Introduction
Worldwide, hepatocellular carcinoma (HCC) is the
fifth most prevalent type of cancer and the third most common cause
of cancer-associated mortality (1). HCC has been shown to be highly
refractory to treatment and has a low five-year survival rate of
~12–15% worldwide (2,3,4).
Early diagnosis is critical for the timely treatment of HCC and
improving the survival rate of patients (5). Therefore, it is essential to identify
novel biomarkers, which may allow for earlier diagnosis of HCC,
provide novel therapeutic targets for HCC treatment and ultimately
improve patient survival (6,7).
Golgi phosphoprotein-3 (GOLPH3) is a member of the
trans-Golgi matrix family. Previous studies have identified GOLPH3
as an oncogene, which has a role in the development of numerous
types of cancer, including lung, ovary, breast, colon and prostate
cancer, as well as melanoma, rhabdomyosarcoma and glioma (8–11).
In addition, it has been reported that GOLPH3 overexpression
promoted cell proliferation and tumorigenesis through activation of
the mammalian target of rapamycin (mTOR) signaling pathway, which
enhanced AKT activity and decreased forkhead box protein O1 (FOXO1)
transcriptional activity (8,12,13).
However, studies regarding the correlation between GOLPH3
expression and the prognosis of patients with HCC are limited. In
the present study, immunohistochemical analysis of human HCC and
adjacent non-cancerous hepatic tissues was conducted in order to
determine the expression of GOLPH3, as well as to investigate
whether there was a correlation between GOLPH3 expression and
clinicopathological factors associated with HCC prognosis. In
addition, HCC cell lines were used to explore the effect of GOLPH3
silencing on cell proliferation, migration and invasion in HCC.
Patients and methods
Patients and specimens
Paired tissue specimens of HCC and adjacent
non-cancerous hepatic tissues (distance from tumor, ≥1.5 cm) were
obtained from 180 patients with primary HCC who underwent surgical
resection without pre-operative treatment at the Department of
Hepatobiliary Surgery, Affiliated Hospital of Weifang Medical
University (Shandong, China) between 2006 and 2008. The
participants consisted of 118 male and 62 female patients, with a
median age of 52.6 years (range, 34–83 years). In addition to these
180 patients, another group consisted of 30 fresh HCC specimens and
paired adjacent non-cancerous tissue, which were assessed for
GOLPH3 protein expression using western blot analysis. The present
study was approved by the ethics committee of the Affiliated
Hospital of Weifang Medical University and conformed to the
Declaration of Helsinki. Written informed consent was obtained from
each patient or their legal guardians. Clinical parameters (as
shown in Table I) were obtained by
consulting the hospital records. The overall survival was measured
as the time from surgery until the patient succumbed to HCC, or the
last observation taken. For patients who survived, the data were
censored at the last follow-up appointment. Following surgery,
patients were followed up every 3 months for the first year, then
every 6 months for the subsequent two years and then annually.
After surgery the patients received neoadjuvant chemotherapy,
radiation therapy or immunotherapy, based on the National
Comprehensive Cancer Network recommendations.
 | Table ICorrelation between GOLPH3 expression
and clinicopathologic features in cancerous tissue from 180
patients with hepatocellular carcinoma. |
Table I
Correlation between GOLPH3 expression
and clinicopathologic features in cancerous tissue from 180
patients with hepatocellular carcinoma.
| Clinicopathologic
variable | Number of cases | GOLPH3 expression
| P-value |
|---|
| Low | High |
|---|
| Gender | | | | 0.230 |
| Male | 118 | 48 | 70 | |
| Female | 62 | 26 | 36 | |
| Age (years) | | | | 0.586 |
| ≤60 | 135 | 60 | 75 | |
| >60 | 45 | 14 | 31 | |
| Liver cirrhosis | | | | 0.425 |
| Presence | 136 | 59 | 77 | |
| Absence | 44 | 15 | 29 | |
| Capsular
formation | | | | 0.861 |
| Presence | 95 | 44 | 51 | |
| Absence | 85 | 30 | 55 | |
| Tumor size | | | | 0.187 |
| ≤5 cm | 89 | 39 | 50 | |
| >5 cm | 91 | 35 | 56 | |
| Tumor nodule
number | | | | 0.316 |
| Multiple | 104 | 56 | 48 | |
| Solitary | 76 | 18 | 58 | |
| Edmondson-Steiner
grade | | | | 0.006 |
| Stage I–II | 99 | 57 | 42 | |
| Stage III–IV | 81 | 17 | 64 | |
| Vascular
invasion | | | | 0.002 |
| Absence | 97 | 44 | 53 | |
| Presence | 83 | 30 | 53 | |
| α feto-protein
levels | | | | 0.015 |
| <400
μg/l | 59 | 15 | 44 | |
| ≥400
μg/l | 121 | 59 | 62 | |
Immunohistochemistry (IHC)
IHC analysis was performed in order to detect GOLPH3
expression in 180 HCC tissues. Paraffin-embedded specimens (4
μm sections) were administered 3% hydrogen peroxide in
methanol in order to quench endogenous peroxidase activity and then
