Introduction
Glioblastoma multiforme (GBM) is the most prevalent
lethal intracranial tumor in adults (1). It is characterized by extensive
intracranial invasion and the patients have been reported to
tolerate conventional and advanced treatments during therapy
(2). Despite the improved
strategies for diagnosis and the aggressive tumor treatments used,
the median survival of patients with GBM remains to be
approximately one year (3,4). Therefore, more effective targeted
therapies are crucial for improving the prognosis of patients with
GBM.
MicroRNAs are small non-coding RNA molecules that
are comprised of 16~22 nucleotides and downregulate translation by
targeting mRNAs (5). These
microRNAs bind with the 3′ untranslated regions (3′UTRs) to block
complementary sites on their mRNA targets and therefore serve
important inhibitory functions in the post-transcription of gene
expression, in a similar capacity to that of RNAi (6). Previous studies have indicated that
this novel class of gene regulators has an involvement in human
cancer progression and tumorigenesis (7).
Previous studies reported that microRNA-206
(miR-206) expression was markedly reduced in osteosarcoma and lung
cancer, and that it was necessary for cell growth, migration,
apoptosis and invasion (8,9). In human breast cancer and
rhabdomyosarcoma, downregulated miR-206 was associated with cell
proliferation, migration and metastasis (10–13).
However, the function of miR-206 in gliomas remains
to be fully elucidated. In the present study, the aim was to
investigate the functional role of miR-206 in gliomas and to
further elucidate the mechanism of miR-206 in tumorigenesis and
progression. The present study hypothesized that miR-206 acts as a
tumor suppressor and suppresses glioma cell proliferation via
cyclinD2.
Materials and methods
Patients and tissue collection
All patient information was obtained from the
Chinese Glioma Genome Atlas (CGGA; www.cgga.org.cn/).
The microRNA microarray analysis was conducted on 198 patients with
glioma and based on the gene expression microarray and cyclinD2
expression was analyzed in 225 patients with grade II glioma or
high-grade gliomas (HGGs), of which 158 patients also underwent
microRNA microarray analysis. In the present study, the patients
underwent a resection operation between January 2006 and December
2010 and subsequently received adjuvant treatment of temozolomide
combined with radiotherapy. The present study was approved by the
Beijing Tiantan Hospital (Beijing, China) institutional review
board and written informed consent was obtained from all
patients.
Cell lines and cell transfection
The human LN229 GBM cell line was purchased from the
Chinese Academy of Sciences Cell Bank (Kunming, China). LN229 cells
were cultured in Dulbecco’s modified Eagle’s medium supplemented
with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and
1% glutamine (all Hyclone, GE Healthcare, Little Chalfont, UK). All
cells were incubated at 37°C in an atmosphere supplemented with 5%
CO2 and passaged every 2–3 days. The Homo sapiens
(hsa)-miR-206 mimics were synthesized by Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China). The following primer sequence was used in
the present study: UGGAAUGUAAGGAAGUGUGUGG. Cells were cultured at a
density of 8×104 cells/well in six-well plates. miR-NC
and hsa-miR-206 oligosaccharides were transfected into LN229 cells
at a final concentration of 50 nmol/l using riboFECTTM CP reagent
(Guangzhou RiboBio Co., Ltd.) according to the manufacturer’s
instructions. The miR-NC contained a scrambled sequence for the
control.
Luciferase reporter assay
For the luciferase assays, the luciferase reporter
vector cyclinD2-3′UTR and the negative control were specifically
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
cells were cultured in 12-well plates and transfected with
hsa-miR-206 and co-transfected with the luciferase reporter vector.
Following incubation for 48 h, cells were harvested and analyzed
for Renilla luciferase activity using the Dual Luciferase
Reporter Assay System (Promega Corp., Madison, WI, USA) in
accordance with the manufacturer’s instructions. Renilla
luciferase was used for normalization.
Cell proliferation assays
Cell proliferation was assessed using MTT
(Sigma-Aldrich, St. Louis, MO, USA) and colony formation assays.
