Introduction
Hypoxia is considered to be a significant
microenvironmental factor in the promotion of tissue fibrosis,
which results in organ dysfunction in diseases including systemic
sclerosis, liver cirrhosis, cardiac fibrosis, idiopathic pulmonary
fibrosis and progressive kidney disease (1–4). The
development of interstitial fibrosis, which is caused by ischemia
or hypoxia, is a common complication and a leading cause of graft
failure following kidney transplantation (3,5).
Therefore, the protection of cells or tissues against fibrosis
plays an essential role in the management of diseases, including in
patients undergoing kidney transplantation.
Renal fibrosis, particularly tubulointerstitial
fibrosis, results in a final outcome of renal failure (6). Renal tubulointerstitial fibrosis
undergoes the processes of tubular atrophy, myofibroblast
accumulation and extracellular matrix (ECM) deposition, in which
fibroblasts are the primary producers of ECM components (3,7). The
process by which epithelial cells are converted into myofibroblasts
and matrix-producing fibroblasts is known as epithelial-mesenchymal
transition (EMT), which plays a key role in the progression of
renal interstitial fibrosis (8–10).
This involves the replacement of E-cadherin, which is lost
gradually, by alpha-smooth muscle actin (α-SMA), which is the main
mesenchymal marker in EMT (11,12).
With regard to the regulation of EMT, transforming growth factor
beta (TGF-β) was characterized as a key mediator (13). TGF-β1 not only stimulates the
production of collagens, fibronectin and proteoglycans by
myofibroblasts, but also triggers its own production (14,15).
It was thus speculated that therapies that targeted the TGF-β1
pathways may provide effective strategies to slow the progression
of fibrosis (3).
MicroRNAs (miRNAs), composed of ~22 nucleotides,
regulate ~60% of gene expression by targeting the 3′-untranslated
regions (3′-UTRs) to result in negative modulation of relevant mRNA
expression (16). miRNAs have a
significant effect not only on cellular biology and
pathophysiological regulatory pathways, but also on the
pathogenesis of fibrosis (17–19).
Previous articles have reported that certain miRNAs function as
regulatory factors in various fibrotic disorders; for example liver
cirrhosis, cardiac fibrosis and idiopathic pulmonary fibrosis
(20–22). In renal fibrosis, miR-200a and
miR-449a/b are characterized as anti-fibrotic factors due to their
repression of the expression of TGF-β and ECM synthesis,
respectively (23). Certain miRNAs
play pro-fibrotic roles in renal fibrosis, including miR-21 and
miR-192 (24,25). Therefore, the properties of miRNAs
involved in the regulation of renal fibrosis are unclear. It is
documented that miR-155 is abnormally expressed in renal cancer,
end-stage renal disease and renal transplantation undergoing acute
cellular rejection (26–28). Most significantly, a study by
Pottier et al reported the effect of miR-155 expression
level on lung fibrosis (29). In
accordance with these previous studies on the functions of miR-155,
interest has been aroused as to whether miR-155 is involved in
hypoxia-associated renal fibrosis.
In the current study, proximal tubule cells (HK-2
cells) were exposed to hypoxia. Then, the expression of miR-155 and
fibrosis-associated factors was analyzed. Next, we investigated the
association of miR-155 and HIF-1α to reveal the effect of hypoxia
on the expression of miR-155. Finally, the study focuses on the
mechanisms involved in the correlations between miR-155 and
fibrosis in hypoxic tubule cells.
Materials and methods
Cell culture
Human renal proximal tubule (HK-2) cells were
acquired from the American Type Culture Collection (ATCC; Manassas,
VA, USA) and cultivated in Dulbecco’s modified Eagle’s medium
supplemented with 10% fetal bovine serum at 37°C in humidified 5%
CO2. The cells were subcultured at 80% confluence by
0.05% trypsin with 0.02% EDTA. After cultivating to ~70%
confluence, cells were treated with hypoxia in Anoxomat chambers
(Mart Microbiology, Lichtenvoorde, Netherlands) for physiological
hypoxia (5% O2) or normoxia (21% O2) at 37°C
for the indicated times.
Cell treatments
miR-155 mimics (miR-mimics), anti-miR of miR-155
(anti-miR) (GMR-miR™, Shanghai, China) and miRNA control (control)
were purchased from GenePharma (GenePharma, Shanghai, China)
(30). HK-2 cells were transfected
with miR-mimics (20 nM), miRNA control or anti-miR (50 nM) using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). HIF-1α siRNA
(Santa Cruz Biotechnologies, Santa Cruz, CA, USA) was used to knock
down the HIF-1α genes (HIF-1α-si), and non-specific siRNA (Santa
Cruz Biotechnologies) was used as a negative control (si-control).
