Introduction
Hypertrophic scarring (HS) results from fibroblast
proliferation and activation and involves the deposition of a
substantial quantity of abnormally secreted extracellular matrix
(ECM) proteins and the secretion of several types of cytokines and
chemicals. HS is regarded as a multifactorial fibrotic disorder;
however, the specific mechanisms underlying fibrogenesis remain to
be elucidated (1). Enhanced and
prolonged inflammation is a common pathogenic feature of HS, which
has been described in previous studies (2–4).
Numerous therapeutic approaches, including surgical excision,
hormone therapy, cytotoxic drugs, superficial compression therapy
and radiation therapy, have been used in the clinical treatment of
HS (5). However, none of these
therapeutic approaches provide effective results.
Topical inflammation contributes markedly to the
formation of HS. During the early proliferation stage, the
infiltration and retention of inflammatory cells can be observed by
histological examination (6). The
primary inflammatory cells involved are monocytes, lymphocytes and
mast cells (7). In addition,
several types of inflammatory cytokine, including monocyte
chemoattractant protein (MCP)-1, tumor necrosis factor (TNF)-α and
interleukin (IL)-6, are secreted by these inflammatory cell and
mediate the interaction of inflammation and fibroblast activation.
These processes, combined with mechanical forces, are important
elements, which lead to the formation of HS (8–11).
Therefore, since the inflammatory response has an important role in
the development of HS, treatment that is able to terminate the HS
inflammatory response offers a potentially promising therapeutic
approach in the treatment of HS.
Emodin is a major component of the widely used
Chinese herb, rhubarb. It has been used to treat several diseases,
including inflammation and cancer. The potential therapeutic
effects of emodin have been investigated in pancreatitis, asthma,
arthritis, atherosclerosis, myocarditis, glomerulonephritis and
Alzheimer’s disease (12,13). The signal transduction pathways
affected include nuclear factor-κB and phosphoinositide 3-kinase
(PI3K)/AKT (13). Previous studies
have demonstrated that emodin can markedly suppress in vitro
fibrotic activities of rat kidney fibroblasts and hepatic stellate
cells (14,15). However, few studies have examined
the effectiveness of emodin in the treatment of HS.
Based on previous findings, the present study
hypothesized that emodin may have a positive effect on HS by
attenuating the HS inflammatory response. Therefore, the aim of the
present study was to investigate whether emodin can be used as an
effective drug for the treatment of HS.
Materials and methods
HS models and materials
Female wild-type C57BL/6 mice (eight-weeks-old) were
purchased from the Shanghai Laboratory Animal Center (Shanghai,
China). All the mice were maintained under standard conditions at
23–26°C, 12-h light/dark cycle, 150 lux with access to standard
food and clean water ad libitum, according to the guidelines
approved by the Shanghai Jiao Tong University Animal Care and Use
Committee (Shanghai, China). The study was approved by the medical
ethics committee office of Jiangxi Provinicial People’s Hospital,
Nanchang, China.
Hypertrophic scar models were created, as described
previously (16). Briefly, two 2
cm linear full-thickness incisions (1.25 cm apart) were made on the
dorsal midline of each mouse and sutured using 4-0 silk threads
(Shanghai Gold Medical Supplies Co., Ltd, Shanghai, China).
Subsequently, 4 days post-incision, the sutures were removed from
the scars and two mechanical stress devices, produced by our lab
based on a previously published method (16), were sutured overlying the
incisions. Equal stretching forces were applied between days 4 and
14 post-incision in one of the two scars of each mouse and the
other scar remained untreated. A total of 20 mice were divided into
two groups, one group was intraperitoneally injected with emodin
(10 mg/kg; Sigma-Aldrich, St Louis, MO, USA) and the other group
was intraperitoneally injected with an equal quantity of Dulbecco’s
modified Eagle’s medium (DMEM; cat. no. 11965; Gibco-BRL, Carlsbad,
CA, USA) once each day between days 4 and 14 post-incision. Half of
the mice from each group were sacrificed at day 14 and the other
half were sacrificed at day 28 post-incision. Mice were sacrificed
by cervical dislocation. Scar specimens and normal specimens were
then harvested for further analyses.
Cell culture
Hypertrophic scarring fibroblasts (HSFs) and normal
fibroblasts (NFs) were obtained from the above-described specimens
using trypsin (Trypsin-EDTA, 0.05%; Gibco-BRL), and cultured in
DMEM supplemented with 10% fetal bovine serum (Gibco-BRL), 100 U/ml
penicillin and 100 mg/ml streptomycin (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). THP-1 human acute
monocytic leukemia cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and maintained in RPMI 1640
medium (Gibco-BRL) supplemented with 10% fetal bovine serum, 100
U/ml penicillin, 100 mg/ml streptomycin and 0.5 mM
β-mercaptoethanol (Gibco-BRL). The HSF and NF cell lines were
incubated at 37°C in a humidified atmosphere containing 5%
CO2. Primary fibroblasts between passages six and eight
were used.
