Introduction
Melanoma was reported to have the highest mortality
rate of all skin cancers; however, it only accounts for <5% of
skin cancer cases (1). The
incidence of melanoma in the general population was shown to be
increasing rapidly, with the estimated number of cases expected to
treble over the next 30 years (2).
Therefore, the identification of melanoma-specific genes, which may
result in the elucidation of novel markers for monitoring the
disease status or novel therapeutic targets, is of great interest
(3).
Transforming growth factor (TGF)-β is secreted as a
latent precursor, which becomes proteolytically activated to form a
homodimer of two 12.5 kDa polypeptides, which are linked by a
disulfide bond (4,5). Three isoforms of TGF-β have been
identified in humans, which include TGF-β1, TGF-β2 and TGF-β3
(6). Active TGF-β isoforms were
found to be highly selective and bind with high affinity to the
membrane-spanning serine/threonine kinase receptor, TGF-β receptor
type II (TβRII), which subsequently recruits and activates TGF-β
receptor type I (5). TGF-β
signaling was reported to downregulate epithelial tumorigenesis
through inhibiting cell cycle progression, enhancing apoptosis as
well as establishing genomic stability and cellular senescence
(7).
Numerous studies have demonstrated the roles of
TGF-β isoforms in melanoma (8–10).
However, to the best of our knowledge, no systemic studies to date
have elucidated the association between in situ or plasma
TGF-β isoforms and the Chinese Han melanoma patients. The aim of
the present study was to investigate the in situ and plasma
levels of TGF-β1, β2 and β3 in melanoma patients and healthy
volunteers. In addition, the present study aimed to improve the
current understanding of the association between tumor and
inflammation.
Materials and methods
Subjects
Tissue specimens were obtained from 20 melanoma
patients, prior to treatment, at the Department of Plastic Surgery,
The First Affiliated Hospital of China Medical University
(Shenyang, China), between January 2006 and December 2010. Blood
samples were collected from the same patients as well as 20 healthy
volunteers without melanoma (selected according to gender
matching), and added to EDTA-containing tubes (Corning Life
Sciences Co. Ltd, Shanghai, China), which were stored on ice within
30 min. The samples were immediately centrifuged at 3,000 × g for
20 min; the plasma was then frozen and stored at −70°C until
further use. The present study was performed in compliance with the
Helsinki Declaration, all patients provided written informed
consent for participation and the procedure was approved by the
Ethics Committee of The First Affiliated Hospital of China Medical
University. Healthy volunteers were nurses, doctors, students and
patients at The First Affiliated Hospital of China Medical
University.
Immunoblotting
Tissues were lysed in lysis buffer (20 mM Tris-HCl,
150 mM NaCl, 2 mM EDTA and 1% Triton-X100) containing a protease
inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Proteins
(30 μg per lane) were separated using 10% SDS-PAGE (Bio-Rad
Laboratories, Inc., Shanghai, China) and transferred to
polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA,
USA). Western blot analysis was then performed using the following
primary antibodies: Mouse monoclonal immunoglobulin (Ig)
G1 TGF-β1 (1:200; sc-130348), goat polyclonal IgG TGF-β2
(1:200; sc-31610), mouse monoclonal IgM TGF-β3 (1:200; sc-166861)
and mouse monoclonal IgG1 β-actin (1:1,000; sc-47778),
which were all purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The reaction was followed by probing with
peroxidase-coupled secondary antibodies, including monoclonal
anti-goat IgG (1:1000; RPN4301), monoclonal anti-mouse IgM (1:1000;
BR100838) or monoclonal anti-mouse IgG (1:2000; RPN4201) antibodies
(Amersham Biosciences, Needham, MA, USA). Incubation with
antibodies was performed in 1.5% bovine serum albumin
(Sigma-Aldrich) in Tris-buffered saline with 0.1% Tween
(Sigma-Aldrich). Detection of the immune complexes was performed
using an enhanced chemiluminescence western blot detection kit
(Takara Bio, Inc., Dalian, China).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total tissular RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). Complementary
(c)DNA was then synthesized from 1 μg total RNA using
SuperScript II reverse transcriptase (Invitrogen Life Technologies)
according to the manufacturer’s instructions. Expression levels of
tissular TGF-β1, TGF-β2 and TGF-β3 messenger (m)RNA were
quantitated by qPCR using the ABI Prism 7500 Real-Time PCR System
(Applied Biosystems, Life Technologies) with power SYBR®
Green PCR Master Mix (Takara Bio, Inc.). The following primer sets
(Sangon Biotech Co., Ltd, Shanghai, China) were used: TGF-β1 sense,
5′-GTGGAGAATGTATACAAGCAGG-3′ and antisense,
5′-CTAATGTAAGGCATCACAGTC-3′; TGF-β2 sense,
5′-TCTAGGGTGGAAATGGATACACGAACC-3′ and antisense,
5′-TGTTACAAGCATCATCGTTGTCGTCG-3′; TGF-β3 sense,
5′GATGCATCCCACTTGCTG-3′ and antisense, 5′-CAGGTGGCATTGAAGGA-3′; and
GAPDH sense, 5′GAAGGTGAAGGTCGGAGT-3′ and antisense,
5′-CATGGGTGGAATCATATTGGAA-3′. The qPCR conditions were as follows:
One cycle at 95°C for 10 min, followed by 40 cycles at 95°C for 15
sec and 60°C for 1 min. The ΔΔCt methods was used for data
analysis.