Transfection was conducted using Lipofectamine 2000 according to
the manufacturer’s instructions. After 16 h transfection or
incubation with the TGF-β type I receptor kinase inhibitor
LY2157299 (Selleckchem, Houston, TX, USA) at 5 μM, cells
were treated with hypoxia or normoxia for the indicated times.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total mRNA was extracted from cells using RNeasy
kits (Qiagen, Valencia, CA, USA). RT-qPCR was performed on an ABI
7500 with a SYBR Premix Ex Taq™ kit (Takara Bio Inc., Shiga,
Japan). GAPDH was used as the internal control. For quantitation of
miR-155, miRNA extraction was conducted using the mirVana miRNA
isolation kit (Ambion, Austin, TX, USA). The mirVana qRT-PCR miRNA
detection kit (Ambion) was used to quantify the expression level of
miR-155, and U6 small nuclear RNA was used as the internal control.
Data were normalized using the 2−ΔΔCt method for
relative quantification. The used primers were: E-cadherin:
F-TTGCAAATTCCTGCCATTC, R-GCTGGCTCAAGTCAAAGTCC; α-SMA:
F-CTGTTCCAGCCATCCTTCATC, R-GCTGGCTCAAGTCAAAGTCC; TGF-β1:
F-TGAACCGGCCTTTCCTGCTTCTCATG, F-GCGGAAGTCAATGTACAGCTGCCGC; HIF-1α:
F-ATCGCGGGGACCGATT, R-CGACGTTCAGAACTTATCTTTTTCTT; GAPDH:
F-GGAGTCAACGGATTTGGTC, R-GGAATCATTGGAACATGTAAAC.
Western blot analysis
Proteins were collected and subjected to 10%
SDS-PAGE by centrifugation at 12,000 rpm for 30 min at 4°C after
HK-2 cells were lysed. Then, proteins were isolated by
electrophoresis and transferred onto nitrocellulose membranes using
the transfer buffer. The membrane was blocked with 2% bovine serum
albumin to prevent non-specific background binding. Immunoblotting
was performed using human monoclonal E-cadherin antibody, α-SMA
antibody, TGF-β1 (Cell Signaling, Inc., Danvers, MA, USA),
anti-HIF-1α (BD Biosciences Inc., Franklin Lakes, NJ, USA) and
anti-β-actin (Sigma, St. Louis, MO, USA). Goat anti-mouse IgG or
goat anti-rabbit IgG (Pierce Biotechnology, Inc., Rockford, IL,
