Introduction
Bladder cancer is a common malignant tumors of the
urinary system. In 2005, 63,210 new cases of bladder cancer were
identified in America, of which 13,180 succumbed to the disease
(1). With the development of an
aging population, bladder cancer incidence is increasing year after
year as incidence increases with age. Surgery is the main treatment
method for bladder cancer; however, the 5-year average survival
period remains poor (2). A
previous study has demonstrated that the most important prognostic
factors of bladder cancer include the pathological grading and
staging (3). Long-term survival
may be achieved through early detection and treatment. With the
elucidation of the molecular biology of bladder cancer, gene
therapy represents a novel method for the adjuvant treatment of the
disease. Osteopontin (OPN) is a secreted acid glycoprotein with a
variety of functions. OPN was initially isolated from the bone
matrix and subsequently found in the tissues or cells of the
placenta, decidua, smooth muscle and macrophages, as well as in
various body fluids. In addition, OPN is an important component of
the extracellular matrix. Previous studies have demonstrated that
OPN is expressed in various tumor tissues and promotes tumor
proliferation, differentiation, invasion and metastasis (4–9). A
number of studies have shown that shorter survival time and poor
prognosis are associated with high levels of OPN (10–13),
while low plasma levels of OPN in patients are associated with good
prognosis (14,15).
RNA interference (RNAi) is a process of typical
post-transcriptional regulation of gene expression, which is a
conservative behavior in biological evolution, with resistance to
virus invasion and maintaining the role of genetic stability
(16,17,18).
RNAi is induced by double-stranded RNA (dsRNA) gene silencing
(18). When dsRNA is transported
into cells, mRNA degrades due to the homologous sequences of dsRNA,
thereby inhibiting the expression of the gene and resulting in the
silencing of the gene expression (18,19).
In the present study, RNAi was used to reduce the expression of OPN
in T24 human bladder carcinoma cells in order to investigate the
effect of OPN expression on the biological behavior of T24
cells.
Materials and methods
Reagents
α-Minimum essential medium (α-MEM), fetal bovine
serum (FBS), penicillin/streptomycin and TRIzol® reagent
were purchased from Gibco Life Technologies (Rockville, MD, USA).
Rabbit anti-human monoclonal antibodies against total caspase-3,
caspase-8, caspase-9 and p53 (1:1,000) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA), while rabbit
anti-human monoclonal antibodies against OPN and β-actin (1:1,000)
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). All other chemicals and reagents used in the present study
were of analytical grade.
Cell culture
The T24 human bladder cancer cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in α-MEM supplemented with 10% (v/v) FBS and 1% (v/v)
penicillin-streptomycin solution and incubated at 37°C in 5%
CO2 humidified air. The culture medium was refreshed
every three days.
Small interfering (siRNA) for OPN
Four siRNA sequences targeting human OPN and a
negative control siRNA were obtained from Shanghai GeneChem Co, Ltd
(Shanghai, China). The siRNA sequences were designed using software
(OCK-iT™ RNAi Designer) from Life Technologies (Carlsbad, CA, USA)
by Shanghai GeneChem Co, Ltd. The siRNA sequences were as follows:
pSC-1, 5′-GACCATTCTGATGAATCTGAT-3′; pSC-2,
5′-GAGCATTCCGATGTGATTGAT-3′; pSC-3, 5′-GAGGAGTTGAATGGTGCATA-3′;
pSC-4, 5′-CACAAGCAGTCCAGATTATA-3′). The OPN eukaryotic expression
plasmid was constructed and co-transfected with 293T cells
(American Type Culture Collection, Manassas, VA, USA). Based on the
inhibition efficiency of the OPN gene, an effective RNAi sequence
was identified: 5′-GAGCATTCCGATGTGATTGAT-3′. Next, the sequence of
shRNA was designed as follows: Forward,
5′-CCGGGAGCATTCCGATGTGATTGATTTCAAGAGAATCAATCACATCGGAATGCTCTTTTTG-3′,
and reverse,
5′-AATTCAAAAAGAGCATTCCGATGTGATTGATTCTCTTGAAATCAATCACATCGGAATGCTC-3′.
shRNA was synthesized by Shanghai GeneChem Co., Ltd..
Lentivirus plasmid construction
A pGCSIL-green fluorescent protein (GFP) vector
(Shanghai GeneChem Co., Ltd) was digested using AgeI and EcoRI (New
England Biolabs (Beijing) Ltd., Beijing, China) in order to
linearize it. The target gene and the digested linearized vector
were directionally connected and the products were transformed into
bacterial competent cells. Next, polymerase chain reaction (PCR)
identification of positive clones was performed by sequencing and
comparative analysis. Viral vectors containing the various RNAi
sequences were used to transfect 293T cells using
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer’s instructions.
Following transfection for 24 h, the cells were observed by
fluorescence microscopy using an Olympus Micropublisher 3.3 RTV
(Olympus Corp., Tokyo, Japan). After 48 h of transfection, western
blot assay was performed to analyze the target protein expression,
which determined the interference effects of various targets.
Finally, the correct siRNA sequence (pSC-2) for the OPN gene and
RNAi expression vector were constructed.