Subsequent to transfection with miR-NC and hsa-miR-206
oligosaccharides for 24 h, LN229 cells were transplanted at a
density of 2,000 cells/well with five replicated wells for each
group in the 96-well plates. In order to determine relative cell
growth, MTT assays were conducted for five consecutive days by
adding 20 μl MTT solution (5 mg/ml) to each well and incubating at
37°C for 4 h. To solubilize formazan crystals, 150 μl DMSO
(Sigma-Aldrich) was added and the absorbance values were detected
at 490 nm using a microplate reader (GloMax®-Multi
Detection system; Promega Corp.), which were proportional to the
number of live cells.
Colony formation assays were performed with 200
cells/group plated in six-well plates, which were transfected for
24 h. Following 10 days of incubation, each well was washed with
phosphate-buffered saline (Hyclone, GE Healthcare) and stained with
crystal violet (Sigma-Aldrich). All colonies were manually counted
by using a microscope (Leica DM6000 B; Leica Microsystems GmbH,
Wetzlar, Germany).
Western blot analysis
Western blot analysis was conducted to assess
cyclinD2 expression in transfected cells. The total proteins were
isolated from the LN229 cell lines transfected with negative
control plasmids and miR-206 mimics for 48 h. These cells were
isolated with trypsin-EDTA (Hyclone, GE Healthcare), and lysed in
lysis buffer (1% Lgepal CA-630, 150 mM NaCl, 50 mM Tris;
Sigma-Aldrich) for a minimum of two hours on ice. Subsequent to
quantification of protein, an equal amount of protein (10 μg) was
added into the sample wells, separated using 10% SDS-PAGE
(Sigma-Aldrich) and transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Western blotting was
conducted with monoclonal rabbit anti-cylinD2 immunoglobulin G
(IgG) as the primary antibody (1:400; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) incubated at 4°C overnight.
Monoclonal rabbit anti-GAPDH IgG antibody, incubated at a dilution
of 1:1,000 at 4°C overnight (Cell Signaling Technology, Inc.,
Danvers, MA, USA) was used to ensure equal protein loading. The
secondary antibodies were horseradish peroxidase-conjugated
anti-rabbit IgG, diluted at 1:5,000 and purchased from Origene
(Beijing, China)
Cell cycle analysis
LN229 cells (1–2×104) treated with
negative control and miR-206 mimics were plated in six-well plates.
Subsequent to incubation for 48 h, the cells were collected by
trypsinization, fixed for 24 h in 70% ethanol (Beijing Modern
Oriental Fine Chemistry Co., Ltd, Beijing, China) and stained with
propidium iodide Beyotime Institute of Biotechnology, Haimen,
China) for 30 min in the dark in a water bath at 37°C according to
the manufacturer’s instructions (Beyotime Institute of
Biotechnology). The cells were then collected and underwent cell
cycle analysis using a flow cytometer (Cytomics FC 500; Beckman
Coulter, Inc., Brea, CA, US).
Statistical analysis
The overall survival time was counted from the date
of diagnosis with glioma to mortality or the last follow-up visit.
Kaplan-Meier survival curves were analyzed and the overall survival
times were assessed using the log-rank test. Student’s t-test
(two-tailed) was used to estimate significant differences between
groups. P<0.05 was considered to indicate a statistically
significant difference. All experiments were performed a minimum of
three times and data were analyzed using GraphPad Prism, version 5
(GraphPad Software Inc., La Jolla, CA, USA) and SPSS, version 13.0
(SPSS, Inc., Chicago, IL, USA).