USA) was utilized to visualize the results, and ECL detection
systems (Supersignal West Femto, Pierce) were used for the
assay.
Statistical analysis
Independent experiments were performed at least in
triplicate. Data were normalized to the mean ± standard deviation.
Comparisons between two groups were made using Student’s t-test.
ANOVA was utilized to compare differences of multiple samples. SPSS
18.0 (SPSS, Inc., Chicago, IL, USA) was used for general
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Hypoxia induces overexpression of miR-155
and promotes fibrosis in proximal tubule cells
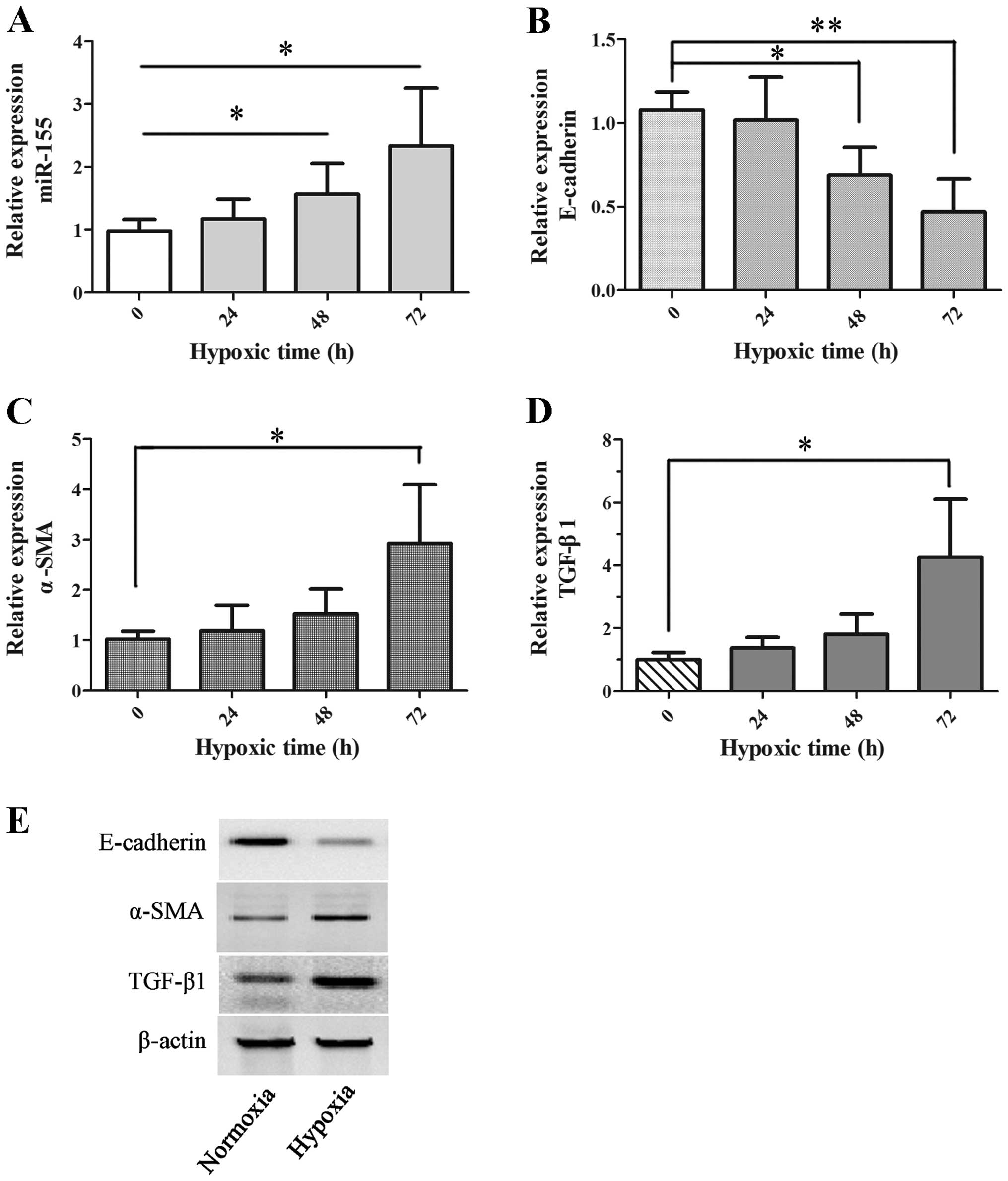
To investigate the hypoxic response of proximal
tubule cells, HK-2 cells were exposed to hypoxia (5% oxygen) for 0,
24, 48 and 72 h. The relative expression of miR-155 was assessed at
the different time points, as shown in Fig. 1A. The data reveal that the
expression of miR-155 was significantly stimulated by hypoxia in
HK-2 cells after 48 h exposure, in contrast with normoxia (P=0.033
at 48 h and P=0.012 at 72 h).
Next, to determine the effects of hypoxia on
fibrosis in proximal tubule cells, the expression levels of
E-cadherin, α-SMA and TGF-β1, which are well characterized as
biomarkers in the process of renal interstitial fibrosis, were
examined at the same time in hypoxic HK-2 cells. The expression of
E-cadherin mRNA was gradually reduced and reached a significant
difference at 48 h (P=0.027) and 72 h (P=0.009) (Fig. 1B). The expression of α-SMA and
TGF-β1 mRNA was enhanced gradually with increasing time and was
significant at 72 h for hypoxia (P=0.049 and P=0.017, respectively;
Fig. 1C and D). Similarly, α-SMA
and TGF-β1 protein expression was increased, while the expression
of E-cadherin protein was shown to be decreased in HK-2 cells on
hypoxic exposure at 72 h by western blot analysis, as shown in
Fig. 1E. All the data reveal that
hypoxia induced overexpression of miR-155 and promoted fibrosis in
proximal tubule cells.