incubated with 1% bovine serum albumin (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China) in order to avoid
non-specific binding. Anti-GOLPH3 (1:50, sc-242931; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was incubated with the
sections overnight at 4°C. Following washing with Tris-buffered
saline with Tween-20 (TBS-T) buffer (Beijing Biosynthesis
Biotechnology Co., Ltd.), the tissue sections were treated with
biotinylated secondary antibodies (sc-2054; Santa Cruz
Biotechnology, Inc.), followed by incubation with a
streptavidin-horseradish peroxidase complex (Santa Cruz
Biotechnology, Inc.). Normal mouse serum (Beijing Biosynthesis
Biotechnology Co., Ltd.) was used as a negative control. Tissue
specimens were scored by two independent pathologists blinded to
the clinical data, using an immunoreactivity scoring system, as
previously described (14). In
case of discrepancies, a final score was established by
reassessment using a double-headed microscope (BH2; Olympus, Tokyo,
Japan). The immunostaining for GOLPH3 was semi-quantitatively
scored, where only cytoplasmic staining was considered positive, as
follows: −, no or <5% positive cells; +, 5–25% positive cells;
++, 26–50% positive cells; and +++, >50% positive cells. For
statistical analysis, the scores of ++ and +++ were classified as
high expression; and scores of − and + as low expression.
Cell culture
Four HCC cell lines (HCCLM3, MHCC97-H, MHCC97-L and
Hep3B) were obtained from the America Type Culture Collection
(Manassas, VA, USA) for use in the present study. Cells were
cultivated in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal calf serum (Sigma-Aldrich, St. Louis,
MO, USA). Cells (1×105 cells/well) were seeded onto
six-well cell culture plates for 48 h until further use.
Western blot analysis
Following surgery the tissues were immediately
frozen in liquid nitrogen and stored at −80°C until further use.
Frozen tumor sections or cells were lysed in cell lysis buffer
(Qiagen, Hilden, Germany). Samples were homogenized and stored at
4°C for 30 min. Cell extracts were then subjected to centrifugation
at 1,5000 × g for 30 min at 4°C and protein concentration was
determined using a bicinchoninic acid assay kit (Qiagen). Total
proteins (60 μg) from each sample were heated at 98°C for 5
min following mixing with SDS loading buffer. Samples were
separated using 12% SDS-PAGE and electrotransferred to
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).
Membranes were blocked with TBS-T containing 5% skimmed milk at
room temperature for 2 h. Membranes were then incubated with goat
polyclonal anti-GOLPH3 (1:2,000, sc-242931) or goat polyclonal
GAPDH antibodies (1:5,000, sc-20356) (Santa Cruz Biotechnology,
Inc.) in TBS-T overnight at 4°C. Following washing with TBS-T, the
membranes were incubated with 5% skimmed milk in TBS-T buffer,
which contained the mouse anti-goat horseradish
peroxidase-conjugated immunoglobulin G secondary antibody (1:30,00,
sc-2355; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Proteins of interest were detected and visualized
using autoradiography (Beijing Biosynthesis Biotechnology Co.,
Ltd.) following various exposure times.
Small interfering (si)RNA
transfection
HCC cells were transfected with GOLPH3 siRNA or
control siRNA (Beijing Biosynthesis Biotechnology Co., Ltd.) using
Lipofectamine 2000® (Invitrogen Life Technologies)
according to the manufacturer’s instructions. Cells transfected
with siRNA (1×105 cells/well) were seeded onto six-well
plates and cultured for 24 h prior to harvesting for further
analysis. The silencing effect of these siRNAs was detected using
western blot analysis, as described previously.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays
Cells (4×103cells/well) were plated onto
96-well plates and each well was incubated with 20 μl MTT (5
mg/ml; Sigma-Aldrich) at 37°C for 4 h. Absorbance was measured at
490 nm using a microplate reader. Cells incubated with culture
medium were used as the control group. Each sample was assayed in
triplicate.
Matrigel invasion assay
Invasion assays were performed using transwell
membrane filter inserts (6.5-mm diameter; 8-μm pore; BD
Biosciences, Franklin Lakes, NJ, USA) in a 24-well tissue culture
plate. Briefly, transfected cells were harvested at 24 h and
resuspended in serum-free RPMI-1640. Cells (2×105
cells/well) in 200 μl growth medium without FBS were added
to the upper chamber, and the bottom chamber was filled with 500
μl growth medium containing 10% FBS. After 48 h,
non-migrating cells were removed from the top of the filter with a
cotton swab. Invading cells on the bottom of the filter were fixed
with methanol, and stained using the Diff-Quick Stain kit (IMEB
Inc., San Marcos, CA, USA) according to the instructions. Stained
cells in 10 random fields were counted using an inverted microscope
(Leica DMI 3000M; Leica, Wetzlar, Germany). Each experiment was
conducted in triplicate.