Results
miR-206 is downregulated in GBM and
associated with poor prognosis in patients with glioma
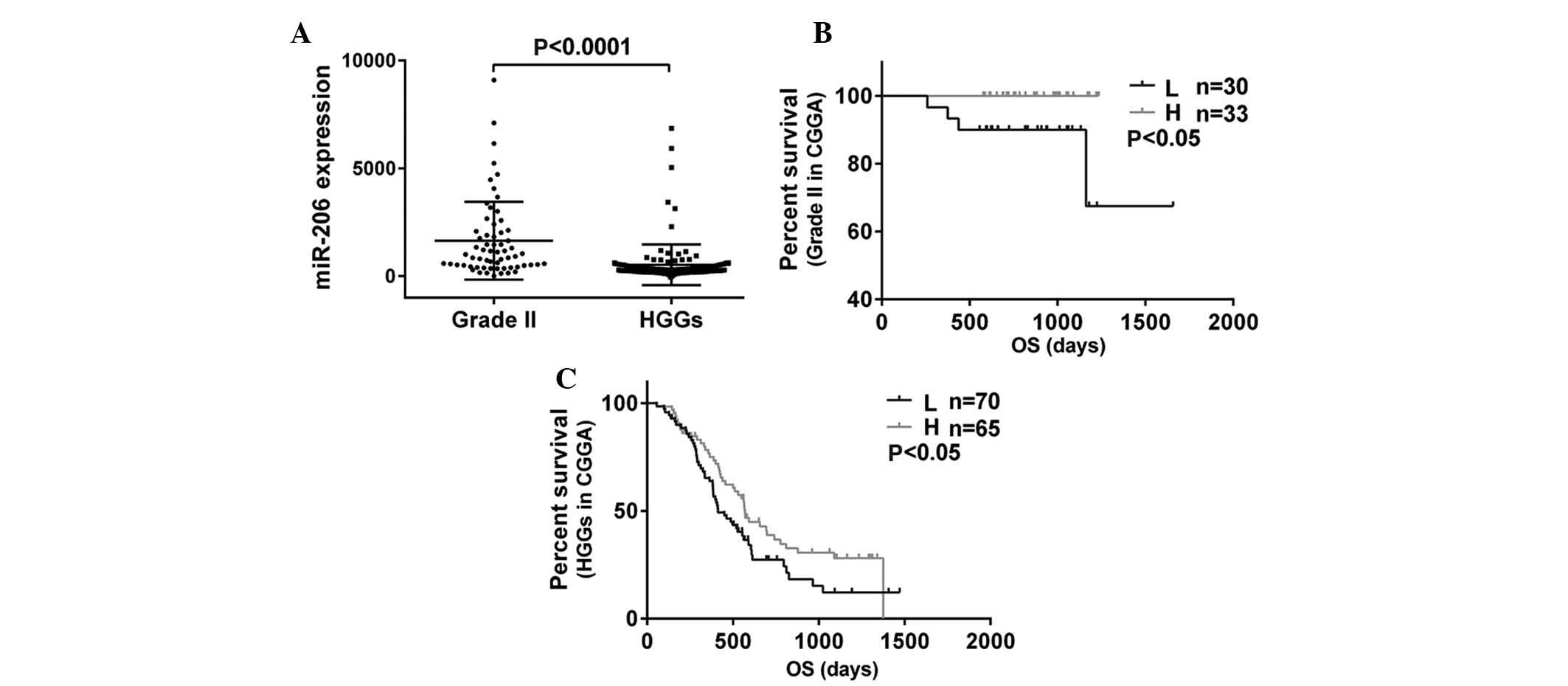
To investigate the tumorigenesis-associated
molecular alterations in glioma, the microRNA expression levels
were analyzed in 63 patients with grade II glioma and 135 patients
with HGGs by microarray analyses. Among these microRNAs, miR-206
expression was observed to be downregulated as the degree of
malignancy in gliomas increased (P<0.0001; Fig. 1A). The overall survival time was
assessed using Kaplan-Meier survival curve analysis and all
survival information used was from the CGGA. The results
demonstrated that patients with glioma grade II or HGGs with high
expression of miR-206 had a markedly increased rate of
progression-free survival as compared with those with low miR-206
expression (P<0.05, P<0.05; Fig.
1B and C). The overall survival curves together with the the
miR-206 expression levels in the 198 patients demonstrated that
reduced expression levels of miR-206 were associated with poor
prognosis in patients with glioma.