Immunohistochemical staining
All reagents used for immunohistochemistry were
obtained from Beyotime Institute of Biotechnology (Beijing, China).
All tissues were fixed in 10% buffered formalin and embedded in
paraffin according to standard methods. Sections (4 μm) were then
deparaffinized in xylene. Endogenous peroxidase was blocked with 3%
hydrogen peroxide in deionized water for 20 min. Antigen retrieval
was performed in citrate buffer (10 mM, pH 6) for 30 min at 95°C.
Sections were immunostained with polyclonal antibodies for TGF-β1
(sc-130348; 1:100), TGF-β2 (sc-31610; 1:50) or TGF-β3 (sc-166861;
1:50) for 60 min at 37°C, followed by biotinylated secondary
antibodies for 30 min and subsequently reacted with horseradish
peroxidase for 30 min. For visualization, hydrogen
peroxide-activated diamino benzidine was applied. Five-minute
washes in phosphate-buffered saline were performed between each
step. Tissue sections were lightly counter-stained with
hematoxylin, dehydrated using a graded series of ethanol, cleared
with xylene and then mounted in mounting medium. Normal tissue was
used as a control and sections treated without primary antibodies
were used as negative controls.
ELISAs
Plasma levels of TGF-β1 (PDB110B; Human TGF-β1
Quantikine ELISA Kit; R&D Systems, Minneapolis, MN, USA),
TGF-β2 (PDB250 Human TGF-β2 Quantikine ELISA Kit; R&D Systems)
and TGF-β3 (OK-0265; OmniKine™ Human TGF-β3 ELISA Kit; Assay
Biotechnology, Sunnyvale, CA, USA) were detected using their
respective commercially available ELISA kits.
Statistical analysis
Values are presented as the mean ± standard
deviation. Differences between groups were analyzed using the
Student’s t-test for continuous variables. Survival time was
calculated from the date of melanoma diagnosis to the date of
succumbing to the disease or last follow-up. The Kaplan-Meier
method (11) was used to evaluate
the effects of TGF-β expression on the overall survival of
patients. Statistical analysis was performed using the SPSS 17.0
software (International Business Machines, Armonk, NY, USA) and
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
TGF-β1, β2 and β3 expression in human
melanoma specimens
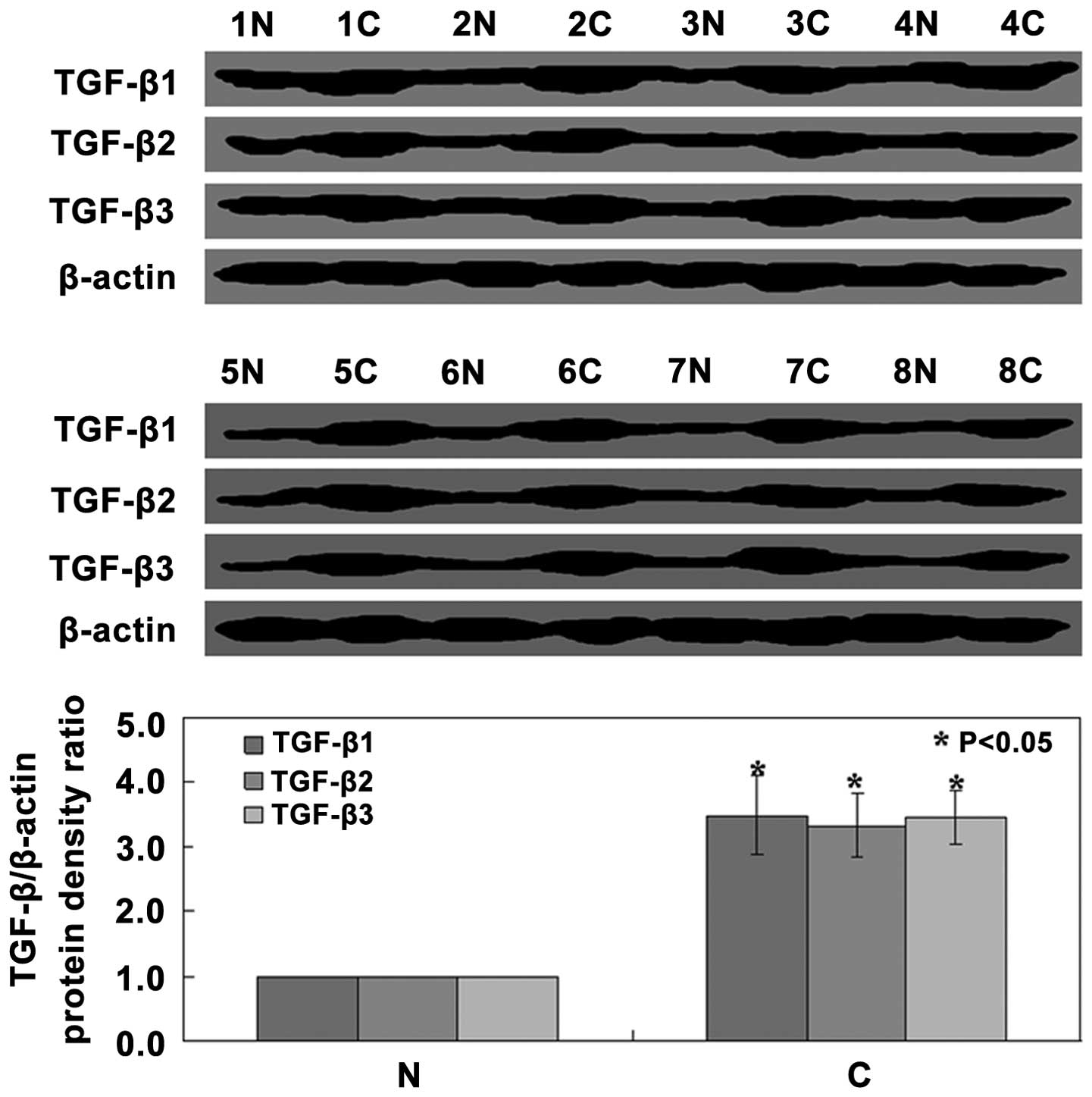
Western blot analysis of tissue samples revealed
that TGF-β1, TGF-β2 and TGF-β3 protein expression levels in cancer
tissue were all significantly increased compared with those in
normal tissue (P<0.05) (Fig.