Cell transfection
The T24 cells were divided into six-well plates
(5×104cells/well) one day prior to the virus infection
and cultured at 37°C in 5% CO2 humidified air. On the
first day of infection, experiments were performed on plasmids
containing RNAi lentiviral particles according to the experimental
design. Briefly, when cell confluence reached 30%, the culture
medium was refreshed. The three lentiviruses were added according
to multiplicity of infection (MOI), ten. The three transfection
groups were as follows: Empty plasmid group, transfected with an
empty lentiviral vector without any sequence; negative control
group, transfected with a lentiviral vector containing a negative
control RNAi sequence (5′-TTATCGACGTATTGGTAGACG-3′); OPN RNAi
group, transfected with a lentivirus containing OPN RNAi sequences.
The cells were transfected for 12 h, if there was no clear
cytotoxic effect induced within this time-period, the incubation
was continued for a further 24 h under identical conditions prior
to refreshment of the medium. If there was a significant cytotoxic
effect following the initial 12 h incubation, the culture medium
was refreshed immediately.
Reverse transcription-quantitative PCR
(RT-qPCR) assay for OPN mRNA
The infection efficiency of OPN-RNAi was detected by
RT-qPCR. After transfection for five days, the cells were collected
and total RNA was isolated using the TRIzol® reagent
(Invitrogen Life Technologies) and quantified by spectrophotometry
(Eppendorf Biospectrometer Basic; Eppendorf, Hamburg, Germany).
Following isolation, 2 μg total RNA from each sample was reverse
transcribed using the HiFi-MMLV cDNA Kit (Beijing CoWin Biotech
Co., Ltd, Beijing, China) according to the manufacturer’s
instructions. The primer sequences of OPN and GAPDH (Generay
Biotech Co. Ltd, Shanghai, China) and the annealing temperatures
used in this study are presented in Table I. RT-qPCR was performed using a
SYBR® Premix Ex Taq™ kit (Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer’s instructions. All RT-qPCR
reactions were performed on an ABI PRISM 7700 sequence detection
system (Applied Biosystems Life Technologies, Grand Island, NY,
USA). In each reaction, 1 μl cDNA, 10 μl SYBR® Premix Ex
Taq™ and 0.4 μM forward and reverse primers in a total volume of 20
μl were used. The reaction conditions were set as follows: one
cycle at 95°C for 15 sec, followed by 25 cycles at 95°C for 5 sec
and 60°C for 30 sec. RT-qPCR was performed in triplicate for each
sample. GAPDH was used as an internal control, and all the results
were analyzed using the standard 2−ΔΔCT method, as
described previously (20).
 | Table ISequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequences
(forward/reverse) | GenBank number | Annealing temperature
(°C) | Product length
(bp) |
|---|
| Osteopontin |
5′-GTTGGTGGAGGATGTCTG-3′/5′-TACTTGGAAGGGTCTGTG-3′ | NM_000582 | 62 | 344 |
| GAPDH |
5′-AACGGATTTGGTCGTATTG-3′/5′-GGAAGATGGTGATGGGATT-3′ | NM_002046 | 62 | 208 |
Western blot assay
Total protein of the collected cells was extracted
six days post-infection. At the end of the treatment, the cell
culture medium was aspirated and the cells were detached in PBS by
scraping. The detached cells were centrifuged at 21,000 × g at 4°C
for 15 min and the cell pellets were then lysed in 300 μl
radioimmunoprecipitation lysis buffer (Cytobuster protein
extraction reagent; P0013; Beyotime Institure of Biotechnology,
Shanghai, China) with 25 mM NaF, 1 mM Na3VO4,
and 1X protease inhibitor cocktail [Aprotinin (2 μg/ml), Leupeptin
(10 μg/ml), Pepstain A (1 μg/ml), PMSF (1 mM), EDTA (5 mM), EGTA (1
mM), Na Fluoride (10 mM), Na Orthovanadate (1 mM); Roche
Diagnostics, Basel, Switzerland] dissolved in distilled water.
Protein concentrations were quantified by spectrophotometry
(Eppendorf BioSpectrometer; Eppendorf, Hamburg, Germany). For
western blot analysis, equal amounts of protein (50 μl) from each
sample were subjected to SDS-PAGE and electrotransferred onto
polyvinylidine fluoride (PVDF) membranes (EMD Millipore, Bedford,
MA, USA). The membranes were blocked with 5% (w/v) bovine serum
albumin (Gibco Life Technologies) in Tris-buffered saline/Tween 20
[10 mM Tris, 150 mM NaCl, and 0.1% (v/v) Tween 20; pH 7.5] for 1 h
at room temperature and incubated with primary antibodies overnight
at 4°C. Incubation with goat monoclonal anti-rabbit secondary
antibody was performed at room temperature for 1 h (1:8,000; Santa
Cruz Biotechnology, Inc.). An enhanced chemiluminescence system
(Beyotime Institute of Biotechnology) was used to detect
immunoreactive protein signals. The protein signals were then
exposed to X-ray films for visualization and photographed (Canon
IXUS 105; Canon Zhuhai, Inc., Zhuhai, China) and quantified using
Image J software version 1.38 (National Institutes of Health,
Bethesda, MA, USA). For re-probing, PVDF membranes were stripped
with 0.2 M NaOH for 10 min before blocking with an additional
primary antibody. The expression levels of the molecules of
interest were determined relative to β-actin and each experiment
was repeated three times.
