Introduction
Sepsis is associated with a high mortality rate
(1,2). Acute lung injury (ALI) is
characterized by an intense inflammatory process in the lung. It is
the most common complication of severe sepsis as it is associated
with the high rates of morbidity and mortality (3–7).
Despite significant advances in understanding the pathogenesis and
management of ALI in sepsis, the mortality rate remains high.
Lipopolysaccharide (LPS), the major endotoxin of
gram-negative bacteria, may enter the bloodstream and elicit an
acute systemic inflammatory response, which leads to sepsis and
shock (8). Pro-inflammatory
cytokines are involved in the development of tissue damage,
metabolic acidosis, hypotension, multiple organ failure and even
fatality during sepsis (9,10). High mobility group box 1 (HMGB1) is
a cytokine mediator that has been observed to have an important
role in delayed endotoxin lethality and systemic inflammation in
murine sepsis models (11–14). HMGB1 is highly expressed in the
nucleus of endothelial cells, which are a crucial source of HMGB1
in inflammation (15,16). As a pro-inflammatory cytokine,
HMGB1 has been hypothesized to be an ideal target in sepsis therapy
(17–21). There is clear evidence that
anti-HMGB1 antibodies may protect against lethality, even if the
first dose of antibody is administered 24 h after infection
(22). A previous study has also
identified chemically synthesized antisense oligonucleotides
(23), which decrease HMGB1
expression and significantly inhibit inflammation in human
umbilical vein endothelial cells (HUVECs). Therefore, gene transfer
has been proposed as a novel method to produce cytokine inhibitors
or antagonists to treat inflammation. However, the gene-silencing
effects of HMGB1 via RNA interference (RNAi) in the development of
ALI in sepsis remain to be elucidated. The aim of the present study
was to determine whether the downregulation of HMGB1 reduced
endotoxin-induced inflammation in mice.
Materials and methods
Animals
A total of 20 male C57BL/6 mice (age, 8–10 weeks)
were obtained from the Experimental Animal Center of China Medical
University (Shenyang, China). The mice were housed in rooms
maintained at 20±2°C in a 12 h light/dark cycle for at least 1 week
to acclimate to their surroundings, during which mice had access to
water and standard mouse chow ad libitum. Mice were fasted
overnight prior to experiments, but had access to water ad
libitum. The animal study protocol was approved by the
Institutional Animal Care and Use Committee of China Medical
University.
HMGB1 small interfering RNA (siRNA)
siRNA sequences targeting the coding regions of
human HMGB1 were generated using the siRNA design center from
GenScript Co., Ltd. (Nanjing, China). The siRNA sequences were
inserted into the pRNA-U6.1/Neo vector attained from GenScript.
Recombinant siRNA plasmids were transfected into HUVECs and the
expression of HMGB1 was verified using western blot analysis
(23). In brief, in order to
construct pRNAU6.1/Neo-HMGB1, the human HMGB1 siRNA corresponding
to the coding region (5′-AAGGTTGAGA GCTATTGCTGA-3′) was synthesized
using GenScript’s siRNA design center (http://www.genscript.com/rnai.html) and inserted into
pRNAU6.1/Neo using BamH1 and HindIII restricted
enzymes. The recombinant pRNA U6.1/Neo-HMGB1 was then transfected
into DH5α cells. Additionally, the pRNA-U6.1/Neo vector was used as
a control.
Animal grouping and treatment
Mice were randomly assigned to one of four groups
(n=5): Control, LPS, LPS plus pRNA-U6.1/Neo-vector and LPS plus
pRNA-U6.1/Neo-HMGB1. The pRNA-U6.1/Neo dosage was considered to be
the optimum quantity based on a previous study (24). pRNA-U6.1/Neo-HMGB1 and
pRNA-U6.1/Neo-vector (3.6×108 plaque forming units/100 g
in 25 μl sterile phosphate-buffered saline solution; SD1201;
GenScript Co., Ltd) was delivered via intravenous injection through
the penile vein 48 h prior to LPS injection. A single dose of LPS
(30 mg/kg body weight in 100 μl saline; Escherichia
coli serotype 055:B5; Sigma-Aldrich, St. Louis, MO, USA) was
administered intraperitoneally.
