Introduction
Malignant glioma is characterized by its highly
invasive growth (1). Due to the
rapid and invasive tumor growth, current treatments for malignant
gliomas, including surgery, chemotherapy and radiation, have not
been successful (2,3). According to statistics, the majority
of malignant gliomas are resistant to chemotherapeutic agents, and
patients have a mean survival time of 12 months following diagnosis
(4). Therefore, it is necessary to
develop novel agents for combating malignant gliomas.
Metastasis involves multiple processes and various
cytophysiological changes; among them, epithelial-to-mesenchymal
transition (EMT) is a key step. EMT involves the loss of epithelial
markers, including E-cadherin. Concomitantly, the expression of a
number of mesenchymal markers is increased, including N-cadherin,
vimentin and matrix metalloproteinase. Through EMT, cancer cells
obtain enhanced motility, which enables metastasis.
Matrine, the molecular formula of which is
C15H24N2O, is derived from
Sophora species of plants and has a long history of use in
traditional Chinese medicine to treat inflammation (5). Matrine has been shown to produce a
wide range of pharmacological effects and has also been used in the
treatment of cancer (5–8). However, there are no studies on the
anti-metastatic effect of matrine on glioma. In the present study,
we investigate the effects and mechanisms of matrine against glioma
invasion.
Materials and methods
Reagents
Phosphate-buffered saline (PBS), dimethyl sulfoxide
(DMSO), polycarbonate membrane filters, polylysine-coated slides,
paraformaldehyde, 3,3′-diaminobenzidine, hematoxylin, lysis buffer
and PVDF membranes were purchased from Shanghai Abcam Biological
Products Co., Ltd (Shanghai, China). Fetal bovine serum (FBS),
penicillin and streptomycin were purchased from Shanghai Worship
Biological Technology Co., Ltd (Shanghai, China). Matrine was
purchased from Shanghai Chuan Xiang Biological Technology Co., Ltd
(Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM) was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
Anti-p38 (AB01165), anti-AKT (AB01313) and anti-β-actin (4967L)
antibodies were purchased from Cell Signaling Technology, Inc.
Anti-p-p38 (sc-166182) was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Anti-E-cadherin (ab11512) and
anti-N-cadherin (ab11339) antibodies were purchased from Shanghai
Abcam Biological Products Co., Ltd (Shanghai, China). Anti-p-AKT
(AP0056) was purchased from Shanghai Seebio Biotech, Inc.
(Shanghai, China).
Cell culture
The human glioma cell lines U251MG and U87MG
(obtained from a cell bank at the Fourth Military Medical
University, Xi’an, China) were cultured in DMEM supplemented with
10% FBS. All cells were incubated at 37°C with 5%
CO2.
Cell viability assays
Cell survival was assessed using standard
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as previously described (9).
Briefly, cells were plated in 96-well culture plates
(3×104 cells per well). The cells were treated with
various concentrations of matrine. After 24 h incubation, the cells
were washed twice with PBS and incubated with 5 mg/ml MTT (Sigma,
St. Louis, MO, USA) for 4 h. Following the incubation period, the
cells were washed with PBS and then solubilized with DMSO. The
optical density was read on an enzyme-linked immunosorbent assay
plate reader (frequency, 3; Victor X2 Multilabel Plate reader,
Perkin-Elmer, Waltham, MA, USA).
In vitro invasion and migration
assays
The in vitro invasion and migration activity
was measured according to the methods described previously
(10). U87MG cells were pretreated
with 0, 0.2, 0.4 and 0.8 mg/ml matrine for 24 h, then surviving
cells were harvested and seeded to Transwell chambers (Corning,
Inc., Corning, NY, USA) at 2×105 cells/well in
serum-free medium and then incubated for 24 h at 37°C. At the
endpoint, the cells on the upper side of the inserts were
completely removed by swabbing, while the cells on the bottom side
of the filter were fixed, stained and measured. For the invasion
assay, 50 μl Matrigel (25 μg/ml; BD Biosciences, Bedford, MA, USA)
was applied to 8-mm pore size polycarbonate membrane filters.
Immunocytochemistry (ICC)
ICC was performed as described previously (11); cells were seeded on
polylysine-coated glass slides, cultured for 2 days, fixed in 4%
paraformaldehyde, then incubated with primary antibody.
Subsequently, sections were incubated with biotinylated secondary
antibody and visualized with 3, 3′-diaminobenzidine; the nuclei
were counter-stained with hematoxylin. Negative controls were
prepared using the same procedure, but PBS was substituted for
primary antibody.