Total protein assay
Following treatment with 20, 40, 80 or 120
μg/ml emodin for 24 h, identical numbers of HSFs
(4×105 cells) were harvested. The microplate
bicinchoninic acid (BCA) method was used to determine the quantity
of total protein using a BCA Protein Assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA) according to the
manufacturer’s instructions.
Histopathological examination
The specimens from the HS murine models were
incised, fixed in 10% neutral-buffered formaldehyde, embedded in
paraffin and stained with either hematoxylin and eosin or
picrosirius red (Fluka Analytical, Buchs, Switzerland). The scar
elevation index (SEI), denoting the ratio of scar thickness to the
thickness of the adjacent normal skin (17), the collagen structure and the level
of inflammation of the HS tissue, was assessed via examination of
the stained sections and scores between 0 and 3 were assigned for
SEI: 0=SEI 0–1, 1=SEI 1–2, 2=SEI 2–3 or 3=SEI≥3; collagen
structure: 0=well organized with no whorl; 1=disorganized with no
whorl; 2=disorganized with one whorl/high power field (HP); or
3=disorganized with ≥two whorls/HP and inflammation: 0=no
monocytes; 1=monocyte or mast cell 2–5/HP; 2=monocyte or mast cell
5–10/HP or 3=monocyte or mast cell >10/HP. The scores for each
factor were then added together to calculate the histopathological
scores of the specimens.
Adhesion assay
Adhesion assays were performed, as described
previously (17,18). The HSFs obtained from the DMEM
treated mice were seeded at 3×105 cells/well in 24-well
plates and grown to 80% confluence. The THP-1 cells were
fluorescently labeled using 2.5 mM calcein AM (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). Subsequently, a 500 μl
THP-1 cell suspension at a density of 1×105 cells/ml in
20, 40, 80 or 120 μg/ml emodin, was added to each well. The
plates were centrifuged at 134 x g for 3 min and then incubated at
37°C for 24 h. The non-adherent THP-1 cells were removed by gently
washing the wells three times with RPMI medium. The adherent THP-1
cells were microscopically quantified (magnification, ×100) in four
randomly selected visual fields of each well. Images were captured
using an Axiovert 200 inverted fluorescence microscope (Carl Zeiss,
Jena, Germany).
Western blotting
Western blotting was performed, as previously
described (19). Briefly, the HSFs
and NFs (3×105 cells/well) were treated with either
emodin (40 μg/ml) or DMEM and incubated at 37°C in a
humidified atmosphere containing 5% CO2 for 24 h. The
cells were lysed using radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) supplemented
with 1 mM phenylmethylsulfonyl fluoride (Adamas, Shanghai, China).
The protein samples were subsequently separated by 12% sodium
dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and
transferred to Hybond enhanced chemiluminescence (ECL) membranes
(GE Healthcare Life Sciences, Chalfont, UK). The membranes were
blocked in 6% nonfat milk dissolved in TBST buffer containing 10 mM
Tris-HCl, (pH 8.0), 150 mM NaCl and 0.05% Tween 20 and incubated
with primary antibodies, including rabbit anti-mouse polyclonal
TNF-α antibody (1:1,000; #3707), rabbit anti-mouse monoclonal IL-6
antibody (1:1,000; #12912), rabbit anti-mouse polyclonal MCP-1
antibody (1:1,000; #2029), rabbit anti-mouse polyclonal PI3K
antibody (1:1,000; #4257), rabbit anti-mouse polyclonal p-PI3K
antibody (1:1,000; #4228), rabbit anti-mouse polyclonal Akt
antibody (1:1,000; #9272) and rabbit anti-mouse polyclonal p-Akt
antibody (1:1,000; #4060) (Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight. The membranes were then
incubated with goat anti-rabbit immunoglobulin G secondary antibody
coupled with horseradish peroxidase at room temperature for 2 h.
The blots were developed using an ECL system (GE Healthcare Life
Sciences). For re-probing, the blots were incubated in stripping
buffer containing 100 mM 2-mercaptoethanol, 2% SDS and 62.5 mM
Tris-HCl (pH 6.7) at 50°C for 30 min.