Sample collection
Following LPS administration for 12 h, mice were
anesthetized via intraperitoneal injection with 0.1 ml/10 g 10%
chloral hydrate and then euthanized through cervical dislocation.
Serum and lung tissue samples were then collected. The superior
lobe of the right lung was excised for histopathological
examination. The middle lobe was excised for analysis of the lung
wet/dry (W/D) weight ratio. The lower lobe was frozen in liquid
nitrogen for a nuclear factor-κB (NF-κB) DNA-binding activity
assay.
Lung W/D weight ratio
The middle lobe of each lung was blotted dry with
filter paper for surface drainage and then weighed to obtain the
wet weight. The lobe was then dried in a drying oven at 65°C for 72
h; subsequently, the lung tissue was weighed to obtain the dry
weight. The W/D ratio was calculated to assess tissue edema.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of tumor necrosis factor-α (TNF-α) and
interleukin-1β (IL-1β) in mouse blood samples were measured with
commercially available ELISA kits (R&D Systems, Minneapolis,
MN, USA) according to the manufacturer’s instructions. The sample
levels were calculated by comparison to a standard curve and
expressed as pg/ml.
Lung histopathology
Lung tissues were fixed in 10% (w/v)
neutral-buffered formalin for 24 h, dehydrated in a graded ethanol
series and subsequently embedded in paraffin. Sequential 5
μm sections were stained with routine hematoxylin and eosin
(H&E) for morphological analysis. The slides were investigated
under a light microscope (BA400 Binocular Microscope; Motic,
Xiamen, China).
Measurements of myeloperoxidase (MPO)
activity and malondialdehyde (MDA) in lung tissue
Frozen lung tissues were thawed, homogenized in cold
saline at a ratio of 1:19 (weight/volume) and then centrifuged at
800 × g for 10 min at 4°C. The tissue supernatants were then
collected for biochemical analyses. MPO activity and MDA levels in
the lung tissue were determined using commercially available kits
(Jiancheng Bioengineering Institute, Nanjing, China). For the MPO
activity assays, the supernatants were incubated with hydrogen
peroxide in the presence of 0.167 mg/ml O-dianisidine
dihydrochloride for 30 min. The change in absorbance at 460 nm for
each sample was recorded with a plate reader (BioTek Instruments,
Inc., Winooski, VT, USA). The MPO activity was defined as the
quantity of enzyme degrading 1 μmol peroxide/min at 37°C and
is expressed as units per gram lung tissue. The MDA content was
determined based on the reaction of MDA with thiobarbituric acid
(Sigma-Aldrich) at 90–100°C. The MDA levels are expressed as
nmol/mg protein.
Electrophoretic mobility shift assay
(EMSA)
NF-κB DNA binding activity was measured with EMSA
using nuclear extracts from lung tissue. Nuclear proteins were
extracted using nuclear and cytoplasmic extraction reagent kits
(Pierce Biotechnology, Rockford, IL, USA). End labeling of
double-strand oligonucleotides containing the NF-κB consensus
sequence (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was performed using
γ-32P-dATP (Amersham Pharmacia Biotech, Piscataway, NJ,
USA) and T4-polynucleotide kinase (Takara Bio Inc., Ohtsu, Japan)
at 37°C for 60 min. Nuclear extracts (20 μg) were incubated in a 20
μl reaction mixture of 4% glycerol, 1 mM MgCl2, 05 mM
EDTA, 0.5 mM DTT, 50 mM NaCl, 10 mM Tris-HCl (pH 7.5) and 50 μg/ml
poly(deoxyinosinic-deoxycytidylic) acid (P4929; Sigma-Aldrich),
with or without unlabeled oligonucleotide for 10 min at room
temperature. 32P-labeled oligonucleotide probes were
then added and incubated at room temperature for 20 min.