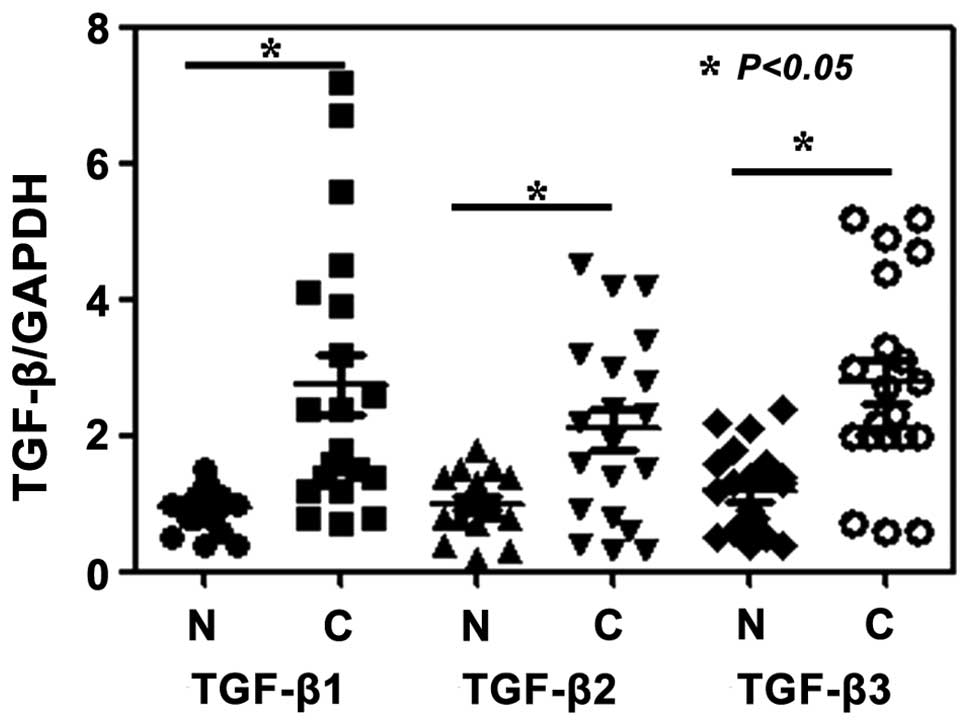
1). In order to determine whether TGF-β1, TGF-β2 and TGF-β3
mRNA expression levels were also increased, RT-qPCR analysis was
performed. The results showed that the expression levels of TGF-β1,
TGF-β2 and TGF-β3 mRNA were coincident with those of the protein