MTT assay for the proliferation of T24
cells
When the T24 cell confluence reached 30%, the medium
was refreshed and lentiviral transfection of the three groups was
performed according to the aforementioned protocol. After 12, 24,
48, 72 or 96 h, MTT (20 mg/ml; Amresco LLC, Solon, OH, USA) was
added and incubated for 4 h, followed by addition of 150 μl
dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA). After
shaking for 10 min, the absorbance was measured at 570 nm
using a microplate reader (Thermo Multiskan MK3; Thermoelectric
Electronics Co., Ltd, Shanghai, China).
Flow cytometric analysis for
determination of cell cycle progression and apoptosis
Flow cytometric analysis (FACS Calibur; BD
Biosciences, Franklin Lakes, NJ, USA) was used to detect the cell
cycle progression and apoptosis. The cells were stained with
propidium iodide (PI; KGI Biotechnology Development Co. Ltd.,
Nanjing, China), followed by detection of the cell cycle
progression. The cell suspension was prepared and the degree of
apoptosis was detected using an Annexin V-APC kit (Promega,
Madison, WI, USA). Briefly, cells were scraped, washed twice with
PBS and centrifuged. Cells were incubated in 1X binding buffer
containing Annexin V-APC and PI in dark for 15 min. Apoptotic cells
were examined using flow cytometry. Each sample was analyzed in
triplicate.
Matrigel Transwell assay for cell
invasion
Matrigel invasion chambers (Promega) were hydrated
for 4 h prior to the initiation of the invasion assay. After
transfection for ~72 h, log-phase cells (4×104/ml)
cultured in α-MEM without FBS were seeded in the upper chamber of
the wells, while the lower chambers were filled with 500 μl
complete α-MEM supplemented with 10% FBS. The cells were allowed to
migrate for 10 h, followed by the invasion assay. Three fields in
the central and surrounding parts of each membrane were randomly
selected for counting. The experiments were performed in triplicate
and the data were compared with the negative control group.
Enzyme-linked immunosorbent assay
(ELISA)
After transfection for ~72 h, the T24 cells were
collected for ELISA. The concentrations of matrix metalloproteinase
(MMP)-9 and MMP-2 in the cell culture supernatants were quantified
using MMP-9 and MMP-2 ELISA kits (R&D Systems, Minneapolis, MN,
USA). Each assay was performed five times.
Statistical analysis
SAS 9.0 software (SAS Institute, Inc., Cary, NC,
USA) was used for statistical analysis. Western blots were
quantified by measuring the relative density of protein bands
recognized by a particular antibody using the Image J software. The
results are expressed as the mean ± standard deviation. Statistical
analysis was conducted with Student’s t-test for comparison
of two groups and P<0.05 was considered to indicate a
statistically significant difference.
Results
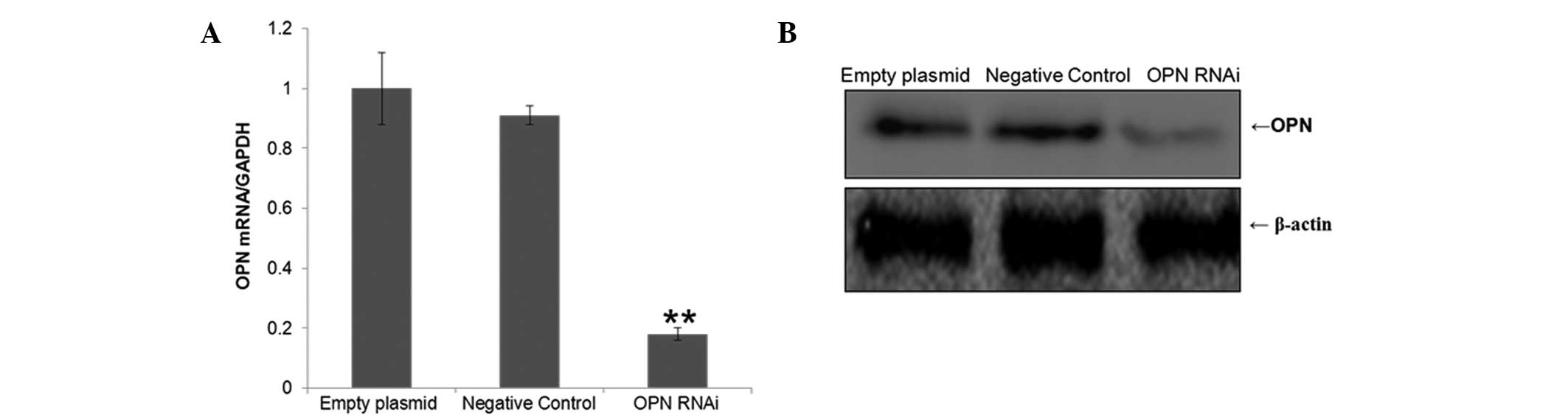
Expression of OPN is clearly inhibited by
RNAi
T24 cells were cultured in vitro for 24 h.