Western blot analysis
Cells were suspended in lysis buffer (40 mmol/l
Tris-HCl, 1 mmol/l EDTA, 150 mmol/l KCl, 100 mmol/l
NaVO3, 1% Triton X-100, 1 mmol/l PMSF, pH 7.5),
following treatment with various concentrations of matrine. The
proteins were separated by 10% or 8% SDS-polyacrylamide gel
electrophoresis and transferred onto PVDF membranes. The membranes
were subsequently blocked in defatted milk at room temperature for
1 h and then incubated with antibodies against E-cadherin,
N-cadherin, p38, p-p38, AKT, p-AKT or β-actin at 4°C overnight. The
membranes were then incubated with a horseradish peroxidase goat
anti-mouse or anti-rabbit IgG antibody for 1 h at room temperature.
The bands were detected with an enhanced chemiluminescence kit
(Amersham, ECL Plus, Freiburg, Germany) and exposed by
autoradiography. The densitometric analysis was performed using
Image J software (GE Healthcare, Buckinghamshire, UK), and the
results were expressed as arbitrary units.
Statistical analysis
All experiments were repeated three times. The
statistical significance of differences throughout this study was
analyzed by the one-way ANOVA test to compare differences between
treatments, and followed up using Dunnett’s multiple comparison
post hoc test. All statistical tests and corresponding P-values
were two-sided. P<0.05 was considered to indicate a
statistically significant difference. Correlation analysis was
performed using the Z-test.
Results
Matrine inhibits the proliferation of
U87MG glioblastoma cells
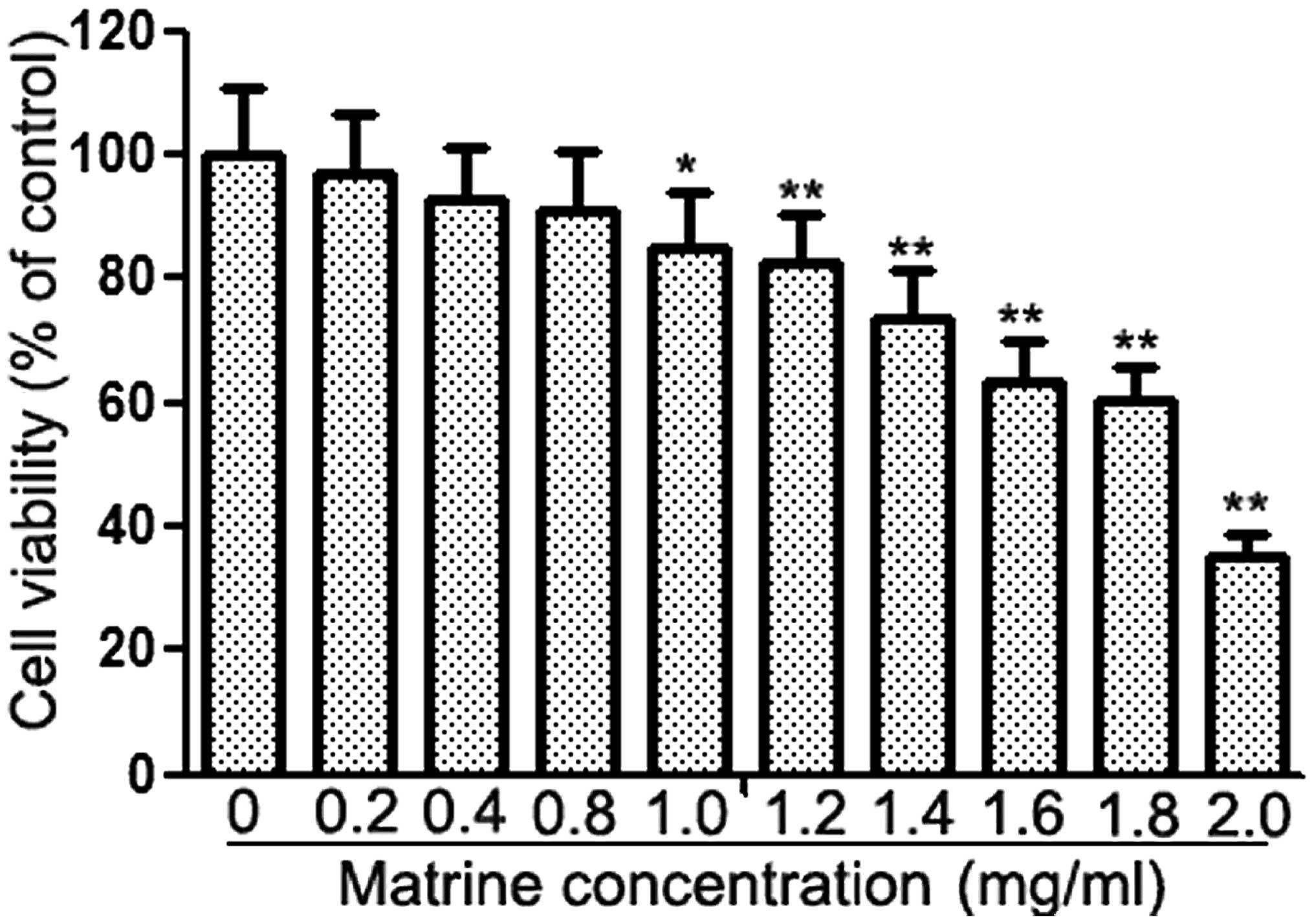
The effects of matrine at various concentrations (0
to 2 mg/ml) on the proliferation of U87MG cells are shown in
Fig. 1. At 1 mg/ml, matrine
clearly inhibited the proliferation of U87MG cells, while at