expression, namely significantly increased compared with those in
normal tissue (P<0.05) (Fig.
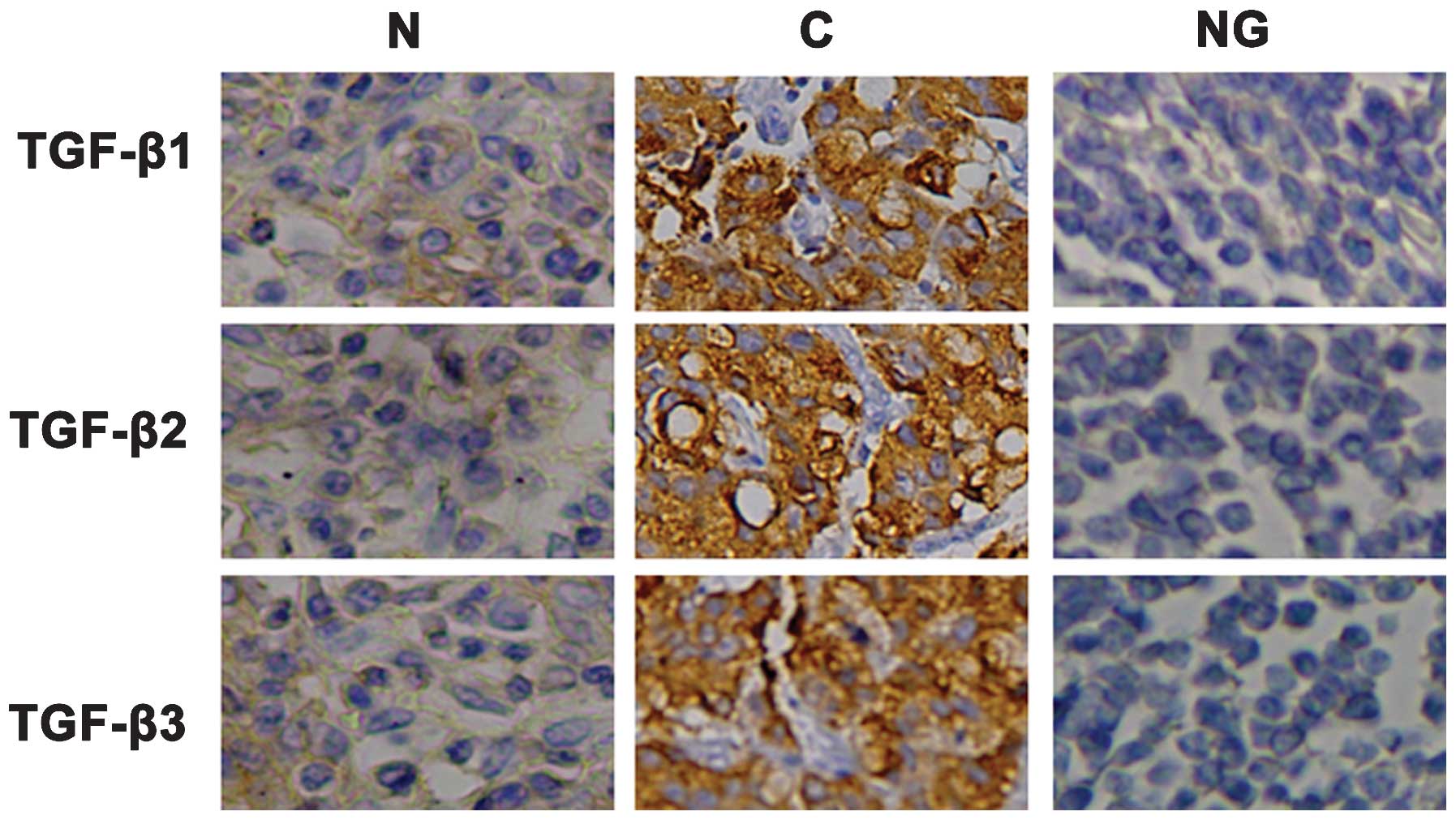
2). As shown in Fig. 3,
immunohistochemical staining revealed that TGF-β1, TGF-β2 and
TGF-β3 proteins were located in the cytoplasm of cancer cells.