Cell migration assay
The cell migration assays were performed in a
chamber system consisting of polycarbonate membrane inserts with an
8-μm pore size (Corning, New York, NY, USA) placed in
24-well cell culture insert companion plates. The migration assay
was conducted at 48 h after the cells were infected with siRNA. The
cells (in 200 μl of growth medium without FBS) were placed
in the upper chamber and 500 μl of growth medium with 5% FBS
was placed in the lower chamber. The cells were incubated at 37°C
for 24 h. Following the incubation, the insert membranes were fixed
with 75% methanol for 30 min, stained with 0.5% crystal violet, and
counted. The stained cells were counted under the inverted
microscope (10 fields per membrane). Each experiment was performed
in triplicate.
Statistical analysis
Data were assessed using a paired Student’s t-test
or one-way analysis of variance for multiple comparisons, and
χ2 test for 2×2 tables was used to compare the
categorical data. Survival curves were compared using the
Kaplan-Meier method and log-rank test. The Cox proportional hazard
model was used for univariate and multivariate analysis in order to
explore the effect of GOLPH3 expression on survival. Statistical
analysis was conducted using SPSS 16.0 software (Internation
Business Machines, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
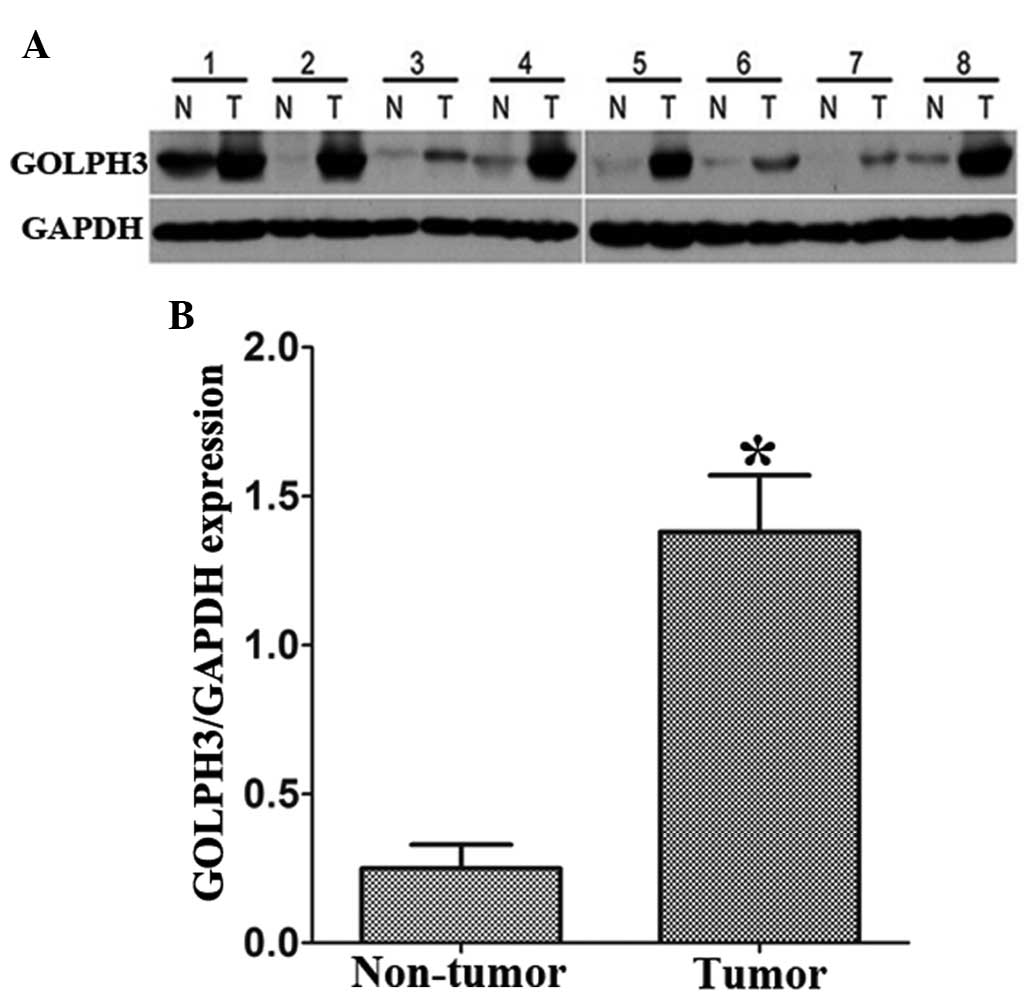
Western blot analysis of GOLPH3
expression in HCC tissues
A total of 30 fresh HCC specimens and paired
adjacent non-cancerous tissues were examined for GOLPH3 protein
expression. The results showed that GOLPH3 expression at the
protein level was markedly increased in HCC tissues compared with
that of the paired adjacent non-tumor tissues (P<0.01; Fig. 1).