CyclinD2 is a direct target of
miR-206
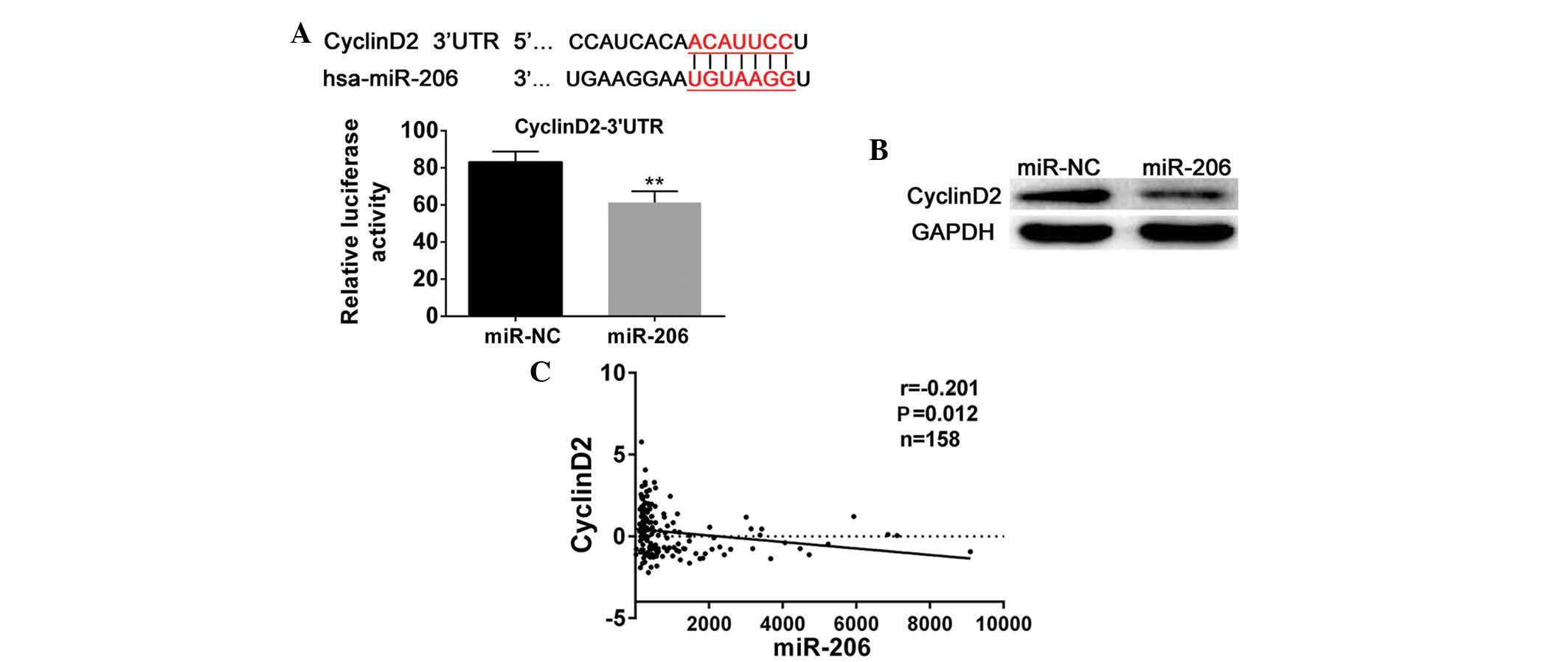
Based on the above analysis, the possible targets of
miR-206 were searched with TargetScan (http://www.targetscan.org/), leading to the
identification of cyclinD2. CyclinD2 was observed to share seven
imperfect complementary sites with miR-206 and was identified to be
important in the cell cycle; therefore, a luciferase reporter assay
was designed to verify this. Subsequent to co-transfection with the
miR-206 mimics and cyclinD2-3′UTR-plasmids, relative luciferase
activity was observed to be significantly reduced (P<0.01;
Fig. 2A). The luciferase reporter
experiment suggested that cyclinD2 may be a potential target of
miR-206. Western blot analysis was also conducted in order to
confirm the role of cyclinD2. The results confirmed that miR-206
mimics-transfected cells exhibited reduced cylinD2 expression
corresponding to that in the negative control cells (Fig. 2B). Finally, correlation analysis
was conducted to investigate the association between the expression
of miR-206 and cyclinD2 in 158 patients, and the results
demonstrated that there was an inverse correlation between miR-206
and cyclinD2 in gliomas (r=−0.201, P=0.012; Fig. 2C). Based on these results, cyclinD2
was suggested to be a direct target of miR-206 in gliomas.