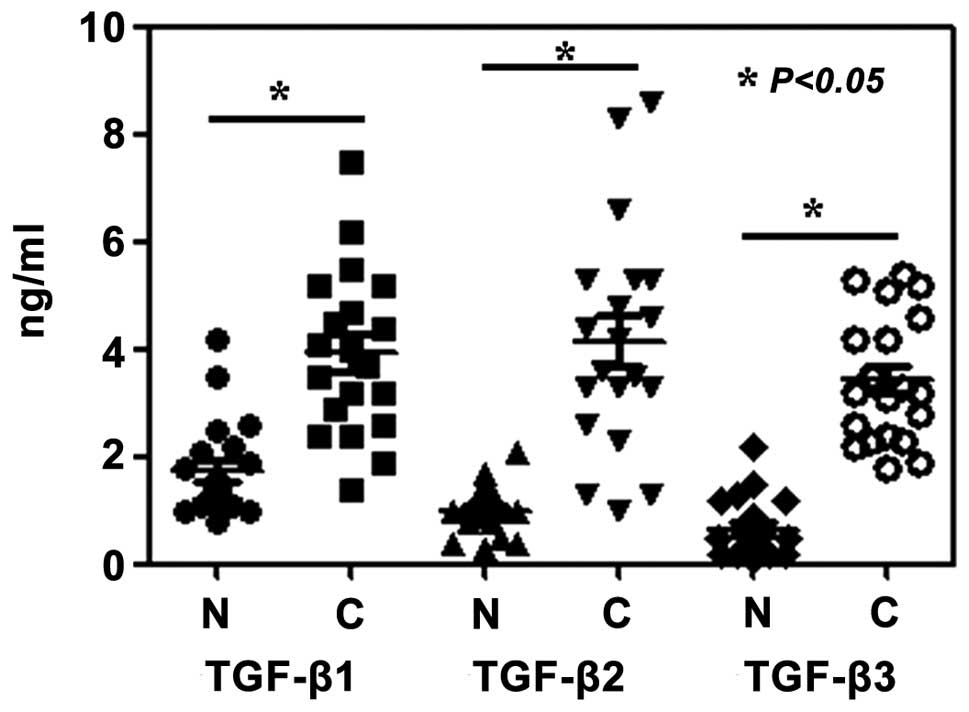
Furthermore, the plasma levels of TGF-β1, TGF-β2 and TGF-β3 in
malignant melanoma patients were significantly elevated compared
with those in the healthy volunteers (P<0.05) (Fig. 4).