Electrophoresis of samples was performed on a 5% polyacrylamide gel
and visualized directly by autoradiography following drying the
gel. Quantification was performed by image analysis using
densitometry (ChemiDoc system; Bio-Rad, Hercules, CA, USA).
Statistical analysis
The results are expressed as the mean ± standard
deviation of at least three separate experiments. A one-way
analysis of variance with Dunnett’s multiple comparison tests was
performed using SPSS software, version 10.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Downregulation of HMGB1 attenuates
LPS-induced histopathological changes in lung tissue
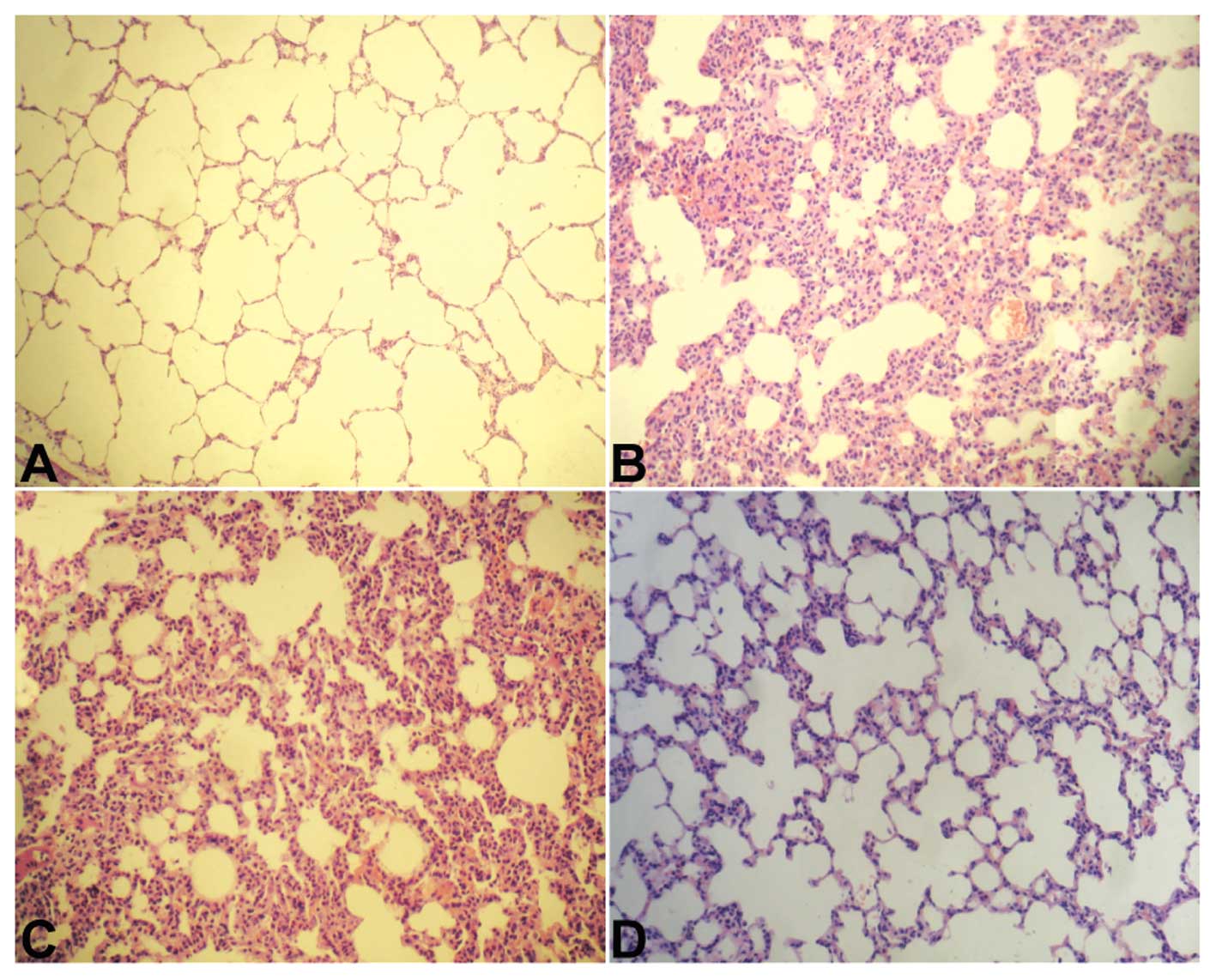
To evaluate the histopathological characteristics of
lung tissue in LPS-challenged mice, lung tissue sections were
stained with H&E. As shown in Fig.