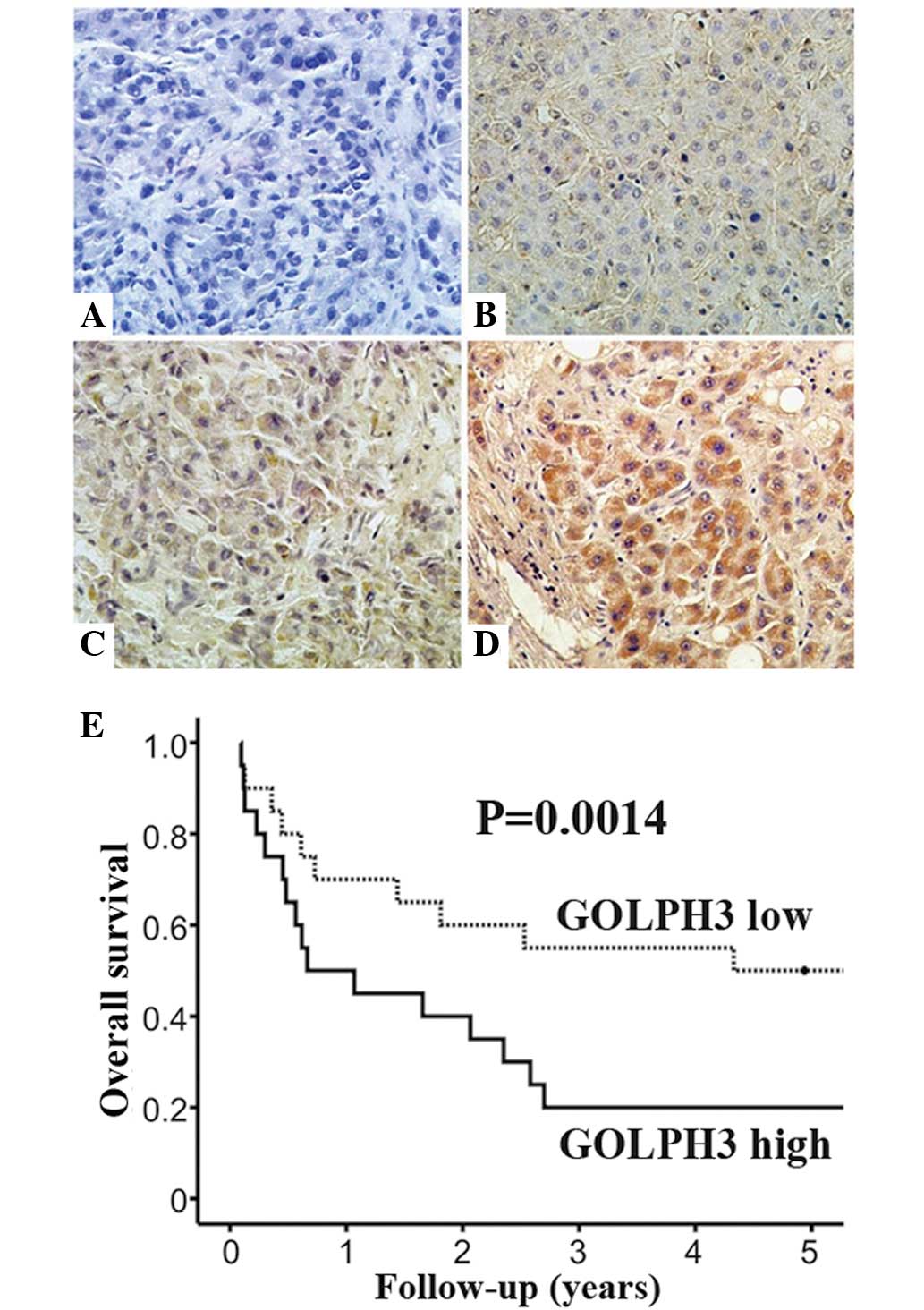
Correlation of GOLPH3 expression with
clinicopathological features in HCC patients
Immunohistochemical staining was used to detect
GOLPH3 expression in the cytoplasm of HCC tissues (Fig. 2A–D). In addition, Kaplan-Meier
analysis indicated that patients with high GOLPH3 expression had a
poorer overall survival rate (P=0.0014) compared with that of
patients with low GOLPH3 expression (Fig. 2E). The results revealed that high
GOLPH3 expression was observed in 58.8% (106 of 180) of HCC tissues
samples. By contrast, high expression of GOLPH3 was only observed
in 7.7% (14 of 180) of non-cancerous tissues (P<0.001). In line
with the results of the western blot analysis, these data indicated
that GOLPH3 expression was significantly upregulated in HCC
samples.
