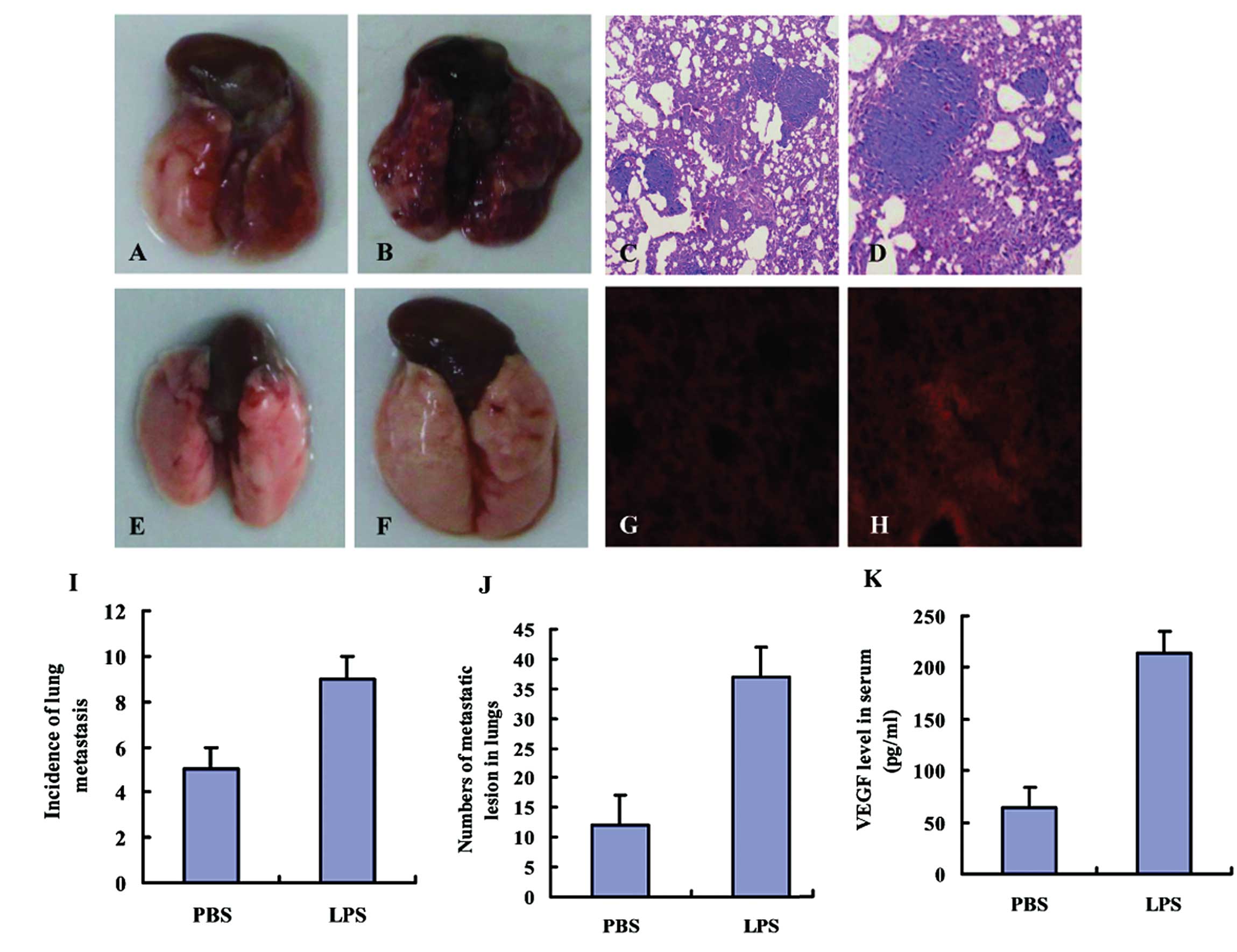
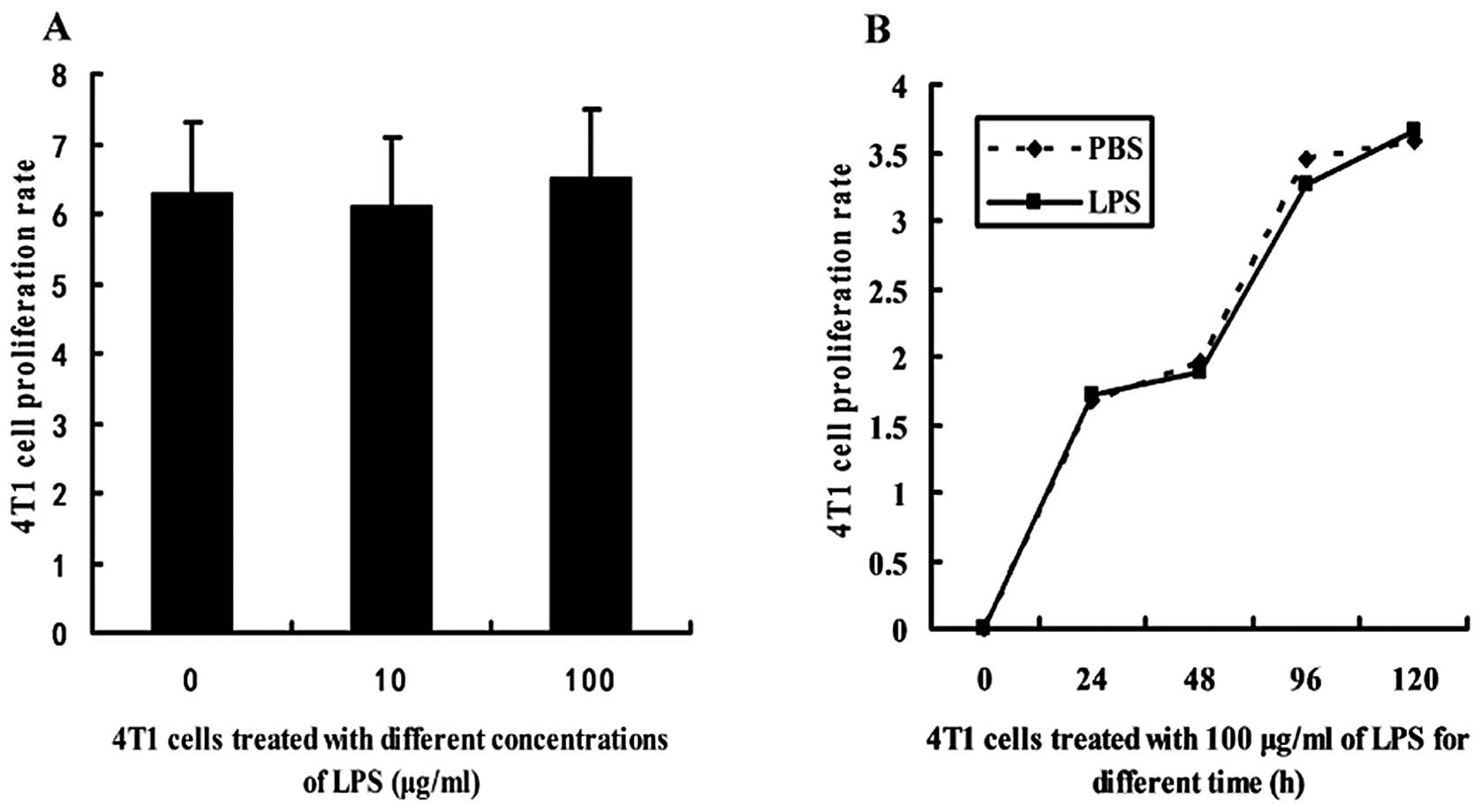
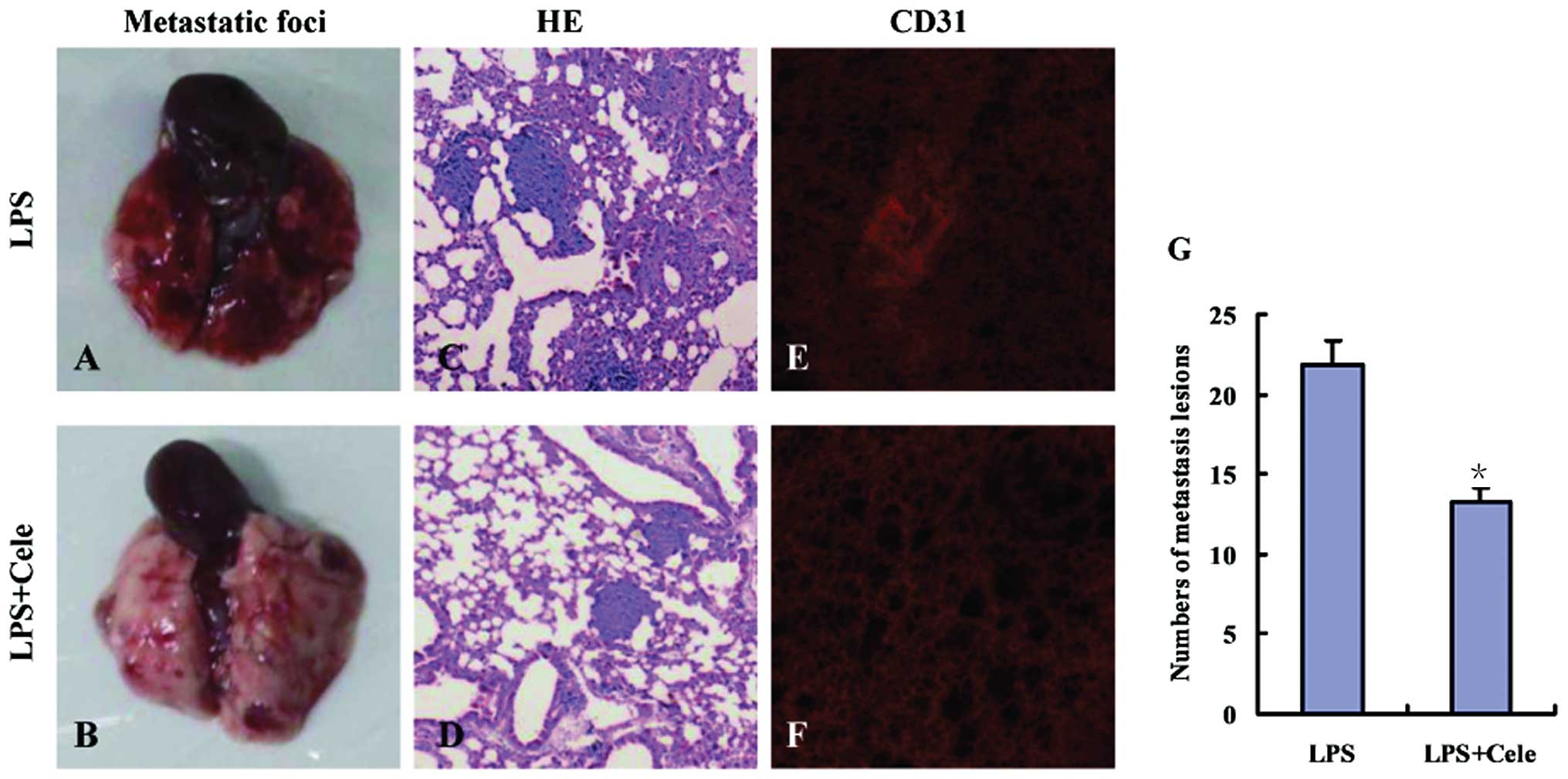
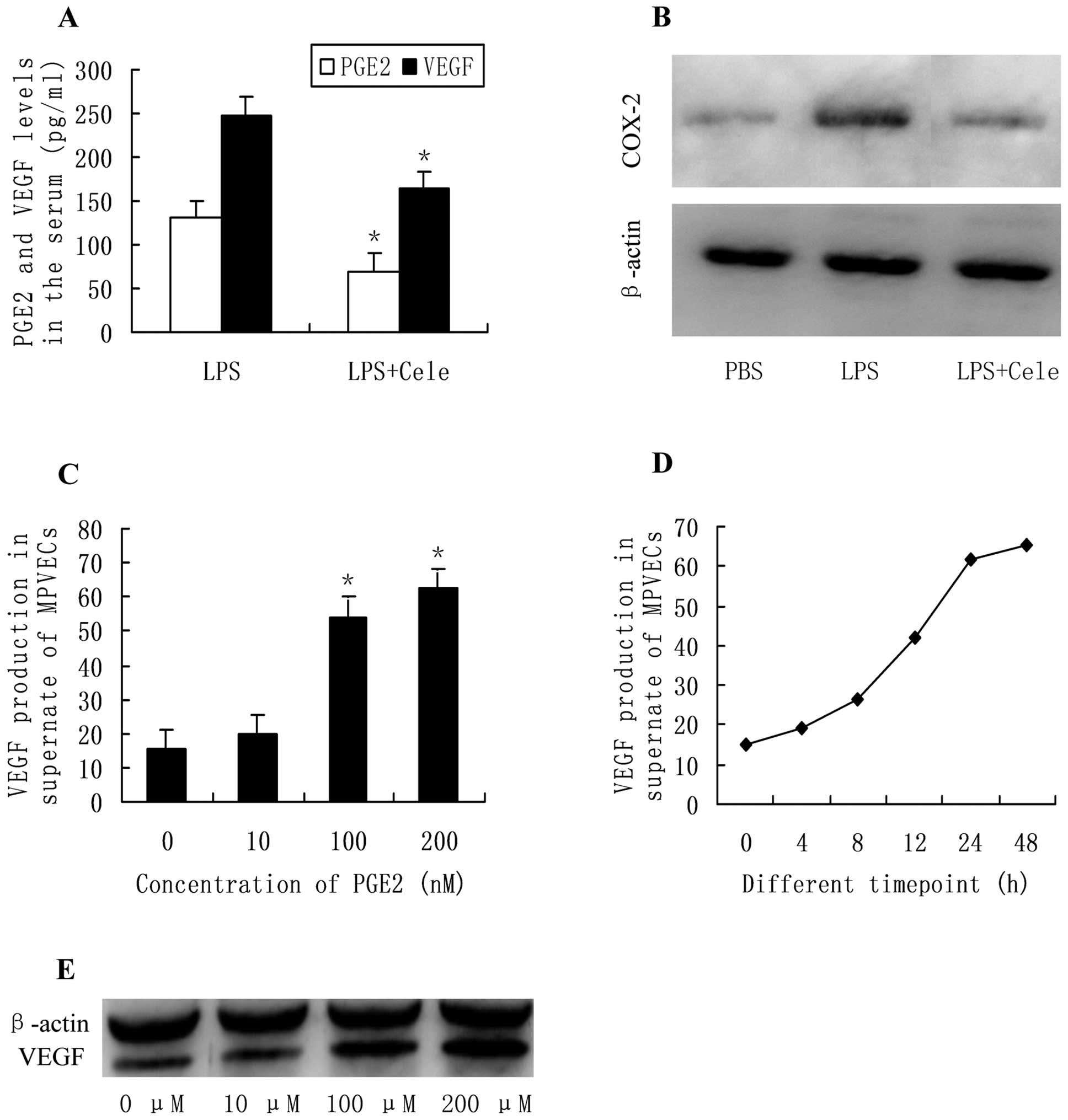
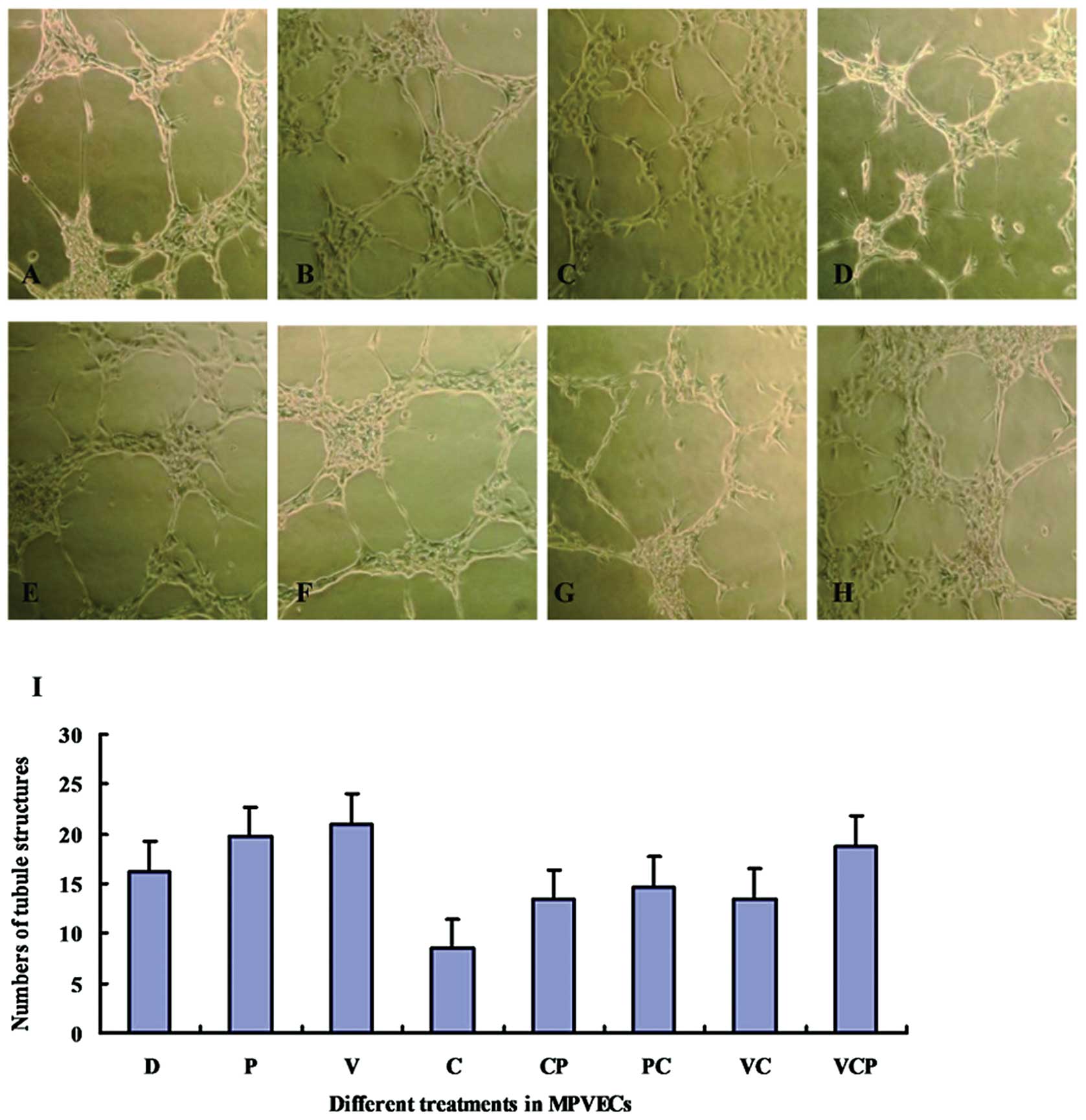
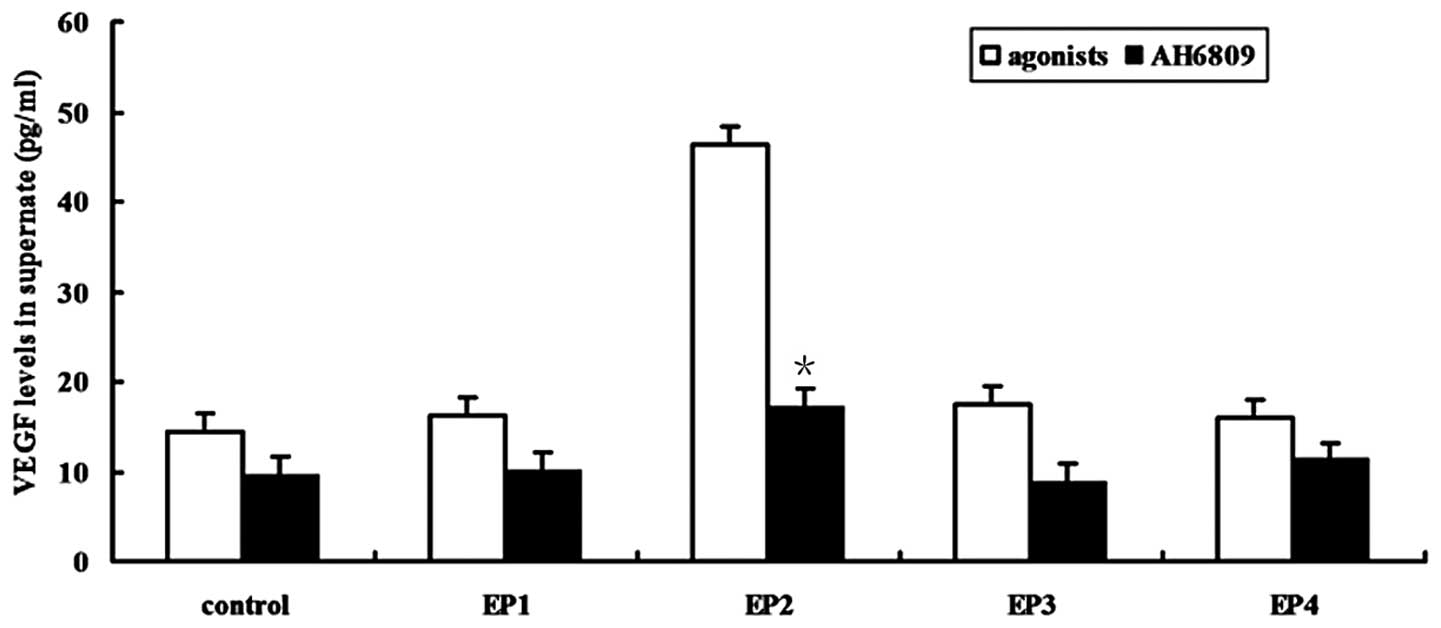
|
1
|
Liu H, Kato Y, Erzinger SA, Kiriakova GM,
Qian Y, Palmieri D, Steeg PS and Price JE: The role of MMP-1 in
breast cancer growth and metastasis to the brain in a xenograft
model. BMC Cancer. 12:5832012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YT: Breast carcinoma: pattern of
metastasis at autopsy. J Surg Oncol. 23:175–180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harmey JH, Bucana CD, Lu W, Byrne AM,
McDonnell S, Lynch C, Bouchier-Hayes D and Dong Z:
Lipopolysaccharide-induced metastatic growth is associated with
increased angiogenesis, vascular permeability and tumor cell
invasion. Int J Cancer. 101:415–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ikebe M, Kitaura Y, Nakamura M, Tanaka H,
Yamasaki A, Nagai S, Wada J, Yanai K, Koga K, Sato N, Kubo M,
Tanaka M, Onishi H and Katano M: Lipopolysaccharide (LPS) increases
the invasive ability of pancreatic cancer cells through the
TLR4/MyD88 signaling pathway. J Surg Oncol. 100:725–731. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He W, Liu Q, Wang L, Chen W, Li N and Cao
X: TLR4 signaling promotes immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance.