CyclinD2 is increased in HGGs and is
correlated with poor prognosis
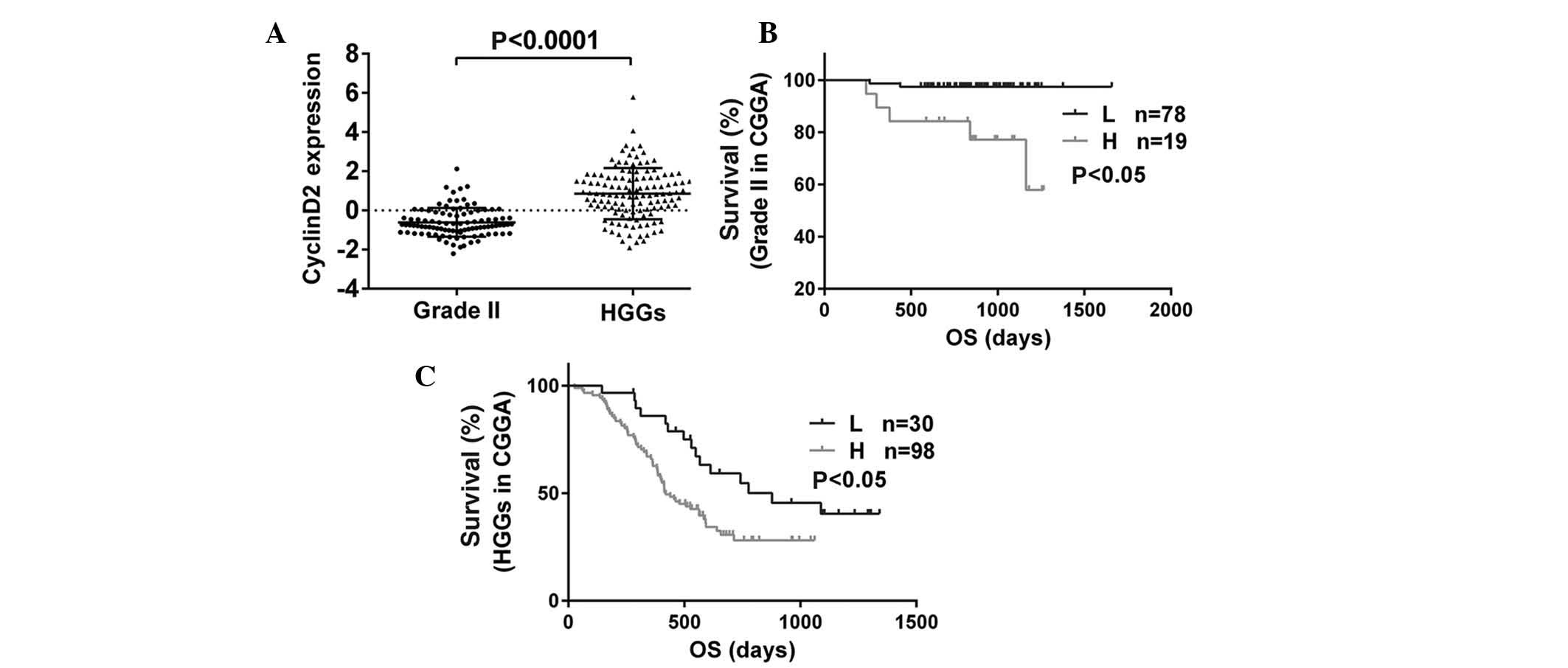
According to the gene expression microarray, it was
observed that with a higher degree of malignancy, cyclinD2
expression was significantly increased (P<0.0001; Fig. 3A). In addition, the correlation
between overall survival time and the expression of cyclinD2 was
analyzed in 225 patients. The results demonstrated that,
independent of the glioma grade, glioma patients with low levels of
cyclinD2 expression exhibited a significantly greater survival
time, while the survival time of patients with high levels of
cyclinD2 was lower (P<0.05, P<0.05; Fig. 3B and C).