Statistical analysis
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was
used for all statistical analyses. Significant differences were
calculated from the means of triplicate samples using a factorial
design analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
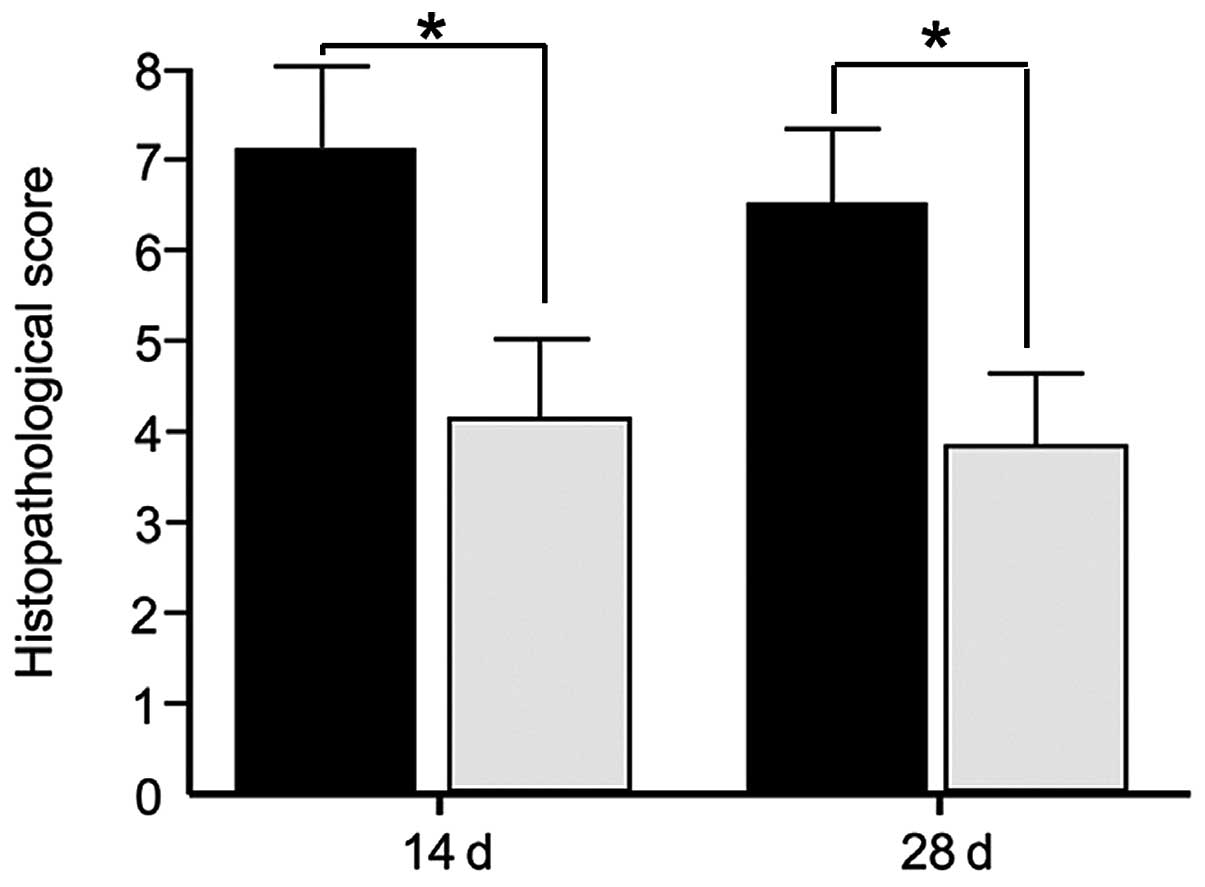
Histopathological assessment
HS is marked by the accumulation of a substantial
quantity of abnormally secreted ECM. HS tissue is characterized by
a deeper collagen fiber thickness, more disorganized collagen
structure and more severe inflammatory response compared with
normal scar tissue (1). The
present study measured the SEI, collagen structure and inflammation
and scored them between 0 to 3, as described previously. The three
parameters were lower, by at least one grade, in the HS specimens
treated with emodin compared with the control tissue. Additionally,
the collagen whorls disappeared and the level of inflammatory cells
was markedly reduced. Treatment with emodin significantly altered
the histological status and histopathological scores, compared with
the control group on day 14 post-incision (Fig. 1). Between days 14 and 28
post-incision, treatment with emodin was terminated, however, the
histopathological scores only marginally improved.