miR-155 is modulated by HIF-1α under
hypoxia
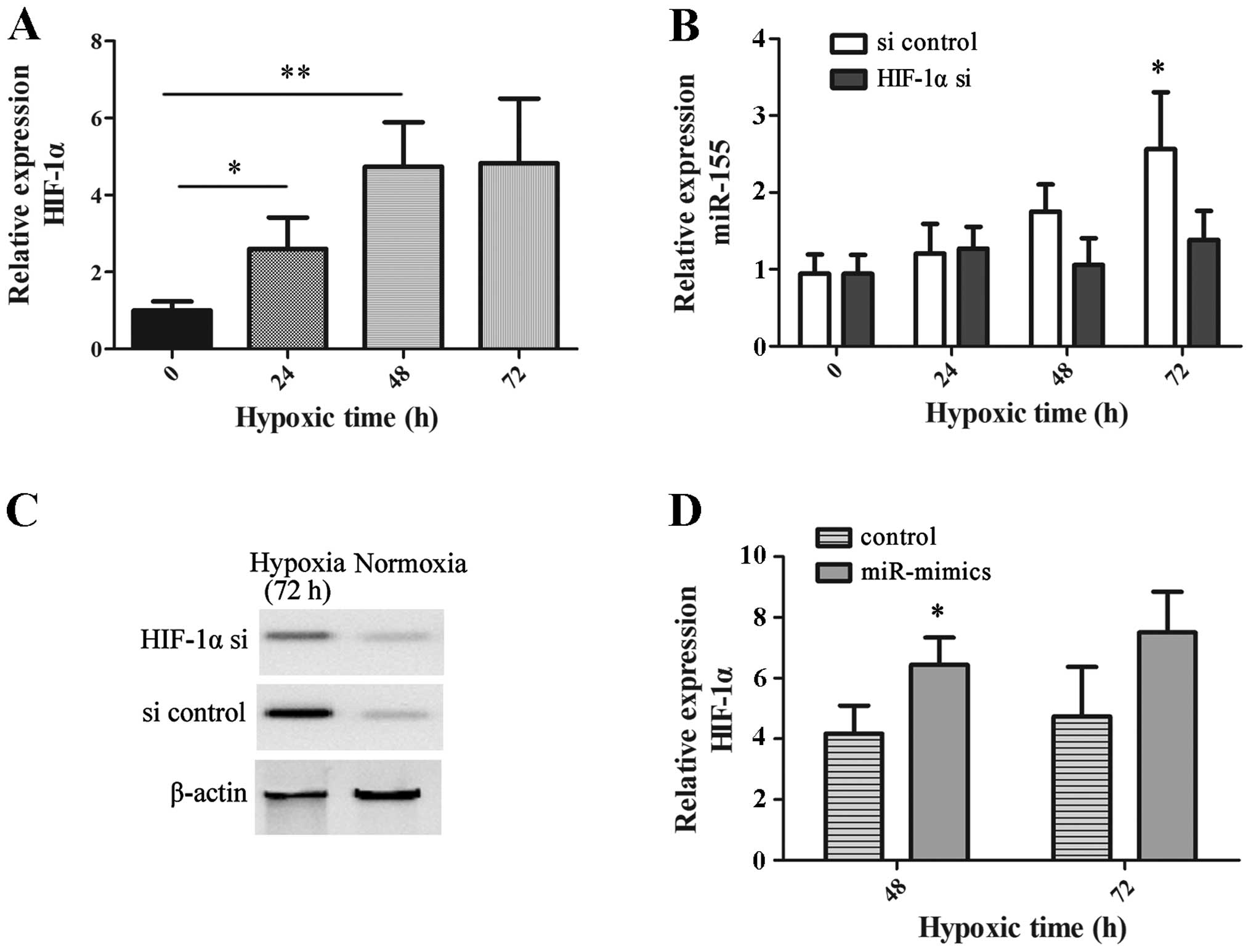
We analyzed whether the upregulated miR-155 was
associated with HIF-1α, which plays a vital role in the cellular
response to hypoxia. Significant overexpression of HIF-1α mRNA was
observed in hypoxic HK-2 cells at the different time points,
compared with normoxic HK-2 cells (0 h; Fig. 2A). Silencing HIF-1α successfully
prevented augmentation in the expression of miR-155 in a hypoxic
environment at 72 h (P=0.031). However, HIF-1α knockdown did not
change the expression of miR-155 in normoxic HK-2 cells (Fig. 2B). Fig. 2C reveals the effectiveness of
HIF-1α knockdown in HK-2 cells by western blot analysis.
In addition, miR-155 mimics were transfected into
HK-2 cells to investigate the effects of miR-155 on the expression
of HIF-1α. The expression of HIF-1α in HK-2 cells with transfection
of the miR-155 mimics was revealed to be significantly higher than
that in HK-2 cells with miR control under hypoxia at 48 h (P=0.038;
Fig. 2D). All of these findings
demonstrate that the expression of miR-155 was modulated in an
HIF-1α-dependent manner in hypoxic HK-2 cells.