concentrations below 1 mg/ml, the inhibition effect was not
significant. Hence we selected a concentration range of matrine
lower than this for all subsequent experiments.
Matrine inhibits the migration and
invasion of U87MG cells
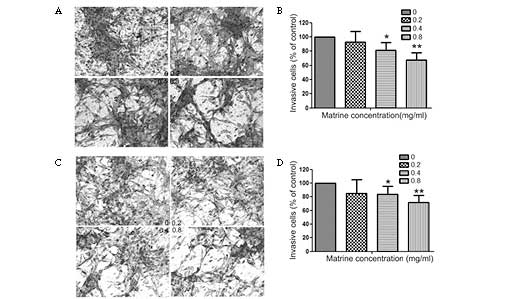
Fig. 2 demonstrates
the effect of matrine on the migration and invasion of U87MG cells
that were treated with 0, 0.2, 0.4 and 0.8 mg/ml matrine for 24 h
in the cell migration and cell invasion assays. The results
revealed that matrine reduced the invasion and migration of U87MG
cells substantially in a concentration-dependent manner. Matrine
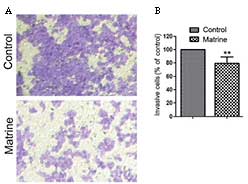
also inhibited the invasion of U251MG (Fig. 3).
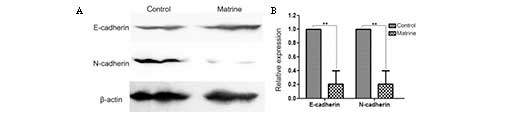
Matrine suppresses the expression of
N-cadherin and increases the expression of E-cadherin
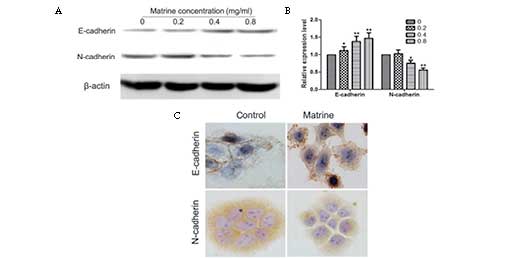
Cells were treated with 0, 0.2, 0.4 and 0.8 mg/ml
matrine for 24 h and then subjected to western blot analysis to
test the expression of E-cadherin and N-cadherin in U87MG cells.
Fig. 4A and B reveal that matrine
significantly reduced the protein levels of N-cadherin and
increased the expression of E-cadherin in a concentration-dependent
manner compared with the control group. Fig. 4C demonstrates the effect of matrine
on the expression of E-cadherin and N-cadherin. From the image, it
can be observed that matrine increased the expression of E-cadherin
and reduced the expression of N-cadherin. Matrine also increased
the expression of E-cadherin and reduced the expression of
N-cadherin in U251MG cells (Fig.
5).
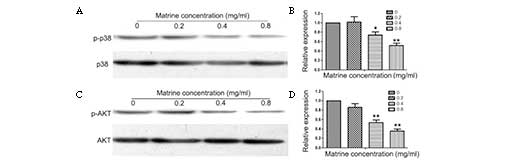
p38 MAPK and AKT are involved in the
anti-metastatic mechanism of matrine
In human glioma cells, activation of p38 is required
for the invasion process (12).