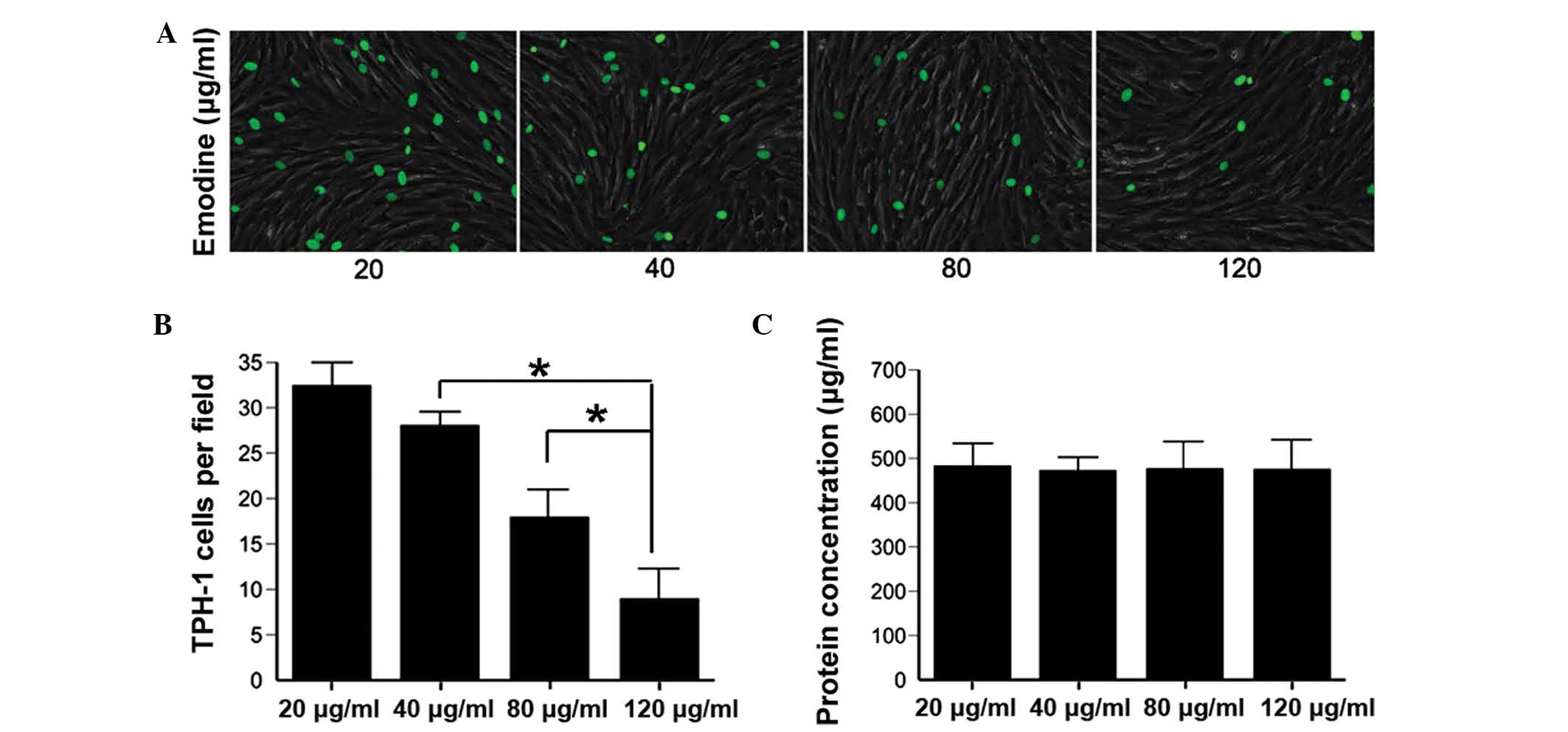
Reduced adhesion of inflammatory cells in
HS
During the early proliferative phase, the
recruitment, adhesion, retention and interaction of inflammatory
cells with topical fibroblasts results in an excessive and chronic
inflammatory response, which contributes to the formation of HS
(4,7). To investigate the direct interaction
between inflammatory cells and fibroblasts treated with various
concentrations of emodin, a cell-cell adhesion assay was performed
using THP-1 cells, a typical type of monocyte. Emodin effectively
reduced the adhesion and mutual reaction of THP-1 and HSFs, in a
dose-dependent manner (Fig. 2A and
B). These results confirmed, to a certain extent, that emodin
inhibited the mechanical stress-induced HS inflammatory response by
reducing the cell-cell interaction between inflammatory cells and
HSFs.
Cytotoxicity of emodin
Emodin has been widely used clinically with few side
effects. To investigate the cytotoxicity of emodin in vitro,
a total protein assay was performed on NFs treated with emodin. No
significant change was observed in the quantity of total protein as
the concentration of emodin increased (Fig. 2C; P>0.10) and no significant
changes were observed in the expression levels of TNF-α, IL-6,
MCP-1, phosphorylated (p-) PI3K/PI3K and p-Akt/Akt in the control
NFs (Figs. 3–6). These results indicated that emodin
was safe for the treatment of HS.