The positive expression rate of GFP was detected by fluorescence
microscopy 48 h after lentiviral infection of T24 cells. The
results indicated that the infection efficiency was >90%. The
relative mRNA expression levels of OPN were detected by RT-qPCR 5
days after lentiviral infection of T24 cells. The relative protein
expression levels of OPN were detected using western blot analysis
6 days subsequent to lentiviral transfection of T24 cells. The
results revealed that the OPN expression level in the interference
group was significantly lower compared with the negative control
and empty plasmid groups (P<0.05; Fig. 1).
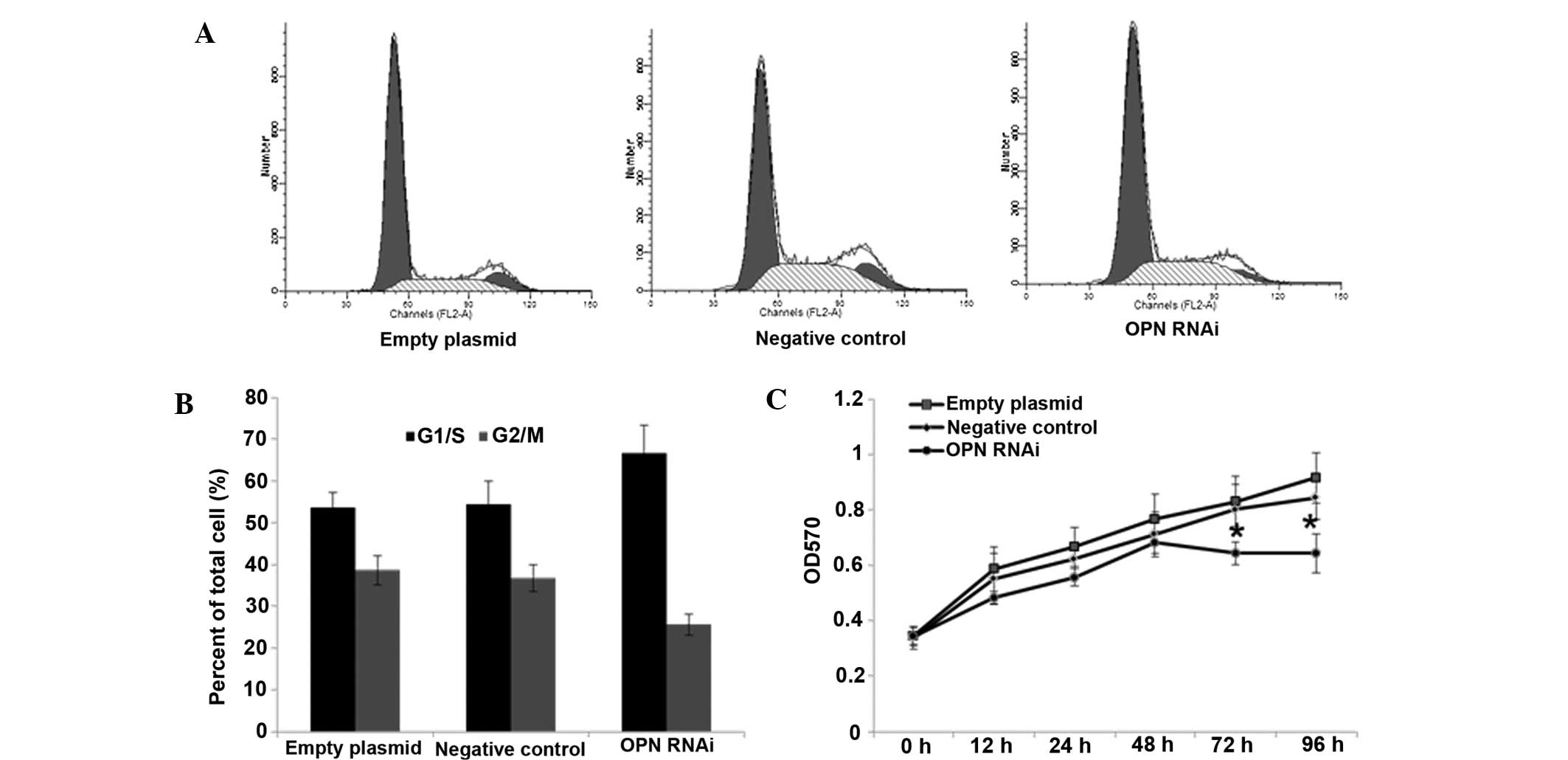
Effect of OPN-RNAi on the growth of T24
cells
The results of the MTT assay demonstrated that the
growth rate of cell proliferation was gradually reduced in the
interference group over 96 h. Cell proliferation was significantly
reduced compared with the control groups after 48 h (P<0.05;
Fig. 2C).
Cell cycle analysis by flow
cytometry
The results of the cell cycle analysis revealed that
a number cells were blocked in G1 phase in the
interference group (Fig. 2). A
significant difference was observed between the number of cells in
the G1/S phase in the interference group compared with
the negative control and empty plasmid groups (P<0.01).
G2/M-phase cells in the interference group were
significantly less compared with those in the negative control and
empty plasmid groups (P<0.05).