Overexpression of miR-155 plays a role in
the process of fibrosis in hypoxic HK-2 cells
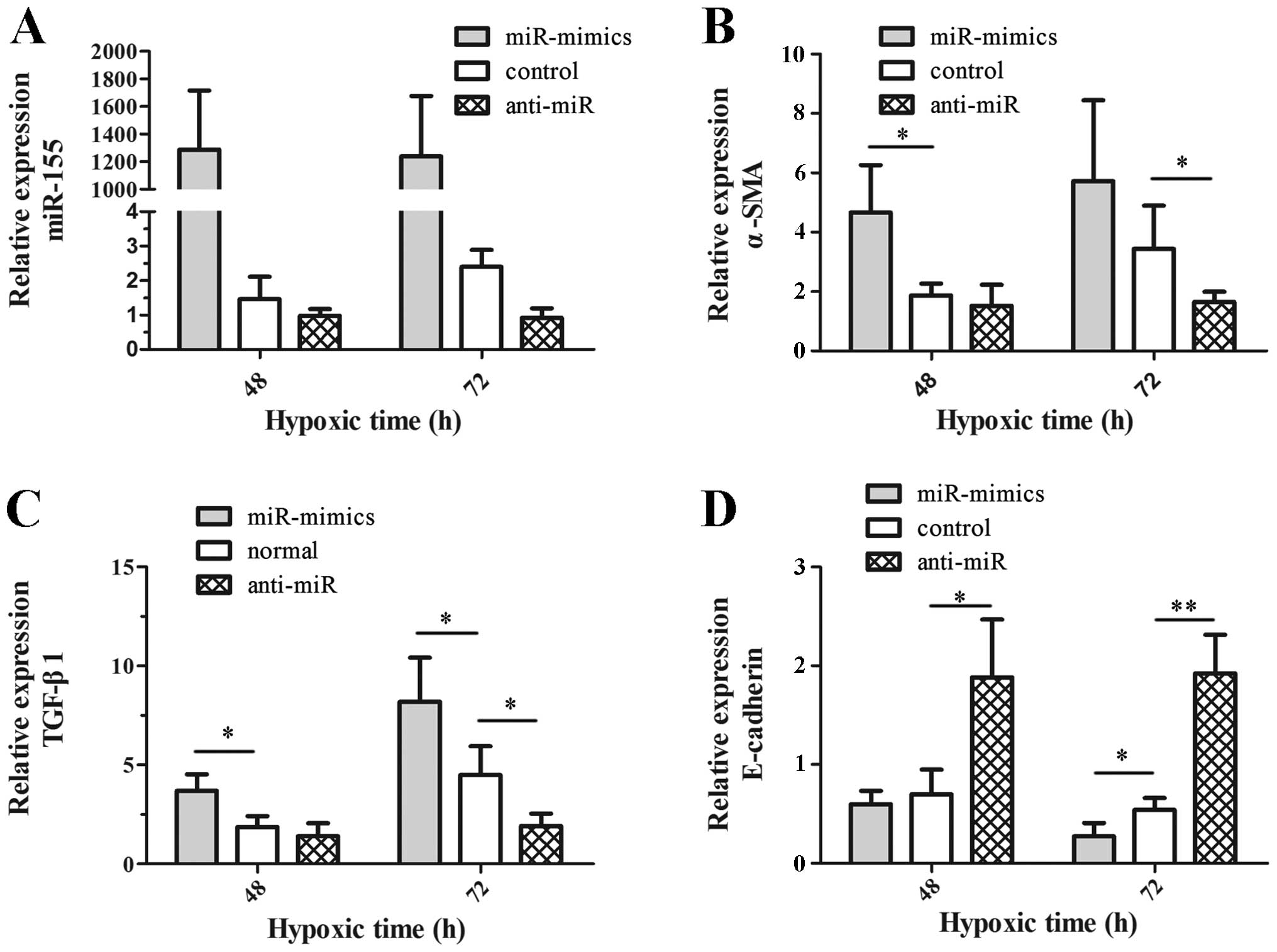
To assess the role of miR-155 in fibrosis, we
investigated the expression of E-cadherin, α-SMA and TGF-β1 mRNA in
hypoxic HK-2 cells in which miR-155 was silenced or ectopically
enhanced (anti-miR group or miR-mimics group). The expression
status of miR-155 was tested, which proved the effectiveness of
cell treatments as shown in Fig.
3A. Next, the expression of E-cadherin, α-SMA and TGF-β1 mRNA
was compared among cells with miRNA control (control group), the
anti-miR group and the miR-mimics group. The data revealed that the
expression of α-SMA (Fig. 3B) and
TGF-β1 (Fig. 3C) mRNA in the
anti-miR group was reduced significantly at 72 h (P=0.033 and
P=0.048, respectively), whereas there was significantly augmented
expression of E-cadherin mRNA at 48 h (P=0.032) and 72 h (P=0.005)
(Fig. 3D). In the miR-mimics
group, the expression of E-cadherin, α-SMA and TGF-β1 mRNA
exhibited the opposite trend to that observed in the anti-miR group
(Fig. 3B–D). All of these findings
illustrate the synergistic effects of upregulated miR-155 on
hypoxia-induced fibrosis in hypoxic HK-2 cells.