Moreover, the mechanism is correlated with EMT; thus, we
investigated the effect of matrine on p38 MAPK and AKT in U87MG
cells. Western blot analysis revealed that matrine reduced the
phosphorylation of p38 in a concentration-dependent manner
(Fig. 6A and B). At the same time,
matrine inhibited the phosphorylation of AKT in a
concentration-dependent manner (Fig.
6C and D).
Discussion
Glioma, in particular glioblastoma multiforme, with
high morbidity and mortality rates, is a serious public health
issue around the world (13).
Matrine has been confirmed as a natural antitumor product in
several types of cancer (14–17);
however, the anti-metastatic effect of matrine and its associated
mechanism(s) in glioma cells remain unclear. In this study we
observed that matrine inhibited the mobility and invasive ability
of glioma cells in vitro by regulating EMT via inhibition of
the p38 MAPK and AKT pathways. To the best of our knowledge, this
is the first scientific study of the anti-metastatic effect of
matrine on glioma.
Transwell chambers (uncoated or coated with
Matrigel) were employed to explore the effect of matrine on glioma
cell migration and invasion. We demonstrated that matrine inhibited
the migration and invasion of glioma cells at non-toxic doses (no
more than 1 mg/ml). These results revealed that the inhibition of
invasion by matrine in U87MG cells was not due to cytotoxicity.
Tumor metastasis and recurrence is one of the most
difficult challenges in the treatment of glioma patients. To
complete the progression of metastasis, carcinoma cells must
complete multiple distinct steps; this process has been termed the
invasion-metastasis cascade, in which EMT plays a significant role
(18). EMT induces epithelial
cells to obtain mesenchymal markers and lose epithelial marker
expression (19). As an epithelial
molecular marker, E-cadherin is responsible for the establishment
of the adherens junction, which forms a continuous adhesive belt
below the apical surface (20). By
mediating interactions with extracellular domains of E-cadherin
molecules in adjacent cells, it forms intercellular junctions. Loss
of E-cadherin by EMT results in the detachment of intercellular
junctions and decreases adhesive force, simplifying the metastasis
of carcinoma cells (20). The
expression of mesenchymal markers, including N-cadherin, increases.
N-cadherin is involved in the maintenance of microvessel stability
and plays a role in blood vessel formation (21). In the present study, we observed
that matrine increased the expression of E-cadherin and decreased
the expression of N-cadherin. The results suggest that the
anti-metastatic effect of matrine on glioma is correlated with
EMT.
The actual implementation of EMT is dependent on the
concomitant activity of a variety of signal transduction pathways,
including the MAPK and AKT signaling pathways (22). p38 MAPK plays a significant role in
the induction of EMT by TGF-β1 (23). p38 maintains E-cadherin expression
by modulating TAK1-NF-κB during EMT in human primary mesothelial
cells (24). In addition to MAPK
signaling, AKT signaling plays a key role in inducing and
maintaining EMT. Squamous cell carcinoma lines, expressing a
constitutively active form of PKB/AKT, the most notable downstream
effector of AKT signaling, underwent EMT, characterized by
downregulation of the epithelial markers desmoplakin, E-cadherin
and β-catenin, and upregulation of the mesenchymal marker vimentin
(25,26). Moreover, AKT signaling may also be
activated by integrins and members of the Rho family of small
GTPases that control cytoskeleton remodeling, a necessity during
the morphogenic process of EMT (27,28).
In our study, we demonstrated that matrine effectively inhibited
p38 MAPK and AKT signaling.
In conclusion, this study demonstrated the
inhibitory effect of matrine on the invasion and metastatic
capabilities of glioma cells. Furthermore, the decrease in the
expression of N-cadherin and the increase in the expression of
E-cadherin induced by matrine is attributed to an inhibition of p38
MAPK and AKT signaling. This mechanism may contribute to the
inhibition of invasion and metastasis in glioma cells by matrine.
These findings reveal a new potential therapeutic application of
matrine in anti-metastatic therapy for glioma.
Acknowledgements
This study was supported by grants from the Second
Affiliated Hospital, School of Medicine, Xi’an Jiaotong University
[YJ (QN) 201219], and Science and Technology Projects in Shaanxi
Province (2013k13-01-09).
References
|
1
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giese A, Rief MD, Loo MA and Berens ME:
Determinants of human astrocytoma migration. Cancer Res.
