Introduction
The second mitochondria-derived activator of caspase
(Smac) protein is a novel protein, which is involved in the
mitochondrial regulation of apoptosis (1,2). It
has been observed that the expression of Smac is reduced in various
types of malignant tissue compared with their normal tissue
counterparts, which suggests that it may function as a novel tumor
suppressor protein (3,4). Several previous studies have
demonstrated that Smac promotes apoptosis by binding to the
inhibitor of apoptosis proteins (IAPs), leading to release of
caspases from the IAPs and to the activation of the caspase cascade
(5–7).
Previous epidemiological, preclinical and clinical
studies have suggested that indomethacin and other non-steroidal
anti-inflammatory drugs (NSAIDs) may possess anticancer activities
(8). A study by Kohli et al
(9) observed that indomethacin
promoted apoptosis in the HCT116 colon cancer cell line and this
effect was attenuated by knockout of the Smac gene, which suggested
that Smac is important in the apoptosis induced by NSAIDs. In
addition, a study by Bank et al (10) demonstrated that Smac sensitizes
NSAID-induced apoptosis by promoting the activation of caspase-3
and the release of cytochrome c. This result further
elucidated the mechanism by which Smac enhances NSAID-induced
apoptosis in colon carcinoma cells.
Esophageal cancer is a common type of human upper
gastrointestinal carcinoma and, in 2008, a total of 400,000
individuals succumbed to mortality, worldwide and 480,000 new cases
were diagnosed (11). Previous
clinical trials have indicated that NSAIDs are able to
significantly reduce the risk of esophageal cancer (12,13).
The effects of indomethacin on the growth, apoptosis and expression
of Smac in esophageal cancer cells remain to be fully elucidated.
In the present study, indomethacin-mediated apoptosis was
investigated in EC109 esophageal cancer cells by overexpression or
knockdown of Smac, to examine the mechanism by which NSAIDs control
the growth and survival of esophageal carcinoma cells.
Materials and methods
Cell culture and cell transfection
The human EC109 esophageal cancer cells were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and maintained at 37°C and 5% CO2 in RPMI 1640
medium (HyClone Laboratories, Inc., Logan, UT, USA) supplemented
with 10% fetal bovine serum (GE Healthcare Life Sciences, Logan,
UT, USA), 100 U/ml penicillin and 100 g/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA). The EC109 cells were
transfected with Lipofectamine™ 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer’s instructions,
during the logarithmic growth phase. Subsequent experiments were
performed 48-h after transfection. The pcDNA3.1-Smac vector was
constructed in our previous work (14). In brief, Smac was amplified from
the mRNA of human testis tissue by reverse transcription polymerase
chain reaction (RT and PCR kits, Takara Bio, Inc., Dalian, China;
S1000 Thermal cyclers, Bio-Rad Laboratories, Inc., Hercules, CA,
USA) using the following primers: Forward
5′-CGGGATCCCACAATGGCGGCTCTGA-3′ and reverse
5′-CCCAAGCTTGGCCCTCAATCCTCACGC-3′. Then, it was digested by
BamHI and HindIII (Takara Bio, Inc.) and inserted
into pcDNA3.1 (Invitrogen Life Technologies) by T4 DNA ligase
(Invitrogen Life Technologies). The small interference RNA (siRNA)
and the control fragment for Smac RNAi were designed and
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
MTT assay
Cell viability was assessed using a methyl-thiazol
tetrazolium (MTT) assay. Exponentially growing EC109 cells were
seeded into 96-well plates at a density of 1×103
cells/well and incubated for 24 h at 37°C. Different concentrations
(0, 50, 100, 200, 400 and 800 μM) of indomethacin
(Sigma-Aldrich) were then added to the wells and incubated at 37°C
for 24, 48 and 72 h. A total of 10 μl sterile MTT (5 mg/ml;
Sigma-Aldrich) was added to each well. Following a further
incubation at 37°C for 4 h, the reaction was stopped by the
addition of 150 μl dimethyl sulfoxide. After 10 min, the
formazan production was determined by measurement of the
spectrometric absorbance at a wavelength of 490 nm using an enzyme
immunoassay analyzer (xMark™ Microplate Absorbance
spectrophotometer; Bio-Rad Laboratories, Inc.).
Flow cytometry assay
The cells were plated into 24-well plates at a
density of 1×105 cells/well and transfected with either
the pcDNA3.1/pcDNA3.1-Smac or siRNA/control vectors using
Lipofectamine™ 2000. After 48 h, indomethacin was added to a final
concentration of 100 μM (overexpression group) and 400
μM (RNA interference group). The following day, the cells
were trypsinized (Sigma-Aldrich) and incubated with 5 μl
propidium iodide (Invitrogen Life Technologies) and 5 μl
annexin V-fluorescein isothiocyanate (FITC; Invitrogen Life
Technologies) for 15 min. Samples were then analyzed for apoptosis
using a FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Western blotting
Following indomethacin treatment, the EC109 cells
were transferred into precooled lysis buffer [50 mM Tris-Cl (pH
7.5), 150 mM NaCl, 1 mM EDTA (pH 8.0), 1% Triton X-100 and protease
inhibitor cocktail; Roche Molecular Biochemicals, Indianapolis, IN,
USA] and incubated for 30 min on ice. Proteins from different
samples (30 μg protein/sample) were separated on 12%
SDS-PAGE gels (Invitrogen Life Technologies) and transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA) by blotting. Subsequent to treatment with 5% bovine serum
albumin (Shanghai Bioleaf Biotech Co., Ltd, Shanghai, China) at
room temperature (25°C) for 1 h, the membranes were incubated with
the primary antibody overnight at 4°C, incubated with horseradish
peroxidase-labeled goat anti-rabbit immunoglobulin G antibody
(1:5,000, sc-45101; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) for 1 h at 25°C, and developed using electrochemiluminescence
detection (ChemiDoc MP System; Bio-Rad Laboratories, Inc.). For the
western blot analysis, the following primary antibodies were used:
Rabbit monoclonal anti-Smac (1:1,000; 1012-1; Epitomics,
Burlingame, CA, USA), rabbit polyclonal anti-Tubulin (1:1,000;
10068-1-AP; ProteinTech Group, Inc., Chicago, IL, USA), rabbit
polyclonal anti-GAPDH (1:5,000; 10494-1-AP; ProteinTech Group,
Inc.), rabbit monoclonal anti-cytochrome c oxidase subunit
IV (COX IV; 1:1,000; 4850; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit polyclonal anti-caspase-3 (1:1,000;
ab2302; Abcam, Cambridge, MA, USA) and rabbit monoclonal
anti-pro-caspase-3 (1:1,000; ab32499; Abcam). Tubulin, COX IV and
GAPDH were used as loading controls western blot analysis of
cytosolic, mitochondria and total protein levels, respectively.