miR-155 modulates the expression of
E-cadherin and α-SMA independently of TGF-β1 in hypoxic HK-2
cells
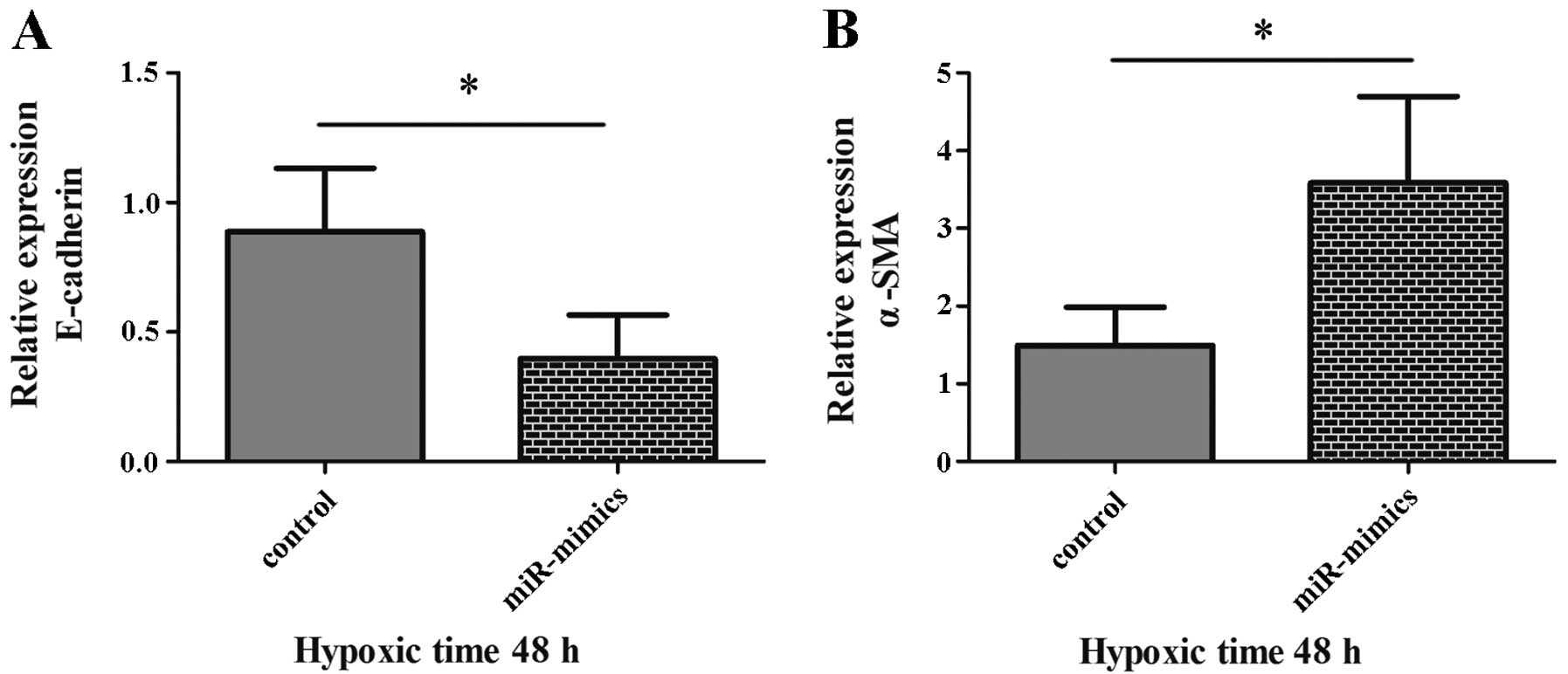
We determined whether the regulatory effects of
miR-155 on the expression of E-cadherin and α-SMA were associated
with TGF-β1, since it is a central mediator of fibrosis (14). Following treatment of cells with
miR-mimics or miR control and incubation with TGF-β type I receptor
kinase inhibitor LY2157299 for 48 h, notably decreased expression
of E-cadherin mRNA (P=0.047; Fig.
4A) and increased α-SMA (P=0.040; Fig. 4B) was observed in the miR-mimics
group (HK-2 cells in which miR-155 was ectopically enhanced),
compared with the control group. These results demonstrated that
upregulated miR-155 was able to promote the enhancement of α-SMA
expression and the reduction of E-cadherin expression, which were
regulated by TGF-β1. It also revealed that miR-155 modulated the
process of EMT independently of TGF-β1 in hypoxic HK-2 cells.
Discussion
Renal tissue hypoxia induces ECM production,
collagen deposition and fibrosis, which are common complications of
renal disease and leading causes of graft failure following kidney
transplantation (5,31). miR-155 has been shown to be highly
correlated not only with hypoxia, but also with fibrosis of the
lung and heart (29,32). Hence, we considered whether miR-155
was correlated with hypoxia-associated renal fibrosis.
In this study, we first analyzed the expression
status of miR-155 and the responsiveness of proximal tubule cells
to hypoxia (HK-2 cells). The results revealed significant
overexpression of miR-155 and dysregulation of fibrosis-associated
genes including E-cadherin, α-SMA and TGF-β1, which demonstrate the
process of tissue fibrosis (Fig.