TGF-β expression and the
clinicopathological variables
The potential associations between the expression of
TGF-β and the clinicopathological characteristics of the patients
enrolled in the present study were then determined. No significant
associations were identified between TGF-β1, TGF-β2 and TGF-β3
proteins and the clinicopathological characteristics of the
patients with melanoma (P>0.05) (Table I). In order to investigate
associations between the expression levels of TGF-β and patient
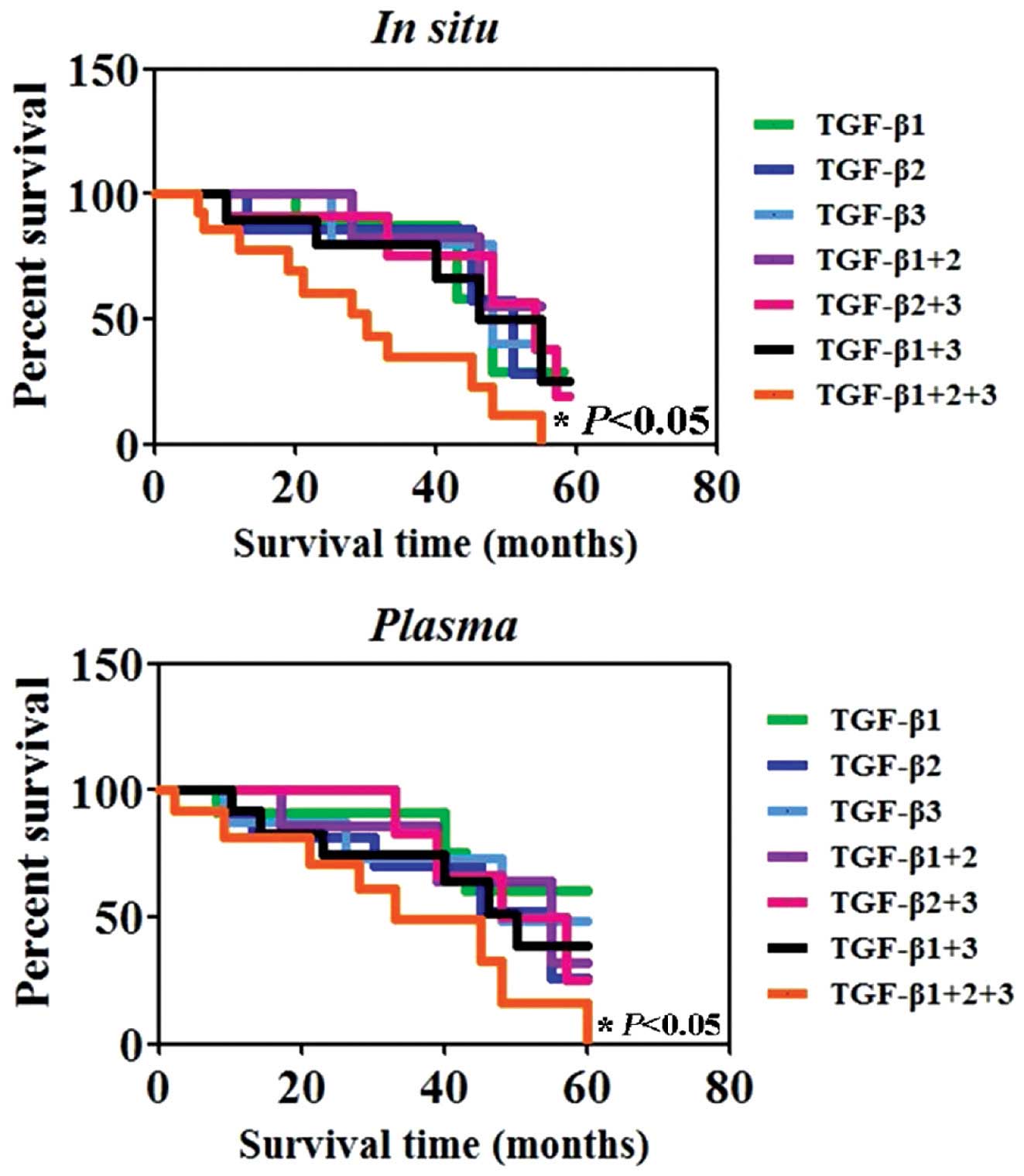
survival, the survival data from the 20 patients with melanoma
enrolled in the present study, were assessed using the Kaplan-Meier
method. The results showed that the survival rate of the
triple-positive (TGF-β1+, TGF-β2+ and
TGF-β3+) patients were markedly decreased compared with
those of patients who were found to be single-(TGF-β1+,
TGF-β2+ or TGF-β3+) or double-positive
(TGF-β1+, TGF-β2+; TGF-β2+,
TGF-β3+; or TGF-β1+, TGF-β3+)
(P<0.05) (Fig. 5), these
results were comparable for in situ and plasma protein
expression.
 | Table IAssociations between the expression of
TGF-β isoforms and the clinicopathological parameters of patients
with melanoma. |
Table I
Associations between the expression of
TGF-β isoforms and the clinicopathological parameters of patients
with melanoma.