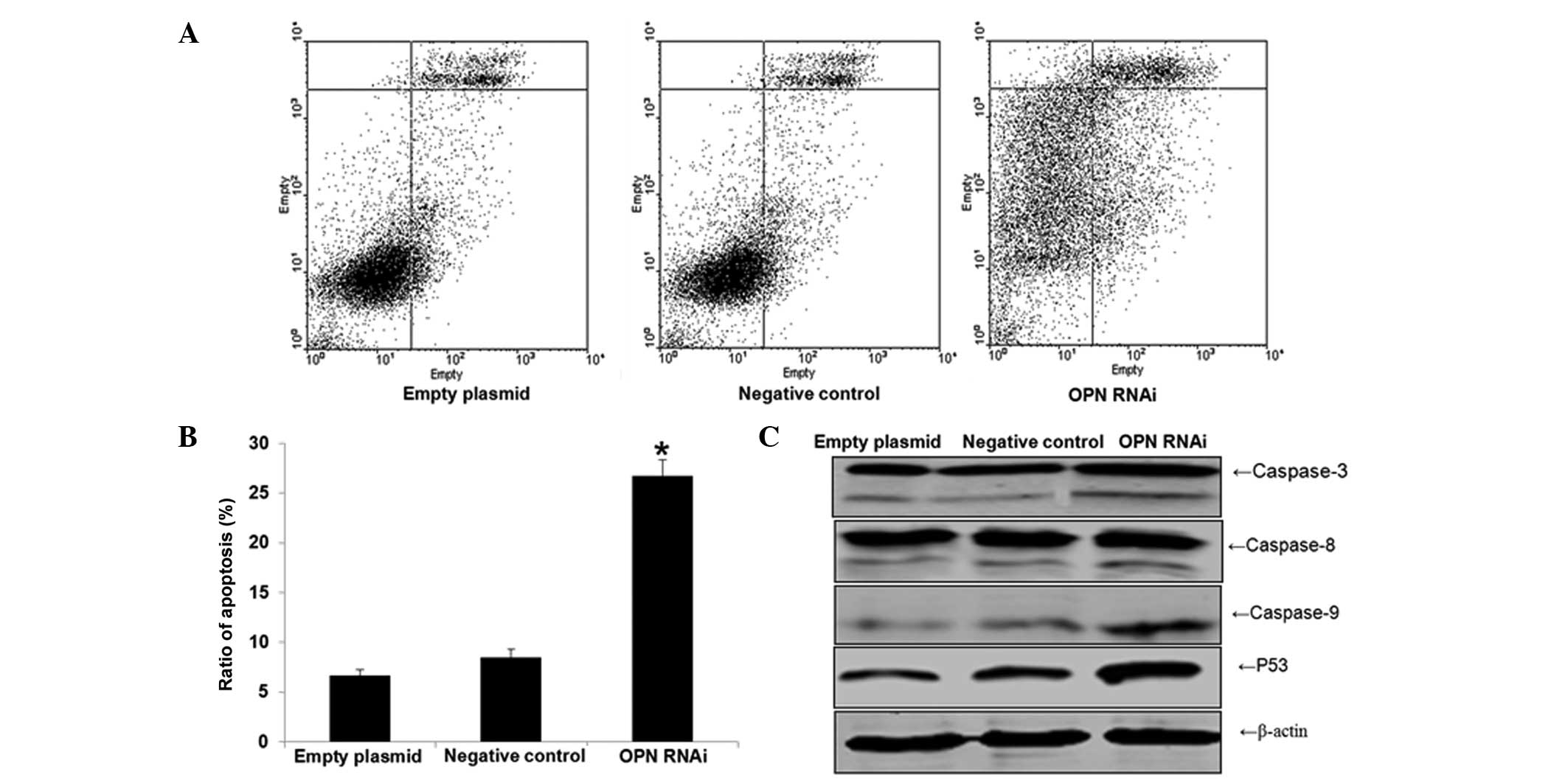
Effect of OPN-RNAi on the apoptosis of
T24 cells
Cell apoptosis was found to be significantly
increased in the interference group compared with the negative
control and empty plasmid group (P<0.05). No significant
differences were identified between the apoptosis rates of the
negative control and empty plasmid groups (P>0.05), as shown in
Fig. 3A. The expression levels of
apoptosis proteins were detected by western blot analysis (Fig. 3B). The results indicated that the
expression levels of caspase-3, caspase-8, caspase-9 and p53
increased significantly upon OPN-RNAi treatment, when compared with
the empty plasmid and negative control groups.