1). All of these findings indicated that hypoxia induced the
fibrosis of HK-2 cells, and were in agreement with a review by
Singh et al concerning the regulatory functions of hypoxia
in fibrosis in acute kidney injury and chronic kidney disease
(31). Allowing for the
overexpression of miR-155, we naturally speculated that aberrant
expression of miR-155 may be of significant relevance to fibrosis
in hypoxic HK-2 cells.
HIF-1α is a key factor in modulating the cellular
response to hypoxia. Through modulation of numerous genes, HIF-1α
has been demonstrated to regulate pathological processes, including
carcinogenesis, immunity, angiogenesis, proliferation and apoptosis
(33–35). Moreover, HIF activation promotes
EMT and renal fibrogenesis (36).
It has also been reported that miR-155 induction contributes to a
negative feedback loop for the resolution of HIF-1α activity in
Caco-2 colonic epithelial cells (37). Conversely, miR-155 promotes the
activity of HIF transcription factors in breast cancer (38). Therefore, the interactions of
miR-155 and HIF-1α may be dependent on the microenvironment, and
the exact mechanism will require further investigation. In this
study, we analyzed the interaction of miR-155 and HIF-1α in hypoxic
HK-2 cells using gain-of-function and loss-of-function approaches.
It was observed that downregulation of miR-155 occurred in hypoxic
HK-2 cells with HIF-1α knockdown (Fig.
2B). In addition, HIF-1α underwent hyper-expression due to
extrinsic upregulated miR-155 in hypoxic HK-2 cells (Fig. 2D). All these results further
indicated that miR-155 is positively modulated by HIF-1α under
hypoxia in HK-2 cells.
TGF-β is a pro-fibrotic cytokine which is not only
widely linked with the expression of ECM, but also plays central
roles in EMT (2). The regulatory
effects of TGF-β1 on EMT and renal fibrosis were confirmed
according to gain-of-function and loss-of-function approaches using
neutralizing TGF-β1 antibodies or ameliorating renal fibrosis in
vivo and in vitro (39). α-SMA was characterized as a
biomarker for the identification of EMT and myofibroblast
differentiation in tubulointerstitial fibrosis. EMT is a
progressive variation in which epithelial cell adhesion (induced by
E-cadherin) decreases gradually and α-SMA increases simultaneously
(11,12,40).
In our study, a positive effect of miR-155 on TGF-β1 was observed
(Fig. 3C). We also detected an
increase of α-SMA (Fig. 3B) mRNA
and a significant reduction of E-cadherin mRNA (Fig. 3D) in the miR-mimics group. These
findings revealed that miR-155 has facilitative functions on the
expression of TGF-β1 and the process of EMT. Allowing for the
fundamental roles of TGF-β1 in EMT, we aimed to clarify whether the
pro-fibrotic functions of miR-155 were due to the regulation of
TGF-β1, which subsequently took effect on fibrosis, or the
regulation of both TGF-β1 and EMT. Notably, our results revealed
that miR-155 modulated the expression of E-cadherin and α-SMA
independently of TGF-β1 in hypoxic HK-2 cells (Fig. 4). In other words, miR-155 is
capable of modulating both TGF-β1 and the process of EMT.
In summary, these results demonstrate that
hypoxia-induced miR-155 is a pro-fibrotic cytokine which is
positively regulated by HIF-1α in proximal tubule cells. In
addition, the data also demonstrates that miR-155 promotes the
fibrosis of proximal tubule cells by regulating both TGF-β1 and the
process of EMT. The results of this study may provide a new
therapeutic target for prohibiting the fibrosis of hypoxic proximal
tubule cells.
Acknowledgments
This study was funded by the 181st hospital of
Chinese People’s Liberation Army.
References
|
1
|
Higgins DF, Kimura K, Bernhardt, et al:
Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of
epithelial-to-mesen-chymal transition. J Clin Invest.
117:3810–3820. 2007.PubMed/NCBI
|
|
2
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
3
|
Wynn TA: Common and unique mechanisms
regulate fibrosis in various fibroproliferative diseases. J Clin
Invest. 117:524–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romano E, Manetti M, Guiducci S,
Ceccarelli C, Allanore Y and Matucci-Cerinic M: The genetics of
systemic sclerosis: an update. Clin Exp Rheumatol. 29:S75–S86.