| Clinicopathological
feature | TGF-β1
| TGF-β2
| TGF-β3
|
|---|
| n | − | + | PR (%) | χ2 | P | − | + | PR (%) | χ2 | P | − | + | PR (%) | χ2 | P |
|---|
| Gender | | | | | 0.42 | 0.52 | | | | 2.22 | 0.14 | | | | 0.42 | 0.52 |
| Female | 5 | 1 | 4 | 80.0 | | | 3 | 2 | 40.0 | | | 2 | 3 | 60.0 | | |
| Male | 15 | 3 | 12 | 80.0 | | | 2 | 13 | 86.7 | | | 2 | 13 | 86.7 | | |
| Age (years) | | | | | 0.31 | 0.57 | | | | 0.00 | 1.00 | | | | 2.81 | 0.09 |
| <60 | 10 | 2 | 8 | 80.0 | | | 2 | 8 | 80.0 | | | 4 | 6 | 60.0 | | |
| >60 | 10 | 2 | 8 | 80.0 | | | 3 | 7 | 70.0 | | | 0 | 10 | 100.0 | | |
| Tumor width (mm) | | | | | 3.78 | 0.29 | | | | 6.16 | 0.10 | | | | 1.88 | 0.60 |
| <1.0 | 2 | 1 | 1 | 50.0 | | | 1 | 1 | 50.0 | | | 1 | 1 | 50.0 | | |
| 1.01–2.00 | 3 | 1 | 2 | 66.7 | | | 2 | 1 | 33.3 | | | 1 | 2 | 66.7 | | |
| 2.01–4.00 | 7 | 2 | 5 | 71.4 | | | 2 | 5 | 71.4 | | | 1 | 6 | 85.7 | | |
| >4.00 | 8 | 0 | 8 | 100.0 | | | 0 | 8 | 100.0 | | | 1 | 7 | 87.5 | | |
| Ulceration | | | | | 0.01 | 0.91 | | | | 0.28 | 0.59 | | | | 1.58 | 0.21 |
| − | 8 | 2 | 6 | 75.0 | | | 3 | 5 | 62.5 | | | 0 | 8 | 10.0 | | |
| + | 12 | 2 | 10 | 83.3 | | | 2 | 10 | 83.3 | | | 4 | 8 | 66.7 | | |
| Site | | | | | 0.11 | 0.74 | | | | 0.61 | 0.44 | | | | 0.11 | 0.74 |
| Sun-protected | 11 | 3 | 8 | 72.7 | | | 4 | 7 | 63.6 | | | 3 | 8 | 72.7 | | |
| Sun-exposed | 9 | 1 | 8 | 88.9 | | | 1 | 8 | 88.9 | | | 1 | 8 | 88.9 | | |
| Subtype | | | | | 0.21 | 0.98 | | | | 0.44 | 0.93 | | | | 3.85 | 0.28 |
| pNO | 4 | 1 | 3 | 75.0 | | | 1 | 3 | 75.0 | | | 2 | 2 | 50.0 | | |
| pNl | 4 | 1 | 3 | 75.0 | | | 1 | 3 | 75.0 | | | 1 | 3 | 75.0 | | |
| pN2 | 6 | 1 | 5 | 83.3 | | | 2 | 4 | 66.7 | | | 1 | 5 | 83.3 | | |
| pN3 | 6 | 1 | 5 | 83.3 | | | 1 | 5 | 83.3 | | | 0 | 6 | 100.0 | | |
Discussion
TGF-β is known to be an important regulator of tumor
progression as well as a variety of biological processes, including
cell proliferation, angiogenesis, migration, invasion and survival
(12,13). In the present study, the in
situ and plasma expression levels of all three TGF-β isoforms
were investigated in melanoma patients. Previous studies have shown
that melanoma cells produced TGF-β1 (14) and that TGF-β1 induced the death of
surrounding healthy cells, thus eliminating their inhibitory
effects on tumor growth (15).
Overexpression of TGF-β1 mRNA was identified in primary and
metastatic melanomas (16); in
addition, TGF-β2 and TGF-β3 were not found to be expressed in
normal melanocytes, whereas they were expressed in nevi as well as
early and advanced primary and metastatic melanomas, where they
were associated with tumor progression (17). In concurrence with these previous
studies, the present study confirmed that the in situ levels
of TGF-β1, TGF-β2 and TGF-β3 protein were significantly increased
in the patients with melanoma.