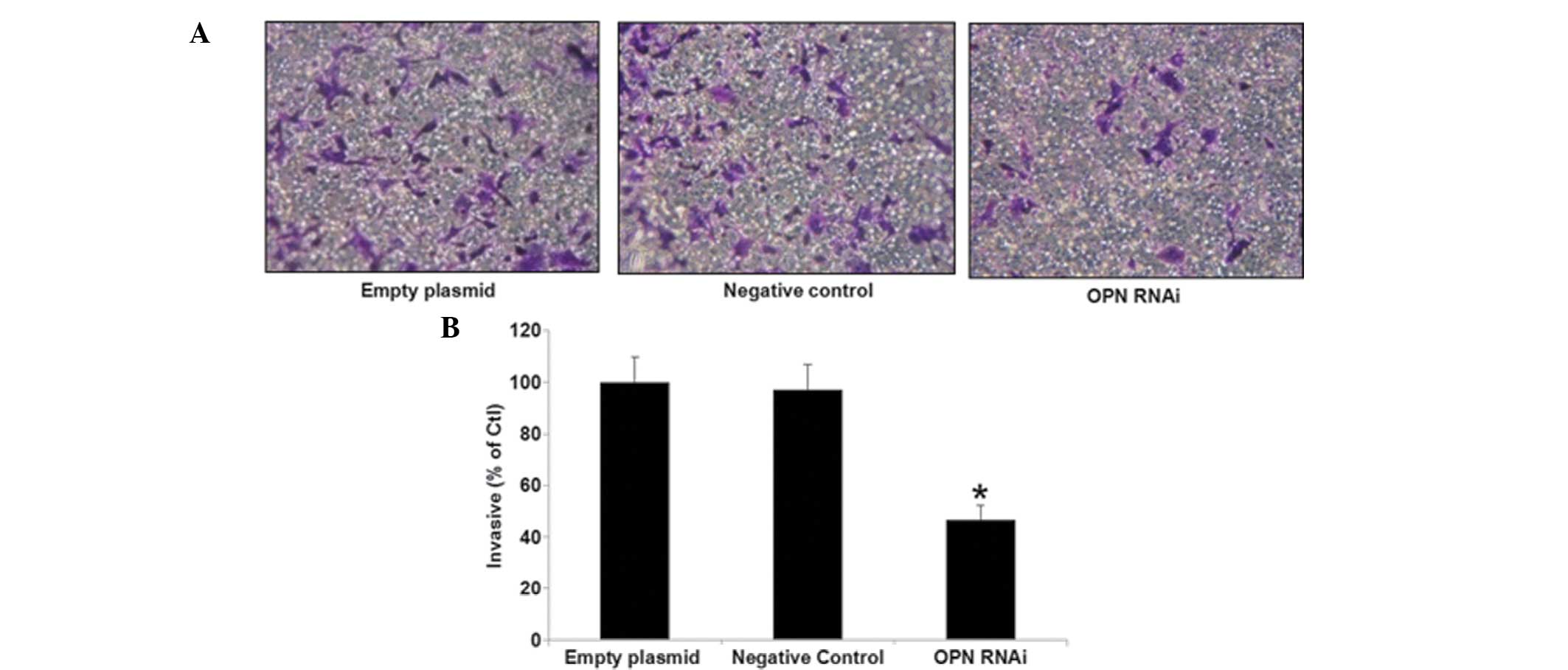
Impact of OPN-RNAi on invasion of T24
cells
The number of cells invading through the membrane in
the interference group was significantly lower compared with the
negative control and empty plasmid groups (P<0.05). The findings
indicated a reduced capacity of tumor invasion in the interference
group in T24 cells, as shown in Fig.
4.
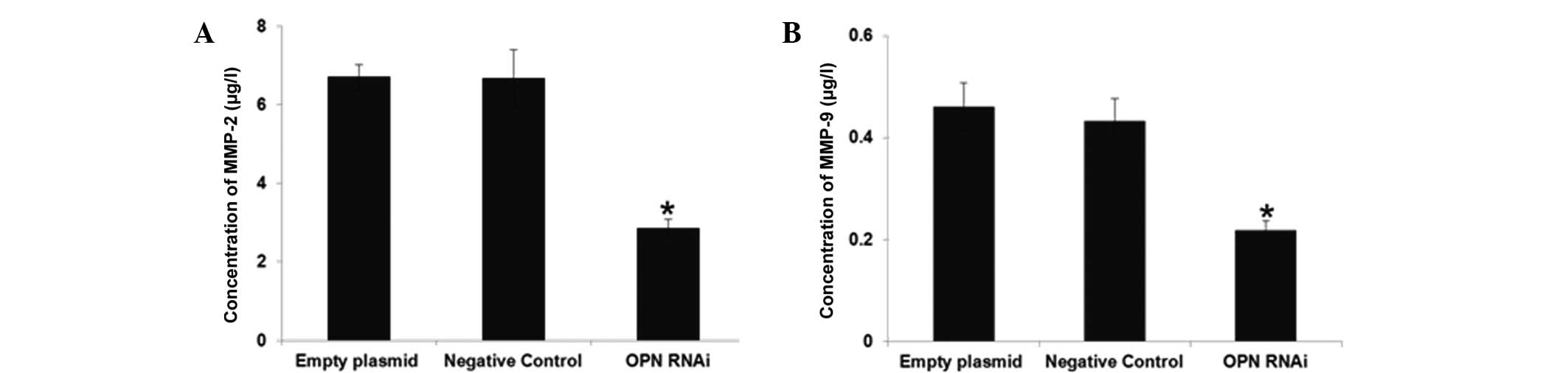
Effect of OPN-RNAi on the expression
levels of migration relate proteins
The concentrations of MMP-2 and MMP-9 were measured
in the cell culture medium. As expected, OPN-RNAi treatment was
found to significantly downregulate the expression of these
proteins (Fig. 5).
Discussion
Osteopontin (OPN) is a secreted acidic glycoprotein
with high expression in various tumor tissues (10–13,
21). Inhibition of the mRNA
expression of OPN has been shown to be satisfactory in a number of
tumors through RNAi technology (8). Previous studies have demonstrated
that inhibition of OPN receptor expression through RNAi
significantly inhibited adhesion, migration and distant metastasis
of human breast cancer cells (22,23).