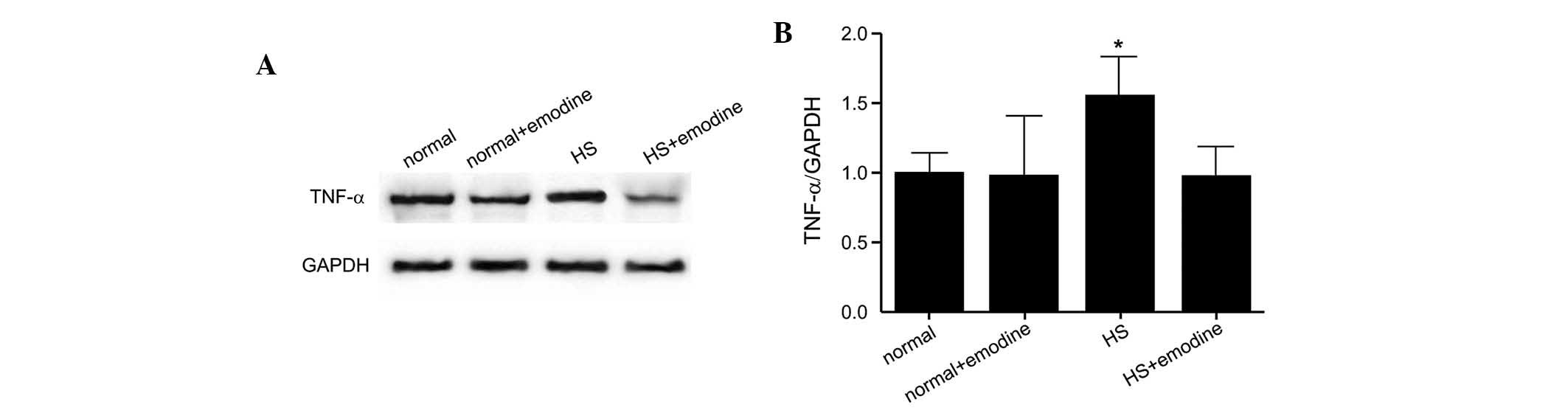
Attenuation of the expression of HS
inflammatory cytokines by emodin
The levels of inflammatory cytokines, which are
closely associated with the HS inflammatory response, were also
assessed in the present study. The protein expression levels of
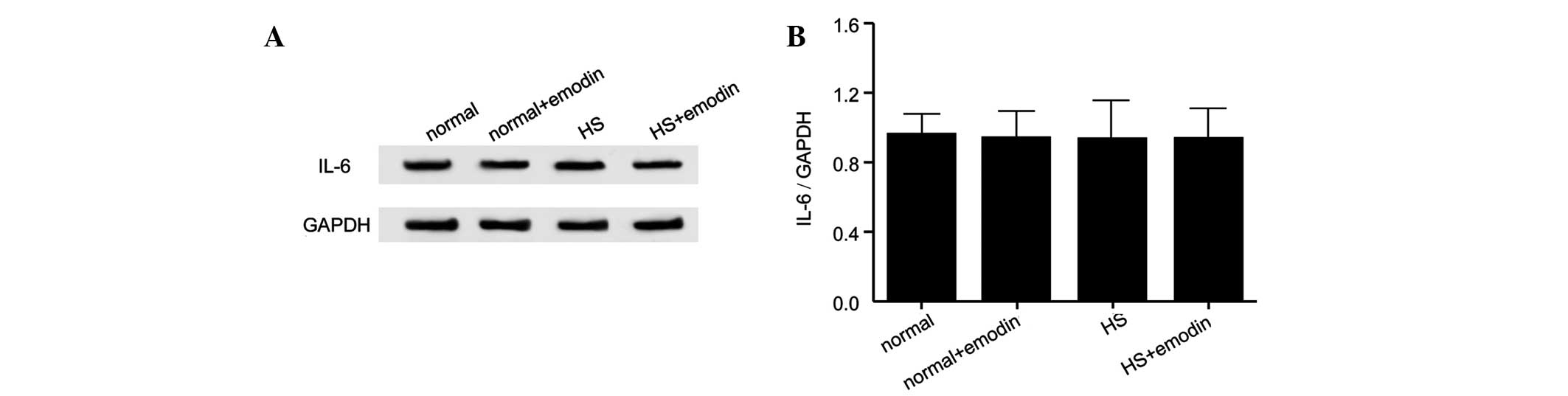
TNF-α, MCP-1 and IL-6 in NFs, NFs treated with emodin and HSFs and
HSFs treated with emodin are presented in Figs. 3–5, respectively. The cytokine expression
levels were significantly increased in the HSF group compared with
the NF group. However, in response to treatment with emodin, the
expression levels of these cytokines were not markedly altered
compared with the controls. These findings demonstrated that emodin
effectively inhibited the HS inflammatory response by attenuating
the production of inflammatory cytokines.