As shown in Table
I, high GOLPH3 expression was found to be positively correlated
with Edmondson-Steiner grade (P=0.006), vascular invasion (P=0.002)
and serum α feto-protein (AFP) levels (P=0.015). In addition,
univariate and multivariate analyses demonstrated that GOLPH3 was
an independent prognostic factor for the overall survival of HCC
patients (hazard ratio, 2.01; 95% confidence interval, 1.26–3.64;
P=0.025) (Table II).
 | Table IIUnivariate and multivariate analysis
of overall survival in 180 patients with hepatocellular
carcinoma. |
Table II
Univariate and multivariate analysis
of overall survival in 180 patients with hepatocellular
carcinoma.
| Variable | Univariate
| Multivariate
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender | 0.89 | 0.37–1.25 | 0.290 | | | |
| Age (years) | 0.71 | 0.22–1.14 | 0.410 | | | |
| Vascular
invasion | 2.18 | 1.14–3.96 | 0.011 | 1.72 | 0.74–3.16 | 0.016 |
| No. of nodules | 0.81 | 0.44–1.29 | 0.190 | | | |
| Cirrhosis | 0.73 | 0.32–1.31 | 0.360 | | | |
| Serum AFP | 1.06 | 0.47–1.72 | 0.130 | | | |
| Edmondson-Steiner
grade | 1.89 | 1.96–3.70 | 0.023 | 1.37 | 1.02–2.19 | 0.476 |
| Vascular
invasion | 2.78 | 1.64–5.98 | 0.001 | 2.29 | 1.02–3.81 | 0.020 |
| GOLPH3
expression | 3.71 | 1.75–6.11 | 0.009 | 2.01 | 1.26–3.64 | 0.025 |