Gao et al (24)
investigated OPN function through the 4T1 and 4T07 mice mammary
gland epithelial cell lines. The authors identified that OPN was
expressed in the 4T1 cells, resulting in tumorigenesis, while the
tumors presented distant metastasis. However, tumors were unable to
transfer in 4T07 cells that did not express OPN. Similar results
have been observed in CT26 colon cancer cells (25). Furthermore, a previous study has
demonstrated that the OPN gene is highly expressed in bladder
cancer (26). Based on previous
studies, OPN-targeting gene therapy was hypothesized to be a
potentially novel treatment method for bladder cancer.
In the present study, OPN RNAi was successfully
performed. An effective target was successfully selected and
transfected with lentivirus, while the efficiency of lentiviral
infection in T24 cells was determined. The lentivirus-mediated RNAi
of OPN gene was investigated in T24 cells using MTT, flow
cytometric and Transwell assays. Five days after transfecting the
T24 cells, the mRNA expression of OPN was found to be significantly
reduced (P<0.05) in the interference group compared with the
untreated groups. In addition, six days post-transfection, the
protein expression of OPN was found to be significantly reduced
(P<0.05) in the interference group compared with the untreated
groups. MTT assay revealed that the proliferation of T24 cells
decreased due to RNAi. Furthermore, cell cycle analysis
demonstrated that cell cycle arrest of T24 cells occurred at
G1/S phase upon OPN gene inhibition. Apoptotic analysis
revealed that OPN gene interference in T24 cells resulted in the
loss of the anti-apoptotic effect of the gene.
OPN plays an important role in cell migration and
invasion processes. In addition, a number of studies have
demonstrated that OPN promoted tumor invasion and metastasis and
regulated MMP-9 secretion (27–31).
OPN was shown to combine with integrin αvβ3 and activate NF-κB
(nuclear factor-κB) through the PI3K/Akt/IKK (κB kinase inhibitor)
signaling pathway, increasing the secretion of urokinase-type
plasminogen activator A (uPA) and promoting tumor invasion
(32,33). In the current study, the results
demonstrated that OPN gene interference inhibited cell invasion and
migration through regulation of the expression levels of MMPs in
T24 cells.
In conclusion, the present study verified that
recombinant lentiviral vectors carrying the OPN-RNAi sequence can
significantly inhibit the proliferation of T24 bladder cancer cells
in vitro. RNAi was found to induce apoptosis in T24 cells
and reduce invasion in vitro. Therefore, OPN may be a
potentially novel target for bladder cancer gene therapy. However,
in vitro studies present certain limitations, such as poor
targets, safety of the carrier and drug delivery. Therefore,
further studies are required prior to the application of the
present study in the clinical treatment of bladder cancer. With the
development of tumor molecular biology and associated disciplines,
as well as the identification of safe and effective vectors, RNAi
technology may play an important role in the treatment of bladder
cancer.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of Luohe Medical College, P.R China (no.
2013-DF-002).
References
|
1
|
Baffa R, Letko J, McClung C, LeNoir J,
Vecchione A and Gomella LG: Molecular genetics ddof bladder cancer:
targets for diagnosis and therapy. J Exp Clin Cancer Res.
25:145–160. 2006.PubMed/NCBI
|
|
2
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
Raghavan D and Skinner DG: Radical cystectomy in the treatment of
invasive bladder cancer: long-term results in 1,054 patients. J
Clin Oncol. 19:666–675. 2001.PubMed/NCBI
|
|
3
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM,
Sesterhenn IA, Tachibana M and Weider J: Bladder cancer:
epidemiology, staging and grading, and diagnosis. Urology. 66(6
Suppl 1): 4–34. 2005. View Article : Google Scholar
|
|
4
|
Hass HG, Nehls O, Jobst J, Frilling A,
Vogel U and Kaiser S: Identification of osteopontin as the most
consistently over-expressed gene in intrahepatic
cholangiocarcinoma: detection by oligonucleotide microarray and
real-time PCR analysis. World J Gastroenterol. 14:2501–2510. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castellano G, Malaponte G, Mazzarino MC,
Figini M, Marchese F, Gangemi P, Travali S, Stivala F, Canevari S
and Libra M: Activation of the osteopontin/matrix
metalloproteinase-9 pathway correlates with prostate cancer
progression. Clin Cancer Res. 14:7470–7480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Das R, Mahabeleshwar GH and Kundu GC:
Osteopontin induces AP-1-mediated secretion of urokinase-type
plasminogen activator through c-Src-dependent epidermal growth
factor receptor transactivation in breast cancer cells. J Biol
Chem. 279:11051–11064. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun BS, You J, Li Y, Zhang ZF and Wang CL:
Osteopontin knockdown suppresses non-small cell lung cancer cell
invasion and metastasis. Chin Med J (Engl). 126:1683–1688.
2013.
|
|
9
|
Yang L, Zhao W, Zuo WS, Wei L, Song XR,
Wang XW, Zheng G and Zheng MZ: Silencing of osteopontin promotes
the radiosensitivity of breast cancer cells by reducing the
expression of hypoxia inducible factor 1 and vascular endothelial
growth factor. Chin Med J (Engl). 125:293–299. 2012.
|
|
10
|
Tuck AB, Chambers AF and Allan AL:
Osteopontin overexpression in breast cancer: knowledge gained and
possible implications for clinical management. J Cell Biochem.