1A, lung tissue from the sham group exhibited a normal
structure and no histopathological changes. In the LPS-treated mice
with or without pRNA-U6.1/Neo-vector injection, the lungs had
widespread increased alveolar wall thickness caused by edema,
severe hemorrhage in the alveolus, alveolus collapse and marked
inflammatory cell infiltration (Fig.
1B and C). However, in the HMGB1 siRNA and LPS-treated groups,
histopathological changes in the lung were attenuated when compared
with those in the LPS plus pRNAU6.1/Neo-vector group, particularly
the inflammatory cell infiltration findings (Fig. 1D). These results suggest that
downregulation of HMGB1 may protect mice against LPS-induced lung
injury.
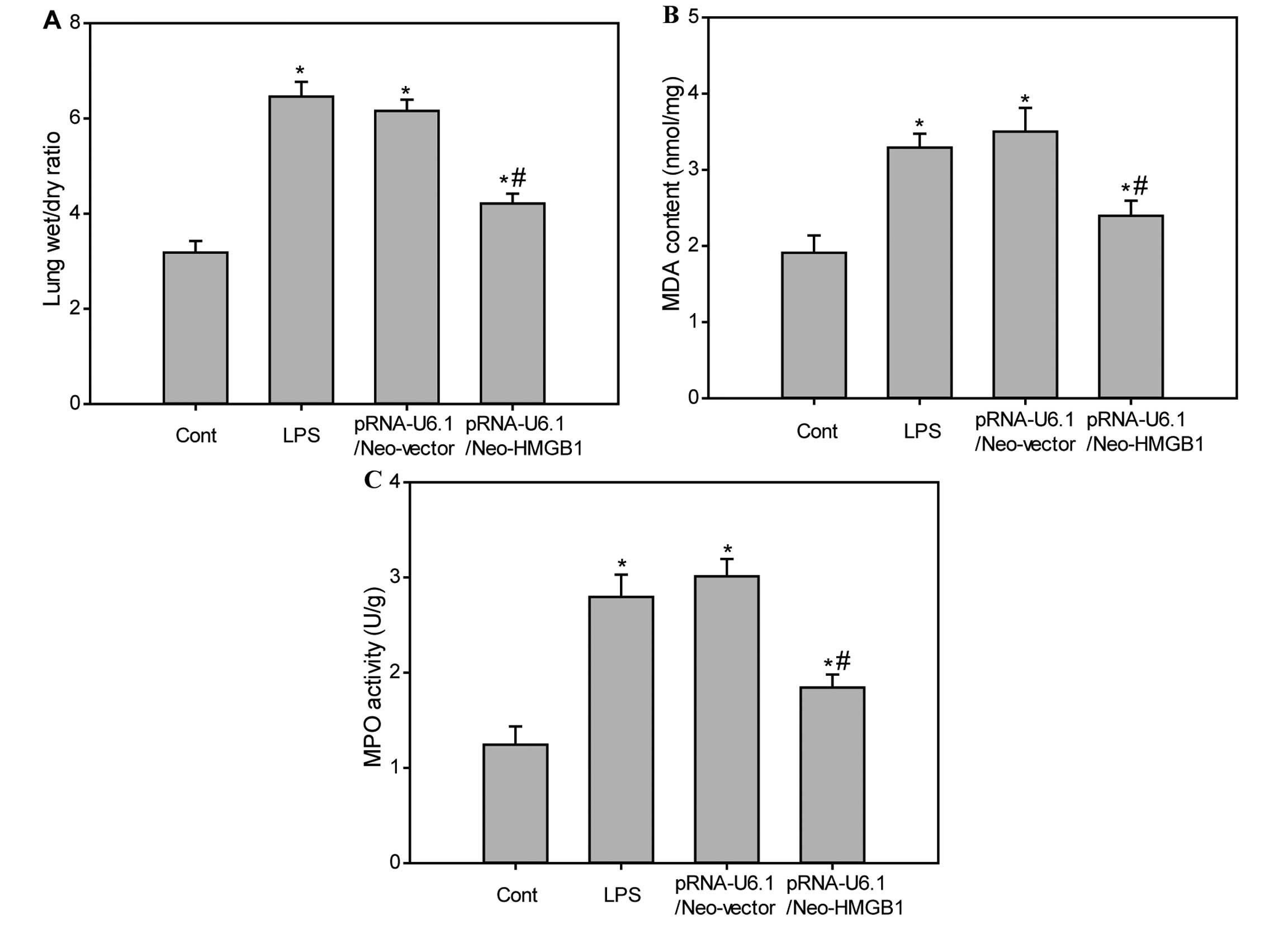
Downregulation of HMGB1 reduces lung W/D
ratio, MDA level and MPO activity in lung tissue of LPS-treated
mice
The W/D ratio was used to evaluate pulmonary
vascular permeability. When compared with the sham group, the lung
W/D ratios were significantly increased in the LPS group and LPS
plus pRNA-U6.1/Neo-vector-treated group. No difference was
identified between the LPS and LPS plus
pRNA-U6.1/Neo-vector-treated groups. However, the increased lung
W/D ratios in the LPS group were significantly reduced by treatment