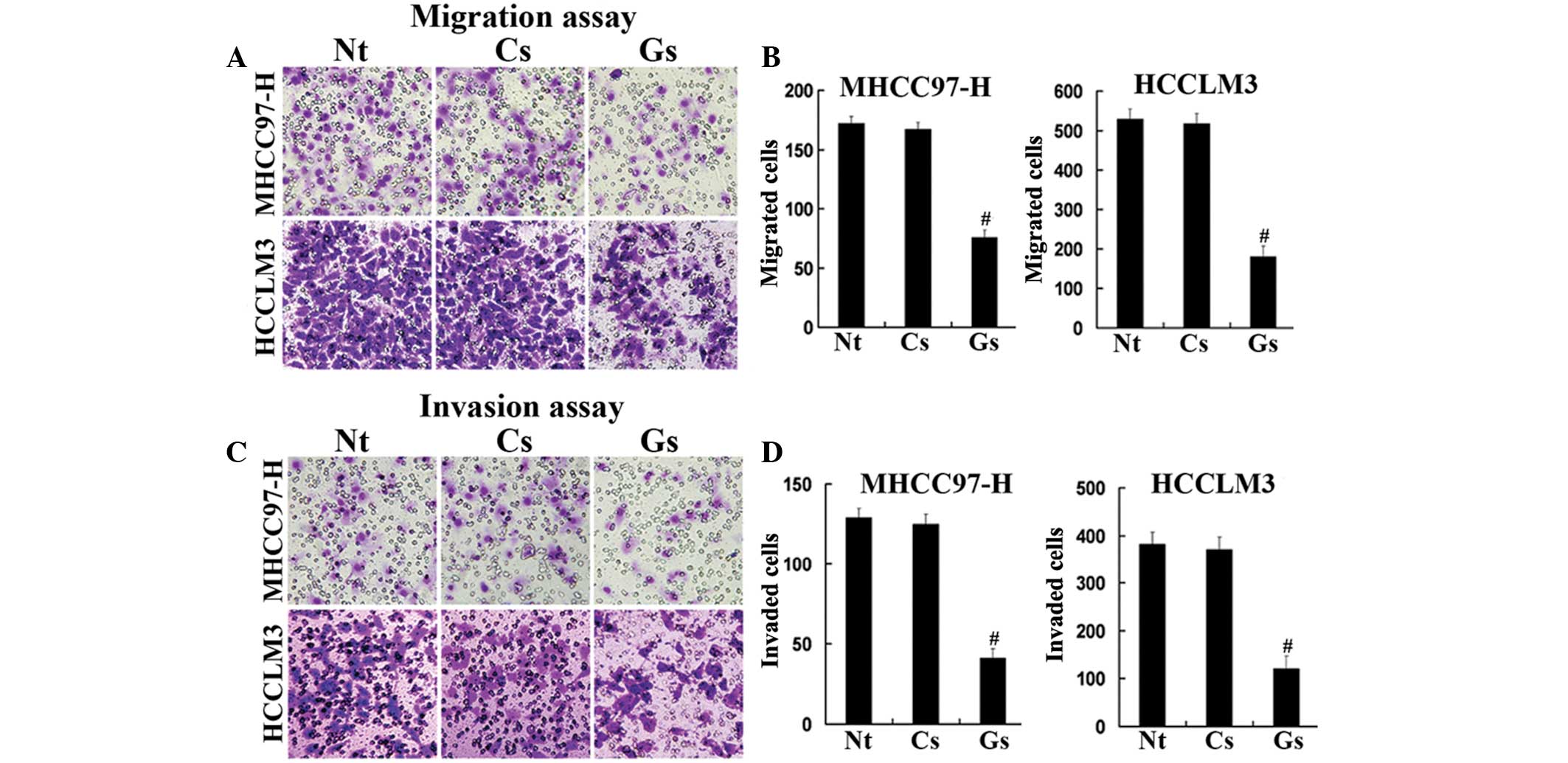
GOLPH3 silencing inhibits the
proliferation, migration and invasion of HCC cell lines
Expression levels of GOLPH3 protein were examined in
four HCC cell lines, HCCLM3, MHCC97-H, MHCC97-L and Hep3B. HCCLM3
and MHCC97-H cells showed higher GOLPH3 expression compared with
that of MHCC97-L and Hep3B cells (Fig.
3A). Therefore, HCCLM3 and MHCC97-H were selected for use in
the GOLPH3 silencing experiments.
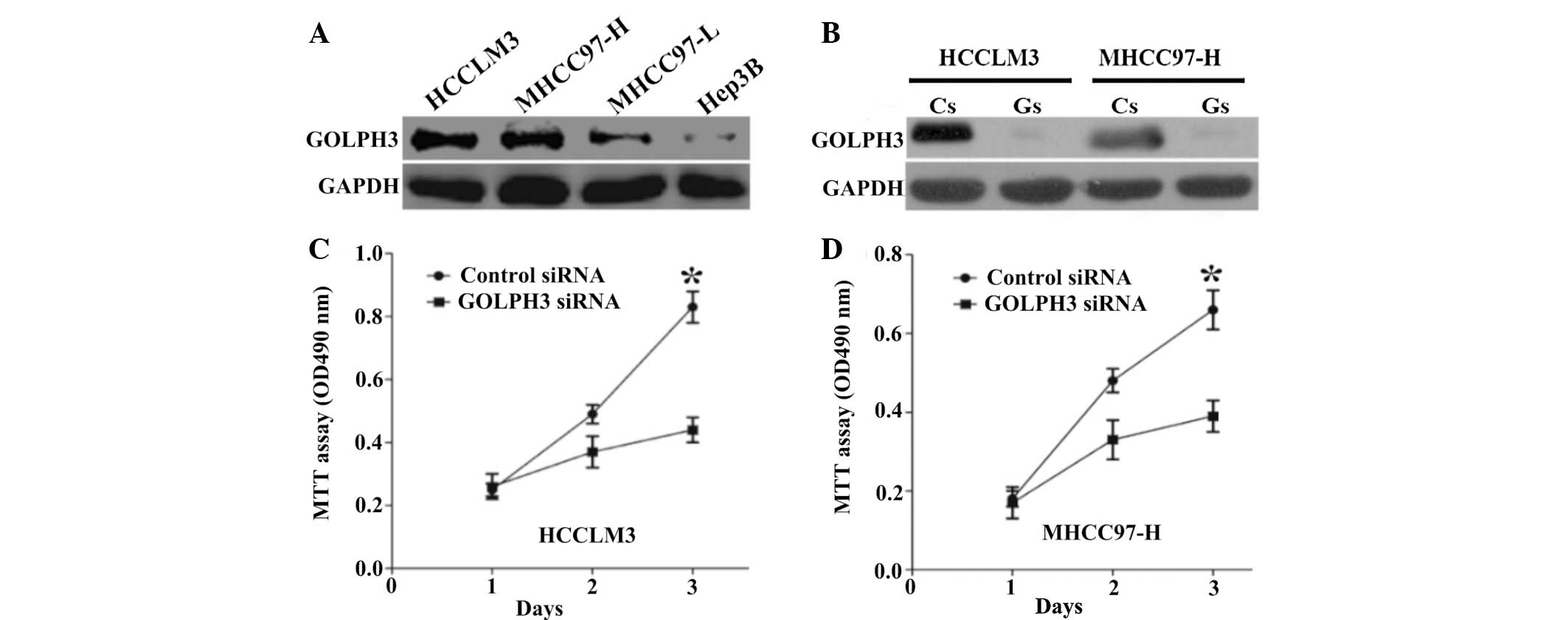 | Figure 3Expression of GOLPH3 in HCC cell lines
and the effect of GOLPH3 silencing on HCC cells. (A) Western blot
analysis of GOLPH3 protein expression in four HCC cell lines,
HCCLM3, MHCC97-H, MHCC97-L and Hep3B. (B) Western blot analysis
confirmed the silencing efficacy of GOLPH3 siRNA. MTT assays showed
that knockdown of GOLPH3 significantly reduced cell proliferation
of (C) HCCLM3 and (D) MHCC97-H cells compared with control siRNA
transfected cells. Values are presented as the mean ± standard
deviation. *P<0.01 vs. control siRNA. GOLPH3, Golgi
phosphoprotein 3; HCC, hepatocellular carcinoma; siRNA, small
interfering RNA; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Cs,
control siRNA; Gs, GOLPH3 siRNA. |
Western blot analysis confirmed the silencing
efficacy of GOLPH3 siRNA (Fig.