The secretion of TGF-β isoforms have been
investigated in established melanoma cell lines (18). Krasagakis et al (19) performed a series of experiments to
determine the systemic levels of TGF-β isoforms in melanoma
patients, the results of which demonstrated a marked increase in
TGF-β1 expression and a moderate increase in TGF-β2 expression in
the plasma of metastatic melanoma patients; however, no elevation
was detected in TGF-β3 plasma expression. By contrast, the results
of the present study showed that the plasma levels of TGF-β1,
TGF-β2 and TGF-β3 proteins were significantly increased in melanoma
patients compared with those of the healthy controls. The present
study demonstrated that the survival rate of the triple-positive
patients was markedly lower compared with that of single- or
double-positive patients.
In conclusion, the results of the present study
revealed that the expression levels of all three TGF-β isoforms
were significantly increased in melanoma patients. In addition,
these results indicated that positive TGF-β1, TGF-β2 and TGF-β3
expression was correlated with a poor survival of melanoma
patients. This therefore suggested that TGF-β1, TGF-β2 and TGF-β3
may serve as promising prognostic markers for patients with
malignant melanoma.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diffey BL: The future incidence of
cutaneous melanoma within the UK. Br J Dermatol. 151:868–872. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sousa JF, Torrieri R, Silva RR, et al:
Novel primate specific genes, RMEL 1, 2 and 3, with highly
restricted expression in melanoma, assessed by new data mining
tool. PLoS One. 5:e135102010. View Article : Google Scholar
|
|
4
|
Massague J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar
|
|
5
|
Derynck R and Zhang YE: Smad-dependent and
Smad independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu MY and Hill CS: Tgf-beta superfamily
signaling in embryonic development and homeostasis. Dev Cell.
16:329–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roberts AB and Wakefeld LM: The two faces
of transforming growth factor beta in carcinogenesis. Proc Natl
Acad Sci USA. 100:8621–8623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Busse A and Keilholz U: Role of TGF-β in
melanoma. Curr Pharm Biotechnol. 12:2165–2175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lasfar A and Cohen-Solal KA: Resistance to
transforming growth factor β-mediated tumor suppression in
melanoma: are multiple mechanisms in place? Carcinogenesis.
31:1710–1717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malaponte G, Zacchia A, Bevelacqua Y, et
al: Co-regulated expression of matrix metalloproteinase-2 and
transforming growth factor-beta in melanoma development and
progression. Oncol Rep. 24:81–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goel MK, Khanna P and Kishore J:
Understanding survival analysis: Kaplan-Meier estimate. Int J
Ayurveda Res. 1:274–278. 2010. View Article : Google Scholar
|
|
12
|
Massagué J: TGFbeta in Cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leivonen SK and Kähäri VM: Transforming
growth factor-beta signaling in cancer invasion and metastasis. Int
J Cancer. 121:2119–2124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Albino AP, Davis BM and Nanus DM:
Induction of growth factor RNA expression in human malignant
melanoma: markers of transformation. Cancer Res. 51:4815–4820.
1991.PubMed/NCBI
|
|
15
|
Bursch W, Oberhammer F and Schulte-Hermann
R: Cell death and its protective role in disease. Trends Pharmacol
Sci. 13:245–251. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmid P, Itin P and Rufli T: In situ
analysis of transforming growth factor-betas (TGF-beta 1, TGF-beta
2, TGF-beta 3), and TGF-beta type II receptor expression in
malignant melanoma. Carcinogenesis. 16:1499–1503. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Belle P, Rodeck U, Nuamah I, Halpern
AC and Elder DE: Melanoma-associated expression of transforming
growth factor-beta isoforms. Am J Pathol. 148:1887–1894.
1996.PubMed/NCBI
|
|
18
|
Rodeck U, Bossler A, Graeven U, et al:
Transforming growth factor b production and responsiveness in
normal human melanocytes and melanoma cells. Cancer Res.
54:575–581. 1994.PubMed/NCBI
|
|
19
|
Krasagakis K, Tholke D, Farthmann B,
Eberle J, Mansmann U and Orfanos CE: Elevated plasma levels of
transforming growth factor (TGF)-b1 and TGF-b2 in patients with
disseminated malignant melanoma. Br J Cancer. 77:1492–1494. 1998.
View Article : Google Scholar : PubMed/NCBI
|