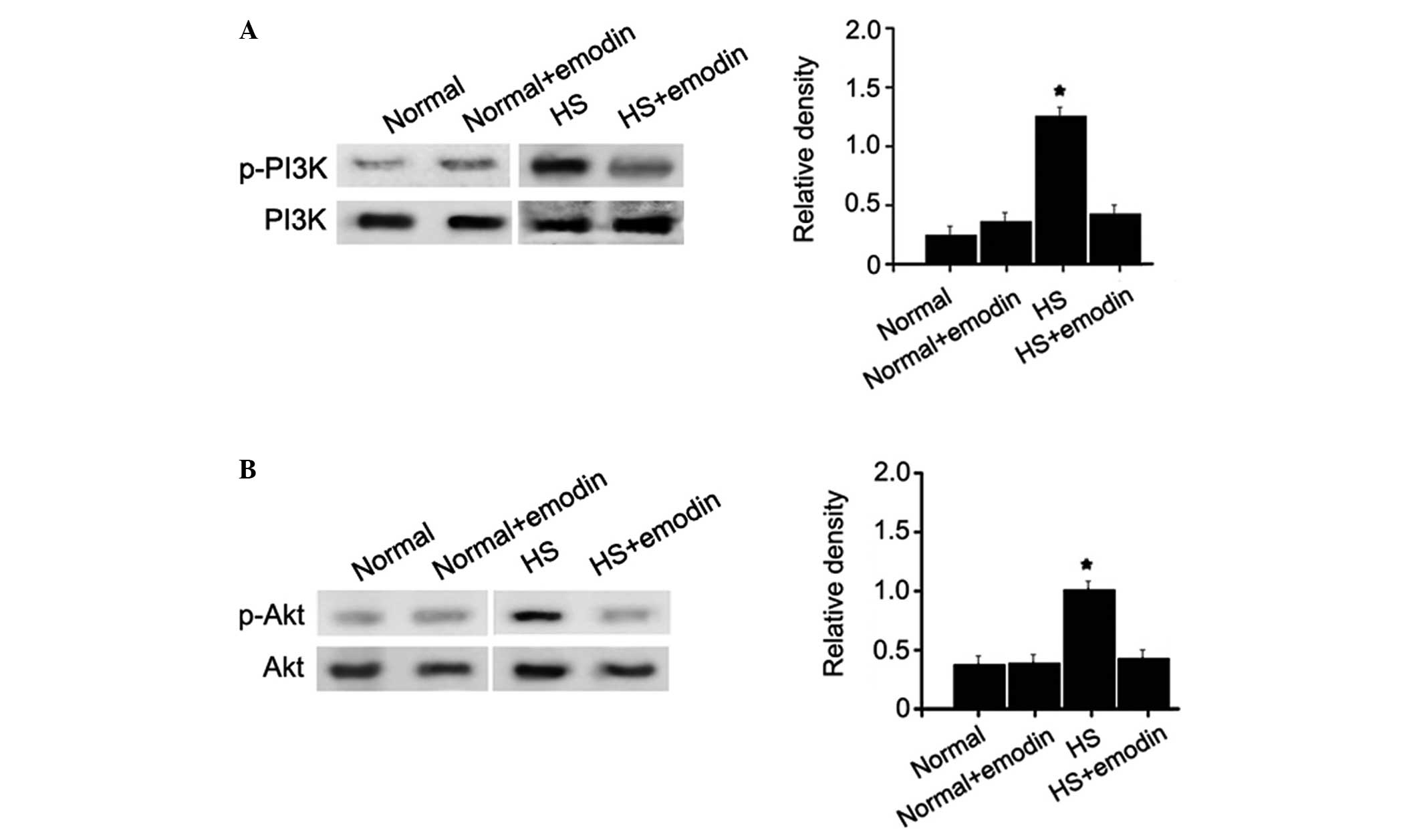
Reduced activation of the expression of
PI3K and Akt
Numerous studies have demonstrated that the PI3K/Akt
signaling pathway is a crucial mediator in the development of
fibrotic diseases, which often involve inflammatory responses
(20–24). To determine the underlying
mechanism by which emodin inhibits the mechanical stress-induced
inflammation of HS, the relative phosphorylation levels of PI3K and
Akt proteins were examined in HSFs and NFs, with or without emodin
treatment. The levels of p-PI3K and p-Akt were significantly higher
in the HSFs compared with the control group (Fig. 6). Treatment with emodin had no
significant effects on the activation of PI3K and Akt in NFs.
However, in the HSFs, treatment with emodin markedly reduced the
activation of these two proteins to levels close to normal. These
findings support the hypothesis that emodin has inhibitory effects
on the mechanical stress-induced inflammation of HS by
downregulating the PI3K/Akt signaling pathway.
Discussion
HS is a fibroproliferative disorder, which can lead
to unsightly scarring or deformities of the organs and extremities.
There is currently no effective treatment for HS, partly due to
limited understanding of the mechanisms underlying HS. Therefore,
the identification of novel antifibrotic activation drugs is
essential.
A previous study demonstrated that inflammation and
mechanical stress are crucial in the initiation, maintenance and
progression of HS (17).
Mechanical stress-induced HS in mice is a novel and
well-characterized model, which is used in the investigation of HS
and was used in the present study (16). Histopathological examinations
indicated that collagen fibers were thicker and larger with a more
disorganized arrangement and collagen whorls developed in
mechanical stress-induced HS. In addition, varying degrees of
inflammatory responses developed, which was marked by the
recruitment and adhesion of monocytes and mast cells. Certain
anti-inflammatory drugs, including corticosteroids, can effectively
inactivate fibroblasts and reduce the inflammatory response in HS
(25). However, due to severe
adverse effects and high costs, the use of these drugs is limited
for the long term and widespread treatment of HS.
The present study examined the effects of emodin, a
cheaper drug with fewer side effects, in HS treatment. Emodin is
extracted from the Chinese herb rhubarb and has been confirmed as
an effective drug for the treatment of fibrosis and the suppression
of inflammatory responses (14,26).