54:3897–3904. 1994.PubMed/NCBI
|
|
3
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jansen M, Yip S and Louis DN: Molecular
pathology in adult gliomas: diagnostic, prognostic, and predictive
markers. Lancet Neurol. 9:717–726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li LQ, Li XL, Wang L, et al: Matrine
inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7
cells. Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Wang X, Wu W, et al: Effects of
matrine on proliferation and apoptosis in gallbladder carcinoma
cells (GBC-SD). Phytother Res. 26:932–937. 2012. View Article : Google Scholar
|
|
7
|
Zhang S, Zhang Y, Zhuang Y, et al: Matrine
induces apoptosis in human acute myeloid leukemia cells via the
mitochondrial pathway and Akt inactivation. PLoS One. 7:e468532012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Zhang J, Wang Y, et al: Matrine, a
novel autophagy inhibitor, blocks trafficking and the proteolytic
activation of lysosomal proteases. Carcinogenesis. 34:128–138.
2013. View Article : Google Scholar
|
|
9
|
Chen K, Zhang S, Ji Y, et al: Baicalein
inhibits the invasion and metastatic capabilities of hepatocellular
carcinoma cells via down-regulation of the ERK pathway. PLoS One.
8:e729272013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang SF, Yang WE, Kuo WH, Chang HR, Chu SC
and Hsieh YS: Antimetastatic potentials of flavones on oral cancer
cell via an inhibition of matrix-degrading proteases. Arch Oral
Biol. 53:287–294. 2008. View Article : Google Scholar
|
|
11
|
Gao Q, Liu W, Cai J, et al: EphB2 promotes
cervical cancer progression by inducing epithelial-mesenchymal
transition. Hum Pathol. 45:372–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demuth T, Reavie LB, Rennert JL, et al:
MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3
and p38 drive glioma invasion and progression and predict patient
survival. Mol Cancer Ther. 6:1212–1222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang ZS, Luo P, Dai SH, Liu ZB, Zheng XR
and Chen T: Salvianolic acid B induces apoptosis in human glioma
U87 cells through p38-mediated ROS generation. Cell Mol Neurobiol.
33:921–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mondal S, Bandyopadhyay S, Ghosh MK,
Mukhopadhyay S, Roy S and Mandal C: Natural products: promising
resources for cancer drug discovery. Anticancer Agents Med Chem.
12:49–75. 2012. View Article : Google Scholar
|
|
15
|
Chandrashekar N, Selvamani A, Subramanian
R, Pandi A and Thiruvengadam D: Baicalein inhibits pulmonary
carcinogenesis-associated inflammation and interferes with COX-2,
MMP-2 and MMP-9 expressions in-vivo. Toxicol Appl Pharmacol.
261:10–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu B, Li J, Huang D, et al: Baicalein
mediates inhibition of migration and invasiveness of skin carcinoma
through Ezrin in A431 cells. BMC Cancer. 11:5272011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang ZD, Huang C, Li ZF, et al:
Chrysanthemum indicum ethanolic extract inhibits invasion of
hepatocellular carcinoma via regulation of MMP/TIMP balance as
therapeutic target. Oncol Rep. 23:413–421. 2010.PubMed/NCBI
|
|
18
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blaschuk OW and Devemy E: Cadherins as
novel targets for anti-cancer therapy. Eur J Pharmacol.
625:195–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen HH, Zhou XL, Shi YL and Yang J: Roles
of p38 MAPK and JNK in TGF-beta1-induced human alveolar epithelial
to mesenchymal transition. Arch Med Res. 44:93–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strippoli R, Benedicto I, Foronda M, et
al: p38 maintains E-cadherin expression by modulating
TAK1-NF-kappaB during epithelial-to-mesenchymal transition. J Cell
Sci. 123:4321–4331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei J, Li Z, Chen W, et al: AEG-1
participates in TGF-beta1-induced EMT through p38 MAPK activation.
Cell Biol Int. 37:1016–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grille SJ, Bellacosa A, Upson J, et al:
The protein kinase Akt induces epithelial mesenchymal transition
and promotes enhanced motility and invasiveness of squamous cell
carcinoma lines. Cancer Res. 63:2172–2178. 2003.PubMed/NCBI
|
|
27
|
Zamir E and Geiger B: Molecular complexity
and dynamics of cell-matrix adhesions. J Cell Sci. 114:3583–3590.
2001.PubMed/NCBI
|
|
28
|
Xia N, Thodeti CK, Hunt TP, et al:
Directional control of cell motility through focal adhesion
positioning and spatial control of Rac activation. FASEB J.
22:1649–1659. 2008. View Article : Google Scholar : PubMed/NCBI
|