with HMGB1 siRNA (Fig. 2A;
P<0.05), suggesting that downregulation of HMGB1 may prevent
LPS-induced increases in lung vascular permeability. To assess
neutrophil accumulation within pulmonary tissue, MPO activity was
measured. Following LPS administration, MPO activity in lung tissue
was significantly increased when compared with the control group.
However, the increased MPO activity was markedly reduced following
downregulation of HMGB1 (Fig. 2B;
P<0.05). In addition, LPS administration significantly raised
MDA levels in lung tissues and the increased MDA levels were
suppressed by downregulating HMGB1 (Fig. 2C).
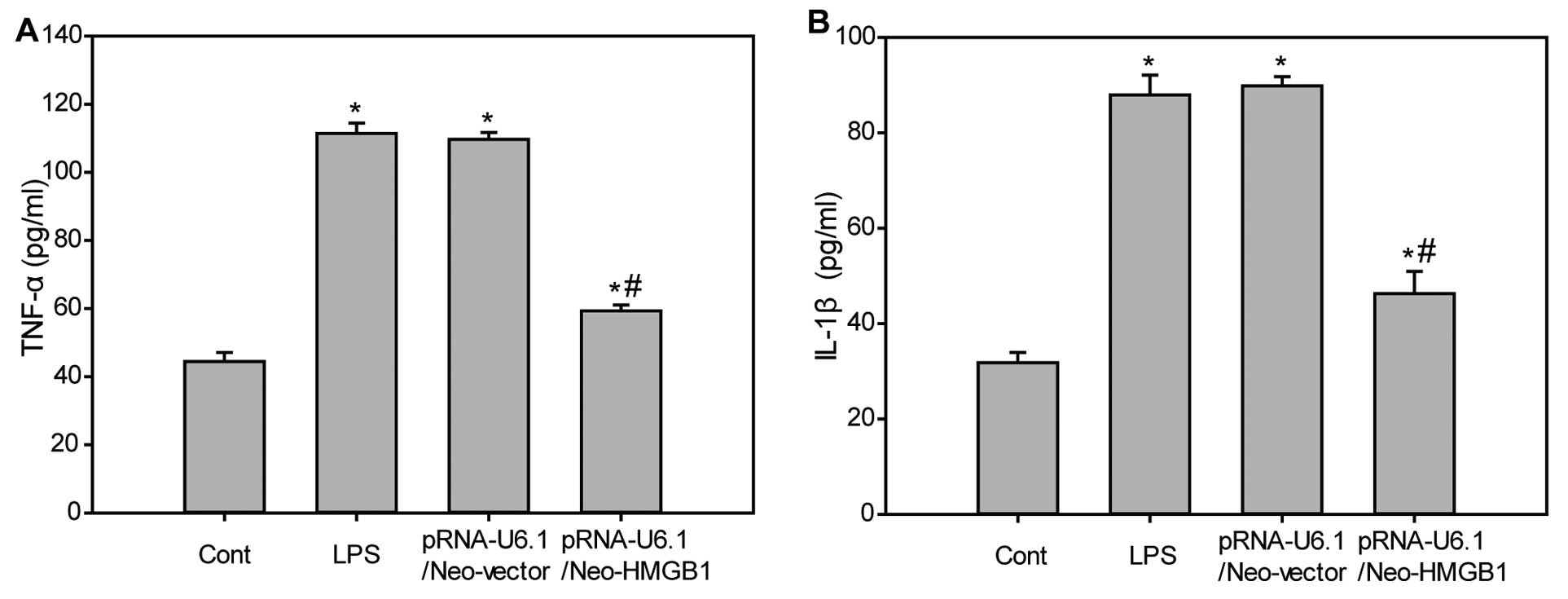
Downregulation of HMGB1 inhibits
production of inflammatory cytokines in LPS-challenged mice
Inflammatory cytokines, such as TNF-α and IL-1β, are
known to be involved in the pathophysiology of LPS-mediated
pulmonary inflammation. Therefore, the serum levels of TNF-α and
IL-1β were determined in LPS-challenged mice. As shown in Fig. 3, levels of serum TNF-α (Fig. 3A) and IL-1β (Fig. 3B) were minimally expressed in the
control group. However, LPS administration markedly increased the
expression of serum TNF-α and IL-1β levels, which were
significantly attenuated following downregulation of HMGB1. These