2011.PubMed/NCBI
|
|
5
|
Zell S, Schmitt R, Witting S, Kreipe HH,
Hussein K and Becker JU: Hypoxia Induces Mesenchymal Gene
Expression in Renal Tubular Epithelial Cells: An in vitro Model of
Kidney Transplant Fibrosis. Nephron Extra. 3:50–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 7:684–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Sun Y, Liu F, et al: Norcantharidin
inhibits renal interstitial fibrosis by blocking the tubular
epithelial-mesenchymal transition. PLoS One. 8:e663562013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kriz W, Kaissling B and Le Hir M:
Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or
fantasy? J Clin Invest. 121:468–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnes JL and Glass WF II: Renal
interstitial fibrosis: a critical evaluation of the origin of
myofibroblasts. Contrib Nephrol. 169:73–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar
|
|
12
|
Nisticò P, Bissell MJ and Radisky DC:
Epithelial-mesenchymal transition: general principles and
pathological relevance with special emphasis on the role of matrix
metalloproteinases. Cold Spring Harb Perspect Biol. 4:a0119082012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
López-Hernández FJ and López-Novoa JM:
Role of TGF-β in chronic kidney disease: an integration of tubular,
glomerular and vascular effects. Cell Tissue Res. 347:141–154.
2012. View Article : Google Scholar
|
|
14
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berk BC, Fujiwara K and Lehoux S: ECM
remodeling in hypertensive heart disease. J Clin Invest.
117:568–575. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chau BN and Brenner DA: What goes up must
come down: the emerging role of microRNA in fibrosis. Hepatology.
53:4–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinzani M, Rosselli M and Zuckermann M:
Liver cirrhosis. Best Pract Res Clin Gastroenterol. 25:281–290.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Koh P, Winbanks C, et al: miR-200a
prevents renal fibrogenesis through repression of TGF-β2
expression. Diabetes. 60:280–287. 2011. View Article : Google Scholar :
|
|
24
|
Zhong X, Chung AC, Chen HY, Meng XM and
Lan HY: Smad3-mediated upregulation of miR-21 promotes renal
fibrosis. J Am Soc Nephrol. 22:1668–1681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kato M, Zhang J, Wang M, et al:
MicroRNA-192 in diabetic kidney glomeruli and its function in
TGF-β-induced collagen expression via inhibition of E-box
repressors. Proc Natl Acad Sci USA. 104:3432–3437. 2007. View Article : Google Scholar
|
|
26
|
Neal CS, Michael MZ, Rawlings LH, Van der
Hoek MB and Gleadle JM: The VHL-dependent regulation of microRNAs
in renal cancer. BMC Med. 8:642010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Peng W, Shen X, Huang Y, Ouyang X
and Dai Y: Circulating levels of inflammation-associated miR-155
and endothelial-enriched miR-126 in patients with end-stage renal
disease. Braz J Med Biol Res. 45:1308–1314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anglicheau D, Sharma VK, Ding R, et al:
MicroRNA expression profiles predictive of human renal allograft
status. Proc Natl Acad Sci USA. 106:5330–5335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pottier N, Maurin T, Chevalier B, et al:
Identification of keratinocyte growth factor as a target of
microRNA-155 in lung fibroblasts: implication in
epithelial-mesenchymal interactions. PLoS One. 4:e67182009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang P, Hou J, Lin L, et al: Inducible
microRNA-155 feedback promotes type I IFN signaling in antiviral
innate immunity by targeting suppressor of cytokine signaling 1. J
Immunol. 185:6226–6233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh P, Ricksten SE, Bragadottir G,
Redfors B and Nordquist L: Renal oxygenation and haemodynamics in
acute kidney injury and chronic kidney disease. Clin Exp Pharmacol
Physiol. 40:138–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kishore R, Verma SK, Mackie AR, et al:
Bone marrow progenitor cell therapy-mediated paracrine regulation
of cardiac miRNA-155 modulates fibrotic response in diabetic
hearts. PLoS One. 8:e601612013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Greer SN, Metcalf JL, Wang Y and Ohh M:
The updated biology of hypoxia-inducible factor. EMBO J.
31:2448–2460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Higgins DF, Kimura K, Iwano M and Haase
VH: Hypoxia-inducible factor signaling in the development of tissue
fibrosis. Cell Cycle. 7:1128–1132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bruning U, Cerone L, Neufeld Z, et al:
MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha
activity during prolonged hypoxia. Mol Cell Biol. 31:4087–4096.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Czyzyk-Krzeska MF and Zhang X: MiR-155 at
the heart of oncogenic pathways. Oncogene. 33:677–678. 2014.
View Article : Google Scholar :
|
|
39
|
Hills CE and Squires PE: The role of
TGF-beta and epithelial-to mesenchymal transition in diabetic
nephropathy. Cytokine Growth Factor Rev. 22:131–139.
2011.PubMed/NCBI
|
|
40
|
Kage H and Borok Z: EMT and interstitial
lung disease: a mysterious relationship. Curr Opin Pulm Med.
18:517–523. 2012.PubMed/NCBI
|