3B). In addition, MTT assays demonstrated that the knockdown of
GOLPH3 significantly reduced cell proliferation in HCCLM3 and
MHCC97-H cells compared with that of the control cells (Fig. 3C and D). Furthermore, GOLPH3
knockdown inhibited hepatocellular carcinoma (HCC) cell migration
and the invasion of HCCLM3 and MHCC97-H cells in vitro
(Fig. 4). These data indicated
that GOLPH3 silencing inhibited the proliferation, migration and
invasion of HCC cells.
Discussion
The results of the present study demonstrated that
GOLPH3 expression was significantly elevated in HCC tissues
compared with that of adjacent non-cancerous liver tissue. In
addition, the high expression levels of GOLPH3 were found to have a
significant positive correlation with Edmondson-Steiner grade,
vascular invasion and serum AFP levels. Furthermore, the
association of GOLPH3 expression with prognosis was assessed and it
was revealed that GOLPH3 was an independent factor for predicting
poor survival in HCC patients.
The GOLPH3 gene was found to be located on
chromosome 5p13 in humans. In addition, this gene was reported to
be frequently amplified in several types of solid tumor, including
lung, ovary, breast, prostate and skin cancers (8). However, the clinical relevance of
GOLPH3 in patients with HCC remained to be fully elucidated.
Previous studies have indicated that high expression levels of
GOLPH3 promoted tumorigenesis and the progression of several types
of malignancies as well as correlated with poor survival rates
(10,14–18).
One study demonstrated that GOLPH3 expression was present in
>50% of the patients with glioma and GOLPH3 expression in the
glioma was associated with the severity of the tumor (10). In addition, overexpression of
GOLPH3 has been associated with the poor prognosis of patients with
cN0 oral tongue cancer; therefore, it was suggested that GOLPH3 may
have potential for use as a novel and useful prognostic indicator
of cN0 oral tongue cancer (14).
Furthermore, high GOLPH3 expression has been associated with the
poor outcome of glioblastoma multiforme patients (15). In vitro siRNA experiments
that downregulated GOLPH3 expression resulted in the suppressed
proliferation and clonogenic growth in a cultured glioblastoma
multiform cell line (15). It was
also reported that GOLPH3 overexpression was associated with the
transition of prostate cancer from the hormone sensitive phase into
the hormone refractory phase; this therefore indicated that GOLPH3
may be an important prognostic indicator for patients with prostate
cancer (16). A marked increase in
GOLPH3 expression was reported to occur in esophageal squamous cell
cancer (ESCC) cell lines and tissues at the mRNA and protein level
(17). In addition, high GOLPH3
expression in ESCC was reported to be positively correlated with
clinical stage, tumor-node-metastasis (TNM) classification,
histological differentiation and vital status; furthermore, the
expression of GOLPH3 was shown to be an independent prognostic
factor for patients with ESCC (17). It has been demonstrated that the
over-expression of GOLPH3 was associated with the size of the
tumor, histological grade, depth of invasion, lymph node
metastasis, distant metastasis and TNM stage in gastric cancer; in
addition, multivariate analysis indicated that GOLPH3 expression
levels were an independent prognostic factor for gastric cancer
patients following radical surgical resection (18). The results of the present study
demonstrated a high GOLPH3 expression rate of 58.8% in HCC tissues.
By contrast, the percentage of patients with high expression of
GOLPH3 in non-HCC tissues signifi-cantly lower at 7.7% (P<0.01).