findings indicated that downregulation of HMGB inhibits LPS-induced
production of inflammatory cytokines.
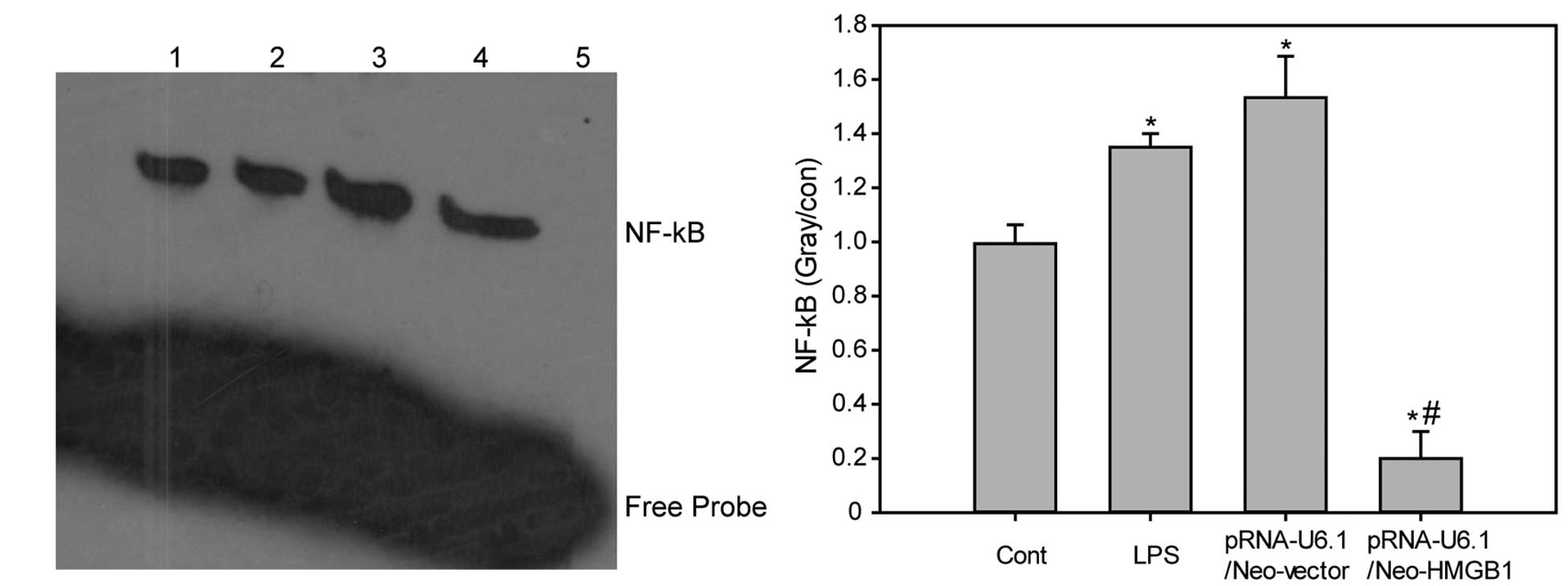
Downregulation of HMGB1 inhibits
LPS-induced activation of nf-κb
The nf-κb DNA binding activity in the
lung tissue samples was determined. The nf-κb DNA binding activity in the
lung tissue of LPS-treated mice with or without negative control
siRNA injection was increased when compared with that of the
control group. By contrast, HMGB1 knockdown decreased LPS-induced
increases in NF-kB DNA binding activity when compared with the LPS
plus pRNAU6.1/Neo-vector group (Fig.
4; P<0.05).
Discussion
In the present study, the effects of HMGB1
downregulation were investigated in LPS-induced ALI in mice. It was
observed that HMGB1 siRNA-treated animals had a decreased lung W/D
ratio compared with the negative control siRNA-treated mice. It is
well-established that the overproduction of pro-inflammatory
cytokines promotes the development of sepsis (25). Among these pro-inflammatory
cytokines, TNF-α and IL-1β, which are released within minutes after
endotoxin exposure, are the most important early response
cytokines. Previous studies have indicated that the persistent
elevation of pro-inflammatory cytokines is associated with a worse
outcome in patients with sepsis (26). In the present study, it was
demonstrated that the serum concentrations of TNF-α and IL-1β
increased significantly following LPS administration. However,
these changes were markedly inhibited by HMGB1 siRNA treatment. In
addition, using pRNA-U6.1/Neo-HMGB1 treatment significantly
improved pulmonary histopathological findings and attenuated the
severity of lung vascular permeability and edema.
NF-κB is a universal transcription factor that has a
crucial role in regulating the transcription of over 200 genes, a
number of which have important roles in the development of septic
shock (27), including TNF-α and
IL-1 (28). In the present study,
it was identified that the downregulation of HMGB1 may inhibit the
activation of NF-κB and consequently downregulate downstream
inflammatory cytokines, relieve endothelial permeability and
attenuate LPS-associated ALI in septic mice.
In conclusion, it was demonstrated that the
downregulation of HMGB1 may inhibit LPS-induced inflammation and
ALI, suggesting that it may provide a novel therapeutic strategy
for sepsis.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301619) and
Shenyang Science and Technology plan projects (grant no.
F13–220–9–11).