miR-206 inhibits cell proliferation and
arrests G1/S transition in the cell cycle via targeting
cyclinD2 in glioma cell lines
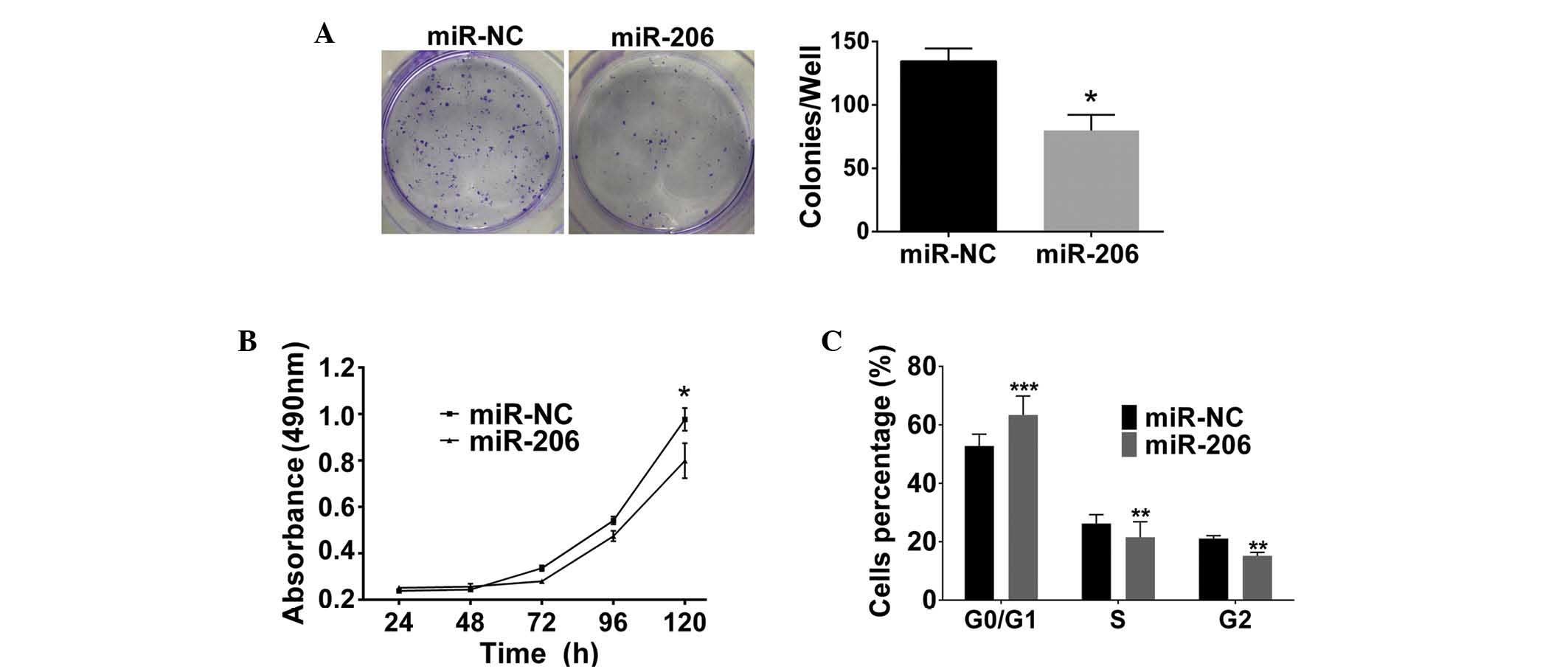
To investigate the biological function of miR-206 in
the progression of glioma, a series of overexpression assays were
conducted in the GBM cell line LN229. A colony formation assay
indicated a significant reduction in colony formation in the
miR-206-transfected cells compared with that in the negative
control cells (P<0.05; Fig.