In response to treatment with emodin, the histopathological score
of the HS was markedly reduced. Furthermore, in vitro cell
adhesion assays of the THP-1 and HSFs further revealed that emodin
attenuated the retention of monocytes and reduced the contact and
interaction between the cells in a dose-dependent manner.
Therefore, it was hypothesized that HS inflammation can be
inhibited by emodin through the suppression of inflammatory cell
recruitment, adhesion, retention and activation.
Several types of cytokine are associated with the HS
inflammatory response, including TNF-α, IL-6, transforming growth
factor-β and MCP-1. In order to investigate the effects of emodin
on inflammatory cytokines in HS, the protein expression levels of
TNF-α, IL-6 and MCP-1 were determined by western blotting. Previous
studies have demonstrated that the expression levels of TNF-α or
the TNF-α receptor are markedly upregulated in HS (9,27,28)
and matrix metalloproteinase (MMP)-1 and MMP-3 are downregulated,
which attenuates the excessive accumulation of collagen formed in
hypertrophic scars by suppressing MMPs under the control of IL-6
(29). These findings are
consistent with those of the present study, which demonstrated that
the expression levels of TNF-α and MCP-1 were markedly increased,
but the expression of IL-6 was not significantly altered in HS. In
response to treatment with emodin, the expression levels of TNF-α
and MCP-1 were gradually restored close to normal levels and the
expression of IL-6 remained unchanged. These results indicated
that, to a certain extent, HS inflammation can be inhibited by
emodin by suppressing the production of inflammatory cytokines.
The PI3K/Akt signaling pathway has a key role in the
inflammatory response and, to a certain extent, the levels of
p-PI3K and p-Akt determine the strength of the inflammatory
response and the formation of HS (30). To further investigate the
mechanisms by which emodin inhibits HS inflammation in the present
study, the levels of p-PI3K and p-Akt were determined. The
phosphorylation of PI3K and Akt in HS was markedly reduced
following treatment with emodin, which was in accordance with the
results of previous studies investigating the effects of emodin on
other systems (31,32). These results suggested that the
PI3K/Akt signaling pathway may mediate the inhibitory effects of
emodin on HS inflammation.
In conclusion, the present study demonstrated that
emodin may inhibit mechanical stress-induced HS inflammation by
reducing histopathological scores, attenuating inflammatory cell
recruitment and adhesion and suppressing the secretion of
inflammatory cytokines by inhibiting the PI3K/Akt signaling
pathway. However, whether emodin can be used clinically to treat
HS, and whether it acts upon other signaling pathways to affect HS
remains to be elucidated. Therefore, further detailed studies are
required to evaluate the therapeutic use of emodin.
Acknowledgments
The present study was supported by the National
Science Foundation of Jiangxi Province (20122BAB205008).
References
|
1
|
Wolfram D, Tzankov A, Pülzl P and
Piza-Katzer H: Hypertrophic scars and keloids - a review of their
pathophysiology, risk factors, and therapeutic management. Dermatol
Surg. 35:171–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahmani-Neishaboor E, Yau FM, Jalili R,
Kilani RT and Ghahary A: Improvement of hypertrophic scarring by
using topical anti-fibrogenic/anti-inflammatory factors in a rabbit
ear model. Wound Repair Regen. 18:401–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang C, Akaishi S, Hyakusoku H and Ogawa
R: Are keloid and hypertrophic scar different forms of the same
disorder? A fibroproliferative skin disorder hypothesis based on
keloid findings. Int Wound J. 11:517–522. 2014. View Article : Google Scholar
|
|
4
|
Eming SA, Krieg T and Davidson JM:
Inflammation in wound repair: molecular and cellular mechanisms. J
Invest Dermatol. 127:514–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gauglitz GG, Korting HC, Pavicic T, et al:
Hypertrophic scarring and keloids: pathomechanisms and current and
emerging treatment strategies. Mol Med. 17:113–125. 2011.
View Article : Google Scholar :
|
|
6
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
7
|
van der Veer WM, Bloemen MC, Ulrich MM, et
al: Potential cellular and molecular causes of hypertrophic scar
formation. Burns. 35:15–29. 2009. View Article : Google Scholar
|
|
8
|
Wong VW, Paterno J, Sorkin M, et al:
Mechanical force prolongs acute inflammation via T-cell-dependent
pathways during scar formation. FASEB J. 25:4498–4510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong VW, Rustad KC, Akaishi S, et al:
Focal adhesion kinase links mechanical force to skin fibrosis via
inflammatory signaling. Nat Med. 18:148–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krötzsch-Gómez FE, Furuzawa-Carballeda J,
Reyes-Márquez R, Quiróz-Hernández E and Díaz de León L: Cytokine
expression is downregulated by collagen-polyvinylpyrrolidone in
hypertrophic scars. J Invest Dermatol. 111:828–834. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Polo M, Ko F, Busillo F, Cruse CW, Krizek
TJ and Robson MC: The 1997 Moyer Award. Cytokine production in
patients with hypertrophic burn scars. J Burn Care Rehabil.