References
|
1
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fry DE: Sepsis, systemic inflammatory
response, and multiple organ dysfunction: the mystery continues. Am
Surg. 78:1–8. 2012.PubMed/NCBI
|
|
3
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011. View Article : Google Scholar :
|
|
5
|
Matthay MA and Zimmerman GA: Acute lung
injury and the acute respiratory distress syndrome: four decades of
inquiry into pathogenesis and rational management. Am J Respir Cell
Mol Biol. 33:319–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Erickson SE, Martin GS, Davis JL, Matthay
MA and Eisner MD; NIH NHLBI ARDS Network: Recent trends in acute
lung injury mortality: 1996–2005. Crit Care Med. 37:1574–1579.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zimmerman JJ, Akhtar SR, Caldwell E and
Rubenfeld GD: Incidence and outcomes of pediatric acute lung
injury. Pediatrics. 124:87–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hudson LD, Milberg JA, Anardi D and
Maunder RJ: Clinical risks for development of the acute respiratory
distress syndrome. Am J Respir Crit Care Med. 151:293–301. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gando S: Microvascular thrombosis and
multiple organ dysfunction Syndrome. Crit Care Med. 38(Suppl 2):
S35–S42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levi M and van der Poll T: Inflammation
and coagulation. Crit Care Med. 38(Suppl 2): S26–S34. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersson U, Wang H, Palmblad K, et al:
High mobility group 1 protein (HMG-1) stimulates proinflammatory
cytokine synthesis in human monocytes. J Exp Med. 192:565–570.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Bloom O, Zhang M, et al: HMG-1 as
a late mediator of endotoxin lethality in mice. Science.
285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Yang H and Tracey KJ:
Extracellular role of HMGB1 in inflammation and sepsis. J Intern
Med. 255:320–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fiuza C, Bustin M, Talwar S, et al:
Inflammation-promoting activity of HMGB1 on human microvascular
endothelial cells. Blood. 101:2652–2660. 2003. View Article : Google Scholar
|
|
15
|
Mullins GE, Sunden-Cullberg J, Johansson
AS, et al: Activation of human umbilical vein endothelial cells
leads to relocation and release of high-mobility group box
chromosomal protein 1. Scand J Immunol. 60:566–573. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae JS and Rezaie AR: Activated protein C
inhibits high mobility group box 1 signaling in endothelial cells.
Blood. 118:3952–3959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasuda T, Ueda T, Takeyama Y, et al:
Significant increase of serum high-mobility group box chromosomal
protein 1 levels in patients with severe acute pancreatitis.
Pancreas. 33:359–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gibot S, Massin F, Cravoisy A, et al:
High-mobility group box 1 protein plasma concentrations during
septic shock. Intensive Care Med. 33:1347–1353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaïni S, Pedersen SS, Koldkjaer OG,
Pedersen C and Møller HJ: High mobility group box-1 protein in
patients with suspected community-acquired infections and sepsis: a
prospective study. Crit Care. 11:R322007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Zoelen MA, Laterre PF, van Veen SQ, et
al: Systemic and local high mobility group box 1 concentrations
during severe infection. Crit Care Med. 35:2799–2804. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karlsson S, Pettilä V, Tenhunen J,
Laru-Sompa R, Hynninen M and Ruokonen E: HMGB1 as a predictor of
organ dysfunction and outcome in patients with severe sepsis.
Intensive Care Med. 34:1046–1053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silva E, Arcaroli J, He Q, et al: HMGB1
and LPS induce distinct patterns of gene expression and activation
in neutrophils from patients with sepsis-induced acute lung injury.
Intensive Care Med. 33:1829–1839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XJ, Luan ZG and Ma XC: shRNAs
targeting high-mobility group box-1 inhibit E-selectin expression
via homeobox A9 in human umbilical vein endothelial cells. Mol Med
Rep. 7:1251–1256. 2013.PubMed/NCBI
|
|
24
|
Luan ZG, Zhang XJ, Yin XH, et al:
Downregulation of HMGB1 protects against the development of acute
lung injury after severe acute pancreatitis. Immunobiology.
218:1261–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cannon JG, Tompkins RG, Gelfand JA, et al:
Circulating interleukin-1 and tumor necrosis factor in septic shock
and experimental endotoxin fever. J Infect Dis. 161:79–84. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Minamino T and Komuro I: Regeneration of
the endothelium as a novel therapeutic strategy for acute lung
injury. J Clin Invest. 116:2316–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rakonczay Z Jr, Hegyi P, Takács T,
McCarroll J and Saluja AK: The role of NF-kappaB activation in the
pathogenesis of acute pancreatitis. Gut. 57:259–267. 2008.
View Article : Google Scholar
|