4A). In addition, the MTT assay demonstrated a significant
reduction in cell growth with cells transfected with miR-206 mimics
compared with those of negative control cells at 120 h (P<0.05;
Fig. 4B). These assays indicated
that miR-206 was associated with glioma cell proliferation. Cell
cycle assays demonstrated that the miR-206-transfected cells had a
significantly increased percentage of cells in the
G0/G1 phase, whereas a significant reduction
in cells in the G2 and S phases was observed compared
with that in negative control cells (P<0.001, P<0.01 and
P<0.01, respectively; Fig. 4C).
In conclusion, the results suggested that miR-206 may arrest
G1/S transition in glioma cell lines via targeting
cyclinD2.
Discussion
Previous studies have indicated that microRNAs
regulate gene expression and may also function as tumor suppressors
or oncogenes (14). By binding to
3′UTRs, microRNAs suppress the expression of their respective
target gene prior to translation, similar to the the mechanism of
RNAi (6,15). Furthermore, previous studies have
demonstrated that these microRNAs are critical in tumorigenesis and
are significant targets for the development of clinical treatments
(16,17). Previous studies have identified
that miR-206 is downregulated in lung cancer, breast cancer and
osteosarcoma (8–10). In breast cancer, miR-206 levels
were shown to be correlated with cell growth, clinical stage and
lymph node metastasis, and affected the overall survival of
patients with breast cancer (10).
In lung cancer, as a tumor suppressor, miR-206 was associated with
tumor cell migration and invasion (9). Similar effects were also observed in
osteosarcoma and in miR-206-transfected cells, where a reduction in
cell viability, promotion of cell apoptosis and inhibition of cell
invasion and migration were identified (11). However, the function of miR-206 in
glioma remains to be fully elucidated.
In the present study, miR-206 was observed to be
downregulated in glioma based on microRNA microarray analysis. In
addition, the overall survival of patients varied significantly
depending on the expression levels of miR-206, suggesting that
patients with a high expression of miR-206 had an improved
prognosis, based on separate statistical analyses in grade II
gliomas and HGGs. Therefore, it was hypothesized that miR-206 may
be important in tumorigenesis and the progression of glioma.
To further investigate the function of miR-206 in
glioma progression, the target-predicting database Targetscan was
searched and cyclinD2 was identified as a potential target of
miR-206, which is associated with the cell cycle. miR-206 was
previously reported to regulate cyclinD2 in rhabdomyosarcoma
(11,12), breast cancer (10,13)
and gastric cancer (18). To
support this association, a luciferase reporter assay was conducted
in the present study, which identified cyclinD2 as a target of
miR-206 in gliomas. The results of the western blot assay were also
in agreement with this, as cyclinD2 expression was found to be
negatively correlated with miR-206 expression. CyclinD2 is a member
of D-type cyclins and is crucial in the progression of the cell
cycle (19). G1
cyclins, including cyclinDs and cyclinEs, combined with
cyclin-dependent kinases CDK4 and CDK6, have been reported to be
activated in the late G1 phase and regulate
G1/S transition (20).
In the process of tumor formation, disruption of cell cycle
progression from G1 to S phase is commonly observed
(21). Based on the results of
previous studies, the overall survival was analyzed separately in
patients with grade II gliomas and HGGS in regard to the expression
of cyclinD2. The results demonstrated that in gliomas, low levels
of cyclinD2 may be associated with lower glioma grades and longer
survival time, and further confirmed that cyclinD2 may act as a
positive regulator in tumorigenesis and function as a tumor
oncogene in gliomas. However, miR-206 exhibited the opposite
effect, indicating that cyclinD2 is inversely correlated with
miR-206 and is negatively associated with the prognosis of gliomas.