102:859–868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boldrini L, Donati V, Dell’Omodarme M,
Prati MC, Faviana P, Camacci T, Lucchi M, Mussi A, Santoro M,
Basolo F and Fontanini G: Prognostic significance of osteopontin
expression in early-stage non-small-cell lung cancer. Br J Cancer.
93:453–457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan HW, Ou YH, Peng SY, Liu SH, Lai PL,
Lee PH, Sheu JC, Chen CL and Hsu HC: Overexpression of osteopontin
is associated with intrahepatic metastasis, early recurrence, and
poorer prognosis of surgically resected hepatocellular carcinoma.
Cancer. 98:119–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schorge JO, Drake RD, Lee H, Skates SJ,
Rajanbabu R, Miller DS, Kim JH, Cramer DW, Berkowitz RS and Mok SC:
Osteopontin as an adjunct to CA125 in detecting recurrent ovarian
cancer. Clin Cancer Res. 10:3474–3478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mack PC, Redman MW, Chansky K, Williamson
SK, Farneth NC, Lara PN Jr, Franklin WA, Le QT, Crowley JJ and
Gandara DR; SWOG. Lower osteopontin plasma levels are associated
with superior outcomes in advanced non-small-cell lung cancer
patients receiving platinum-based chemotherapy: SWOG Study S0003. J
Clin Oncol. 26:4771–4776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartung F and Weber GF: RNA blood levels
of osteopontin splice variants are cancer markers. Springerplus.
2:1102013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dubreuil G, Magliano M, Dubrana MP, Lozano
J, Lecomte P, Favery B, Abad P and Rosso MN: Tobacco rattle virus
mediates gene silencing in a plant parasitic root-knot nematode. J
Exp Bot. 60:4041–4050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stein P, Svoboda P and Schultz RM:
RNAi-based methods for gene silencing in mouse oocytes. Methods Mol
Biol. 957:135–151. 2013. View Article : Google Scholar
|
|
18
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salido-Guadarrama I, Romero-Cordoba S,
Peralta-Zaragoza O, Hidalgo-Miranda A and Rodríguez-Dorantes M:
MicroRNAs transported by exosomes in body fluids as mediators of
intercellular communication in cancer. Onco Targets Ther.
7:1327–1338. 2014.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Sørensen ES, Højrup P and Petersen TE:
Posttranslational modifications of bovine osteopontin:
identification of twenty-eight phosphorylation and three
O-glycosylation sites. Protein Sci. 4:2040–2049. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mi Z, Guo H and Kuo PC: Identification of
osteopontin-dependent signaling pathways in a mouse model of human
breast cancer. BMC Res Notes. 2:1192009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shevde LA, Samant RS, Paik JC, Metge BJ,
Chambers AF, Casey G, Frost AR and Welch DR: Osteopontin knockdown
suppresses tumorigenicity of human metastatic breast carcinoma,
MDA-MB-435. Clin Exp Metastasis. 23:123–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao C, Mi Z, Guo H and Kuo PC: Osteopontin
regulates ubiquitin-dependent degradation of Stat1 in murine
mammary epithelial tumor cells. Neoplasia. 9:699–706. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wai PY, Mi Z, Guo H, Sarraf-Yazdi S, Gao
C, Wei J, Marroquin CE, Clary B and Kuo PC: Osteopontin silencing
by small interfering RNA suppresses in vitro and in vivo CT26
murine colon adenocarcinoma metastasis. Carcinogenesis. 26:741–751.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zaravinos A, Lambrou GI, Volanis D,
Delakas D and Spandidos DA: Spotlight on differentially expressed
genes in urinary bladder cancer. PLoS One. 6:e182552011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castellano G, Malaponte G, Mazzarino MC,
Figini M, Marchese F, Gangemi P, Travali S, Stivala F, Canevari S
and Libra M: Activation of the osteopontin/matrix
metalloproteinase-9 pathway correlates with prostate cancer
progression. Clin Cancer Res. 14:7470–7480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Likui W, Hong W, Shuwen Z, Yuangang Y and
Yan W: The potential of osteopontin as a therapeutic target for
human colorectal cancer. J Gastrointest Surg. 15:652–659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anborgh PH, Mutrie JC, Tuck AB and
Chambers AF: Pre- and post-translational regulation of osteopontin
in cancer. J Cell Commun Signal. 5:111–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramchandani D and Weber GF: An osteopontin
promoter polymorphism is associated with aggressiveness in breast
cancer. Oncol Rep. 30:1860–1868. 2013.PubMed/NCBI
|
|
32
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor-inducing kinase plays a crucial role in
osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear
factor kappaB-mediated promatrix metalloproteinase-9 activation. J
Biol Chem. 279:38921–38935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang X, Li J, Yu B, Su L, Yu Y, Yan M, Liu
B and Zhu Z: Osteopontin splice variants differentially exert
clinicopathological features and biological functions in gastric
cancer. Int J Biol Sci. 9:55–66. 2013. View Article : Google Scholar : PubMed/NCBI
|