propidium iodide staining. J
Cell Biol. 66:188–193. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buchegger F, Dupertuis YM and
Perillo-Adamer F: A pitfall of propidium iodide staining in
fluorescence-activated cell sorting cell cycle analysis? Cancer
Res. 67:5576–5577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung cancers
in association with shortened postoperative survival. Cancer Res.
64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu Z, Chen J, Tian T, et al: Genetic
variants of miRNA sequences and non-small cell lung cancer
survival. J Clin Invest. 118:2600–2608. 2008.PubMed/NCBI
|
|
26
|
Ohdaira H, Sekiguchi M, Miyata K and
Yoshida K: MicroRNA-494 suppresses cell proliferation and induces
senescence in A549 lung cancer cells. Cell Prolif. 45:32–38. 2012.
View Article : Google Scholar
|
|
27
|
Zhao G, Guo W, Zhao X, Wang Y and Hou Y:
Glossy ganoderma spore oil promotes apoptosis of human lung
adenocarcinoma SPC-A1 through downregulation of miR-21. Zhongguo
Zhong Yao Za Zhi. 36:1231–1234. 2011.(In Chinese). PubMed/NCBI
|
|
28
|
Skrzypek K, Tertil M, Golda S, et al:
Interplay between heme oxygenase-1 and miR-378 affects non-small
cell lung carcinoma growth, vascularization, and metastasis.
Antioxid Redox Signal. 19:644–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nonaka M, Kataoka D, Yamamoto S, et al:
Outcome following surgery for primary lung cancer with interlobar
pleural invasion. Surg Today. 35:22–27. 2005. View Article : Google Scholar
|
|
30
|
Hsu YL, Wu CY, Hung JY, Lin YS, Huang MS
and Kuo PL: Galectin-1 promotes lung cancer tumor metastasis by
potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway.
Carcinogenesis. 34:1370–1381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding J, Ge D, Guo W and Lu C:
Diazoxide-mediated growth inhibition in human lung cancer cells via
downregulation of beta-catenin-mediated cyclin D1 transcription.
Lung. 187:61–67. 2009. View Article : Google Scholar
|
|
32
|
Xiong F, Jiang M, Huang Z, et al: A novel
herbal formula induces cell cycle arrest and apoptosis in
association with suppressing the PI3K/AKT pathway in human lung
cancer A549 cells. Integr Cancer Ther. 13:152–160. 2014. View Article : Google Scholar
|