The correlation between the expression levels of these miR-206 and
cyclin D2 is therefore likely to be important in the development of
gliomas. Thus, in order to investigate the function of miR-206 in
cell proliferation, MTT and colony formation assays were conducted
and the results demonstrated that increased miR-206 expression
inhibited cell proliferation in GBM. Cell cycle analysis was also
conducted in order to detect the percentage of cells in different
stages of the cell cycle. This analysis demonstrated that
miR-206-transfected cells exhibited a significantly increased
G0/G1 population and a reduction in the S
phase population as compared with negative control cells. These
results further demonstrated that miR-206 acted as a cell cycle
inhibitor, as an increase in the levels of miR-206 expression
significantly inhibited transition of LN229 cells from
G0/G1 to S phase. In conclusion, cyclinD2 was
a direct target of miR-206 and miR-206 regulated the cell cycle by
promoting G1/S arrest and suppressing cell proliferation
via targeting cylinD2 in gliomas.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that miR-206 suppresses
glioma formation and possibly targets the downstream complementary
sites of cyclinD2 to inhibit cancer cell proliferation. In
addition, the low expression of miR-206 in patients with glioma was
demonstrated to be correlated with poor prognosis. Therefore, it
was concluded that miR-206 acts as a tumor suppressor in glioma and
regulates cell proliferation and cell cycle arrest by targeting
cyclinD2. On the basis of observation and data analysis, miR-206
was suggested to be a novel candidate for use as a prognostic
marker in patients with glioma and to have potential for use as a
therapeutic target in gliomas.
Acknowledgements
The current study was supported by grants in part
from Beijing Municipal Commission of Education (no. KZ201410025021)
and others: The National High Technology Research and Development
Program (grant no. 2012AA02A508), the International Science and
Technology Cooperation Program (grant no. 2012DFA30470), the
National 973 Program (grant no. 2011CB707804) and the National
Natural Science Foundation of China (grant nos. 91229121, 81201993
and 81071626).
References
|
1
|
Parsons DW, Jones S, Zhang X, et al: An
integrated genomic analysis of human glioblastoma multiforme.
Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Furnari FB, Fenton T, Bachoo RM, et al:
Malignant astrocytic glioma: genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Li S, Chen L, et al: Glioblastoma
with an oligodendroglioma component: distinct clinical behavior,
genetic alterations, and outcome. Neuro Oncol. 14:518–525. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang W, Zhang J, Yan W, et al:
Whole-genome microRNA expression profiling identifies a 5-microRNA
signature as a prognostic biomarker in Chinese patients with
primary glioblastoma multiforme. Cancer. 119:814–824. 2013.
View Article : Google Scholar
|
|
5
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao YP, Yi Y, Peng LL, et al: Roles of
microRNA-206 in osteosarcoma pathogenesis and progression. Asian
Pac J Cancer Prev. 14:3751–3755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Ling C, Bai Y and Zhao J:
MicroRNA-206 is associated with invasion and metastasis of lung
cancer. Anat Rec (Hoboken). 294:88–92. 2011. View Article : Google Scholar
|
|
10
|
Kondo N, Toyama T, Sugiura H, Fujii Y and
Yamashita H: miR-206 Expression is down-regulated in estrogen
receptor alpha-positive human breast cancer. Cancer Res.
68:5004–5008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyachi M, Tsuchiya K, Yoshida H, et al:
Circulating muscle-specific microRNA, miR-206, as a potential
diagnostic marker for rhabdomyosarcoma. Biochem Biophys Res Commun.
400:89–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan D, da Dong XE, Chen X, et al:
MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma
development. J Biol Chem. 284:29596–29604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Hong F and Yu Z: Decreased
expression of microRNA-206 in breast cancer and its association
with disease characteristics and patient survival. J Int Med Res.
41:596–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carmell MA, Xuan Z, Zhang MQ and Hannon
GJ: The Argonaute family: tentacles that reach into RNAi,
developmental control, stem cell maintenance, and tumorigenesis.
Genes Dev. 16:2733–2742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wightman B, Ha I and Ruvkun G:
Posttranscriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao J, Wang WY, Mao YW, et al: A novel
pathway regulates memory and plasticity via SIRT1 and miR-134.
Nature. 466:1105–1109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi ZM, Wang XF, Qian X, et al: MiRNA-181b
suppresses IGF-1R and functions as a tumor suppressor gene in
gliomas. RNA. 19:552–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Liu X, Jin H, et al: miR-206
inhibits gastric cancer proliferation in part by repressing
cyclinD2. Cancer Lett. 332:94–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dehay C and Kennedy H: Cell-cycle control
and cortical development. Nat Rev Neurosci. 8:438–450. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sherr CJ: Mammalian G1 cyclins. Cell.
73:1059–1065. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|