Immunohistochemical analysis statistics demonstrated that high
GOLPH3 expression was positively correlated with Edmondson-Steiner
grade, vascular invasion and serum AFP levels. Kaplan-Meier
analysis indicated that patients with high GOLPH3 expression had a
poorer overall survival rate compared with that of patients with
low GOLPH3 expression. In addition, univariate and multivariate
analyses showed that GOLPH3 was an independent prognostic factor
for the overall survival of HCC patients. Furthermore, the results
of the present study also suggested that GOLPH3 enhanced the
proliferation and invasion of HCC cells.
In conclusion, the results of the present study
indicated that GOLPH3 may have an important role in the
pathogenesis of human HCC. In addition, GOLPH3 overexpression was
found to be a novel prognostic marker for HCC; however, further
studies are required in order to explore the underlying mechanisms
of action of GOLPH3 in HCC.
References
|
1
|
Knudsen ES, Gopal P and Singal AG: The
changing landscape of hepatocellular carcinoma: etiology, genetics,
and therapy. Am J Pathol. 184:574–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salgia R and Singal AG: Hepatocellular
carcinoma and other liver lesions. Med Clin North Am. 98:103–118.
2014. View Article : Google Scholar
|
|
4
|
Bosetti C, Turati F and La Vecchia C:
Hepatocellular carcinoma epidemiology. Best Pract Res Clin
Gastroenterol. 28:753–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finn RS: Current and future treatment
strategies for patients with advanced hepatocellular carcinoma:
role of mTOR inhibition. Liver Cancer. 1:247–256. 2012. View Article : Google Scholar
|
|
6
|
Song P1, Gao J, Inagaki Y, Kokudo N,
Hasegawa K, Sugawara Y and Tang W: Biomarkers: Evaluation of
screening for and early diagnosis of hepatocellular carcinoma in
Japan and China. Liver Cancer. 2:31–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shao YY, Hsu CH and Cheng AL: Predictive
biomarkers of antiangiogenic therapy for advanced hepatocellular
carcinoma: Where are we? Liver Cancer. 2:93–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scott KL, Kabbarah O, Liang MC, Ivanova E
and Anagnostou V: GOLPH3 modulates mTOR signalling and rapamycin
sensitivity in cancer. Nature. 459:1085–1090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunigou O, Nagao H, Kawabata N, Ishidou Y
and Nagano S: Role of GOLPH3 and GOLPH3L in the proliferation of
human rhabdomyosarcoma. Oncol Rep. 26:1337–1342. 2011.PubMed/NCBI
|
|
10
|
Li XY, Liu W, Chen SF, Zhang LQ, Li XG and
Wang LX: Expression of the Golgi phosphoprotein-3 gene in human
gliomas: a pilot study. J Neurooncol. 105:159–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romanuik TL, Wang G, Holt RA, Jones SJ,
Marra MA and Sadar MD: Identification of novel androgen-responsive
genes by sequencing of LongSAGE libraries. BMC Genomics.
10:4762009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abraham RT: GOLPH3 links the Golgi network
to mTOR signaling and human cancer. Pigment Cell Melanoma Res.
22:378–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Z, Lin H, Zhao X, Liu G, Wang X, Xu
R, Chen K, Li J and Song L: Overexpression of GOLPH3 promotes
proliferation and tumorigenicity in breast cancer via suppression
of the FOXO1 transcription factor. Clin Cancer Res. 18:4059–4069.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin L, Qin Y, Jin T, Liu S, Zhang S, Shen
X and Lin Z: Significance of NQO1 overexpression for prognostic
evaluation of gastric adenocarcinoma. Exp Mol Pathol. 96:200–205.
2013. View Article : Google Scholar
|
|
15
|
Li H, Guo L, Chen SW, Zhao XH, Zhuang SM,
Wang LP, Song LB and Song M: GOLPH3 overexpression correlates with
tumor progression and poor prognosis in patients with clinically N0
oral tongue cancer. J Transl Med. 10:1682012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Xu T, Qin R, Yan Y, Chen C, Chen
Y, Yu H, Xia C, Lu Y, Ding X, Wang Y, Cai X and Chen J:
Overexpression of Golgi phos-phoprotein-3 (GOLPH3) in glioblastoma
multiforme is associated with worse prognosis. J Neurooncol.
110:195–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hua X, Yu L, Pan W, Huang X, Liao Z, Xian
Q, Fang L and Shen H: Increased expression of Golgi
phosphoprotein-3 is associated with tumor aggressiveness and poor
prognosis of prostate cancer. Diagn Pathol. 7:1272012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JH, Chen XT, Wen ZS, Zheng M, Deng
JM, Wang MZ, Lin HX, Chen K, Li J, Yun JP, Luo RZ and Song LB: High
expression of GOLPH3 in esophageal squamous cell carcinoma
correlates with poor prognosis. PLoS One. 7:e456222012. View Article : Google Scholar : PubMed/NCBI
|