Mol Immunol. 44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gassmann P, Hemping-Bovenkerk A, Mees ST
and Haier J: Metastatic tumor cell arrest in the liver-lumen
occlusion and specific adhesion are not exclusive. Int J Colorectal
Dis. 24:851–858. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Z, Zhu Y and Jiang H: Inhibiting
toll-like receptor 4 signaling ameliorates pulmonary fibrosis
during acute lung injury induced by lipopolysaccharide: an
experimental study. Respir Res. 10:1262009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang EL, Qian ZR, Nakasono M, Tanahashi T,
Yoshimoto K, Bando Y, Kudo E, Shimada M and Santo T: High
expression of Toll-like receptor 4/myeloid differentiation factor
88 signals correlates with poor prognosis in colorectal cancer. Br
J Cancer. 102:908–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan L, Cai Q and Xu Y: The ubiquitin-CXCR4
axis plays an important role in acute lung infection-enhanced lung
tumor metastasis. Clin Cancer Res. 19:4706–4716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Killeen SD, Wang JH, Andrews EJ and
Redmond HP: Bacterial endotoxin enhances colorectal cancer cell
adhesion and invasion through TLR-4 and NF-kappaB-dependent
activation of the urokinase plasminogen activator system. Br J
Cancer. 100:1589–1602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Liang J and Li G:
Lipopolysaccharide promotes adhesion and invasion of hepatoma cell
lines HepG2 and HepG2.2.15. Mol Biol Rep. 37:2235–2239. 2010.
View Article : Google Scholar
|
|
13
|
Brown DM and Ruoslahti E: Metadherin, a
cell surface protein in breast tumors that mediates lung
metastasis. Cancer Cell. 5:365–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W
and Yang Q: Metadherin mediates lipopolysaccharide-induced
migration and invasion of breast cancer cells. PLoS One.
6:e293632011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sethi G, Shanmugam MK, Ramachandran L,
Kumar AP and Tergaonkar V: Multifaceted link between cancer and
inflammation. Biosci Rep. 32:1–15. 2012. View Article : Google Scholar
|
|
16
|
Senger DR, Galli SJ, Dvorak AM, Perruzzi
CA, Harvey VS and Dvorak HF: Tumor cells secrete a vascular
permeability factor that promotes accumulation of ascites fluid.
Science. 219:983–985. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doan HQ, Bowen KA, Jackson LA and Evers
BM: Toll-like receptor 4 activation increases Akt phosphorylation
in colon cancer cells. Anticancer Res. 29:2473–2478.
2009.PubMed/NCBI
|
|
18
|
Wang L, Liu Q, Sun Q, Zhang C, Chen T and
Cao X: TLR4 signaling in cancer cells promotes chemoattraction of
immature dendritic cells via autocrine CCL20. Biochem Biophys Res
Commun. 366:852–856. 2008. View Article : Google Scholar
|
|
19
|
Ferrara N and Henzel WJ: Pituitary
follicular cells secrete a novel heparin-binding growth factor
specific for vascular endothelial cells. Biochem Biophys Res
Commun. 161:851–858. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuji T, Aoshiba K, Yokohori N and Nagai
A: A systemically administered EP2 receptor agonist stimulates
pulmonary angiogenesis in a murine model of emphysema.
Prostaglandins Other Lipid Mediat. 90:85–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato Y, Kanno S, Oda N, Abe M, Ito M,
Shitara K and Shibuya M: Properties of two VEGF receptors, Flt-1
and KDR, in signal transduction. Ann NY Acad Sci. 902:201–207.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perrot-Applanat M and Di Benedetto M:
Autocrine functions of VEGF in breast tumor cells: adhesion,
survival, migration and invasion. Cell Adh Migr. 6:547–553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koch AE: Angiogenesis as a target in
rheumatoid arthritis. Ann Rheum Dis. 62:ii60–ii67. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baeriswyl V and Christofori G: The
angiogenic switch in carcinogenesis. Semin Cancer Biol. 19:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69(Suppl 3): 4–10. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakanishi M, Sato T, Li Y, Nelson AJ,
Farid M, Michalski J, Kanaji N, Wang X, Basma H, Patil A, Goraya J,
Liu X, Togo S, L Toews M, Holz O, Muller KC, Magnussen H and
Rennard SI: Prostaglandin E2 stimulates the production of vascular
endothelial growth factor through the E-prostanoid-2 receptor in
cultured human lung fibroblasts. Am J Respir Cell Mol Biol.