18:477–482. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei WT, Lin SZ, Liu DL and Wang ZH: The
distinct mechanisms of the antitumor activity of emodin in
different types of cancer (Review). Oncol Rep. 30:2555–2562.
2013.PubMed/NCBI
|
|
13
|
Shrimali D, Shanmugam MK, Kumar AP, et al:
Targeted abrogation of diverse signal transduction cascades by
emodin for the treatment of inflammatory disorders and cancer.
Cancer Lett. 341:139–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Q, Noor M, Wong YF, et al: In vitro
anti-fibrotic activities of herbal compounds and herbs. Nephrol
Dial Transplant. 24:3033–3041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin YL, Wu CF and Huang YT: Phenols from
the roots of Rheum palmatum attenuate chemotaxis in rat hepatic
stellate cells. Planta Med. 74:1246–1252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aarabi S, Bhatt KA, Shi Y, et al:
Mechanical load initiates hypertrophic scar formation through
decreased cellular apoptosis. FASEB J. 21:3250–3261. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu XJ, Xu MJ, Fan ST, et al: Xiamenmycin
attenuates hypertrophic scars by suppressing local inflammation and
the effects of mechanical stress. J Invest Dermatol. 133:1351–1360.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pfaff AW, Georges S and Candolfi E:
Different effect of Toxoplasma gondii infection on adhesion
capacity of fibroblasts and monocytes. Parasite Immunol.
30:487–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha MK, Song YH, Jeong SJ, et al: Emodin
inhibits proinflammatory responses and inactivates histone
deacetylase 1 in hypoxic rheumatoid synoviocytes. Biol Pharm Bull.
34:1432–1437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Li Y, Liang C and Yang W: CCN5
overexpression inhibits profibrotic phenotypes via the PI3K/Akt
signaling pathway in lung fibroblasts isolated from patients with
idiopathic pulmonary fibrosis and in an in vivo model of lung
fibrosis. Int J Mol Med. 33:478–486. 2014.
|
|
21
|
Son G, Hines IN, Lindquist J, Schrum LW
and Rippe RA: Inhibition of phosphatidylinositol 3-kinase signaling
in hepatic stellate cells blocks the progression of hepatic
fibrosis. Hepatology. 50:1512–1523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Cras TD, Korfhagen TR, Davidson C, et
al: Inhibition of PI3K by PX-866 prevents transforming growth
factor-alpha-induced pulmonary fibrosis. Am J Pathol. 176:679–686.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Son MK, Ryu YL, Jung KH, et al: HS-173, a
novel PI3K inhibitor, attenuates the activation of hepatic stellate
cells in liver fibrosis. Sci Rep. 3:34702013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon HE, Kim SJ, Kim SJ, Chung S and Shin
SJ: Tempol attenuates renal fibrosis in mice with unilateral
ureteral obstruction: the role of PI3K-Akt-FoxO3a signaling. J
Korean Med Sci. 29:230–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Veer WM, Ferreira JA, de Jong EH,
Molema G and Niessen FB: Perioperative conditions affect long-term
hypertrophic scar formation. Ann Plast Surg. 65:321–325. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kitano A, Saika S, Yamanaka O, et al:
Emodin suppression of ocular surface inflammatory reaction. Invest
Ophthalmol Vis Sci. 48:5013–5022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salgado RM, Alcántara L, Mendoza-Rodríguez
CA, et al: Post-burn hypertrophic scars are characterized by high
levels of IL-1β mRNA and protein and TNF-α type I receptors. Burns.
38:668–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reno F, Sabbatini M, Lombardi F, et al: In
vitro mechanical compression induces apoptosis and regulates
cytokines release in hypertrophic scars. Wound Repair Regen.
11:331–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dasu MR, Hawkins HK, Barrow RE, Xue H and
Herndon DN: Gene expression profiles from hypertrophic scar
fibroblasts before and after IL-6 stimulation. J Pathol.
202:476–485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song J, Xu H, Lu Q, Xu Z, Bian D, Xia Y,
Wei Z, Gong Z and Dai Y: Madecassoside suppresses migration of
fibroblasts from keloids: involvement of p38 kinase and PI3K
signaling pathways. Burns. 38:677–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SU, Shin HK, Min YK and Kim SH: Emodin
accelerates osteoblast differentiation through phosphatidylinositol
3-kinase activation and bone morphogenetic protein-2 gene
expression. Int Immunopharmacol. 8:741–747. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei WT, Chen H, Ni ZL, et al: Antitumor
and apoptosis-promoting properties of emodin, an anthraquinone
derivative from rheum officinale Baill, against pancreatic cancer
in mice via inhibition of Akt activation. Int J Oncol.
39:1381–1390. 2011.PubMed/NCBI
|