46:217–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang S, Wettlaufer SH, Hogaboam C,
Aronoff DM and Peters-Golden M: Prostaglandin E2
inhibits collagen expression and proliferation in patient-derived
normal lung fibroblasts via E prostanoid 2 receptor and cAMP
signaling. Am J Physiol Lung Cell Mol Physiol. 292:L405–L413. 2007.
View Article : Google Scholar
|
|
29
|
Sakurai T, Tamura K, Okamoto S, Hara T and
Kogo H: Possible role of cyclooxygenase II in the acquisition of
ovarian luteal function in rodents. Biol Reprod. 69:835–842. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakurai T, Tamura K and Kogo H:
Stimulatory effects of eicosanoids on ovarian angiogenesis in early
luteal phase in cyclooxygenase-2 inhibitor-treated rats. Eur J
Pharmacol. 516:158–164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bos CL, Richel DJ, Ritsema T,
Peppelenbosch MP and Versteeg HH: Prostanoids and prostanoid
receptors in signal transduction. Int J Biochem Cell Biol.
36:1187–1205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakurai T, Tamura K and Kogo H: Vascular
endothelial growth factor increases messenger RNAs encoding
cyclooxygenase-II and membrane-associated prostaglandin E synthase
in rat luteal cells. J Endocrinol. 183:527–533. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Folkman J and D’Amore PA: Blood vessel
formation: what is its molecular basis? Cell. 87:1153–1155. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Keith RL, Geraci MW, Nana-Sinkam SP,
Breyer RM, Hudish TM, Meyer AM, Malkinson AM and Dwyer-Nield LD:
Prostaglandin E2 receptor subtype 2 (EP2) null mice are protected
against murine lung tumorigenesis. Anticancer Res. 26:2857–2861.
2006.PubMed/NCBI
|
|
35
|
Chang SH, Liu CH, Conway R, Han DK,
Nithipatikom K, Trifan OC, Lane TF and Hla T: Role of prostaglandin
E2-dependent angiogenic switch in cyclooxygenase 2-induced breast
cancer progression. Proc Natl Acad Sci USA. 101:591–596. 2004.
View Article : Google Scholar :
|
|
36
|
Gately S and Li WW: Multiple roles of
COX-2 in tumor angiogenesis: a target for antiangiogenic therapy.
Semin Oncol. 31:2–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma X, Kundu N, Ioffe OB, et al:
Prostaglandin E receptor EP1 suppresses breast cancer metastasis
and is linked to survival differences and cancer disparities. Mol
Cancer Res. 8:1310–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Narumiya S, Sugimoto Y and Ushikubi F:
Prostanoid receptors: structures, properties and functions. Physiol
Rev. 79:1193–1226. 1999.PubMed/NCBI
|
|
39
|
Yang T and Du Y: Distinct roles of central
and peripheral prostaglandin E2 and EP subtypes in blood pressure
regulation. Am J Hypertens. 25:1042–1049. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugimoto Y and Narumiya S: Prostaglandin E
receptors. J Biol Chem. 282:11613–11617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Breyer RM, Bagdassarian CK, Myers SA and
Breyer MD: Prostanoid receptors: subtypes and signaling. Annu Rev
Pharmacol Toxicol. 41:661–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chell S, Kaidi A, Williams AC and
Paraskeva C: Mediators of PGE2 synthesis and signalling downstream
of COX-2 represent potential targets for the prevention/treatment
of colorectal cancer. Biochim Biophys Acta. 1766:104–119.
2006.PubMed/NCBI
|