Results
Indomethacin reduces the viability of the
EC109 cells
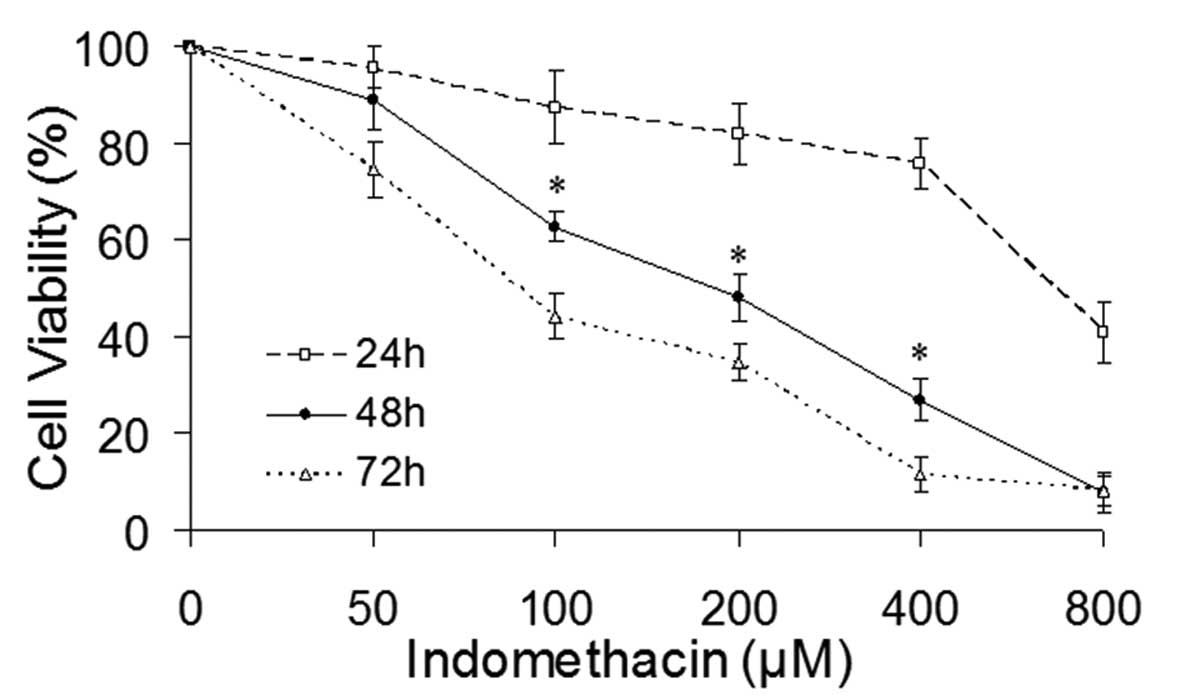
In order to examine the effect of indomethacin on
the EC109 cells, an MTT assay was used initially to investigate the
viability of EC109 cells treated with a range of concentrations of
indomethacin (0, 50, 100, 200, 400 and 800 μM) for 24, 48
and 72 h. Indomethacin treatment resulted in a gradual reduction of
the viability of the EC109 cells as the exposure duration and drug
concentration increased, which indicated that the inhibitory effect
of indomethacin on the EC109 cell survival was dose- and
time-dependent (Fig. 1). No
significant change was observed in the viability of the EC109 cells
following indomethacin treatment, for 24 h while prolonged exposure
of the drug for 72 h resulted in significant cell death. Treatment
with indomethacin for 48 h resulted in a range of EC109 cell
viabilities at different concentrations, between 73.58% (50
μM) and 6.57% (800 μM), with a linear correlation
between the drug concentration and cell viability (Fig. 1).
Indomethacin treatment releases Smac from
mitochondria into the cytosol and activated caspase-3
The EC109 cells were treated with 200 μM
indomethacin for 0, 24 or 48 h and the expression levels of Smac in
the mitochondria and cytosol were analyzed by western blotting. In
the absence of indomethacin, Smac was localized to the
mitochondria, however, following indomethacin stimulation, Smac was
released into the cytosol. The increase in cytosolic levels of Smac
correlated with the activation of caspase-3 during treatment of
EC109 cells with indomethacin (Fig.
2). In addition, the release of Smac and the activation of
caspase-3 were prominent following treatment of indomethacin for 48
h. Therefore, a treatment duration of 48 h was selected for the
subsequent experiments.
Smac knockdown inhibits
indomethacin-induced apoptosis
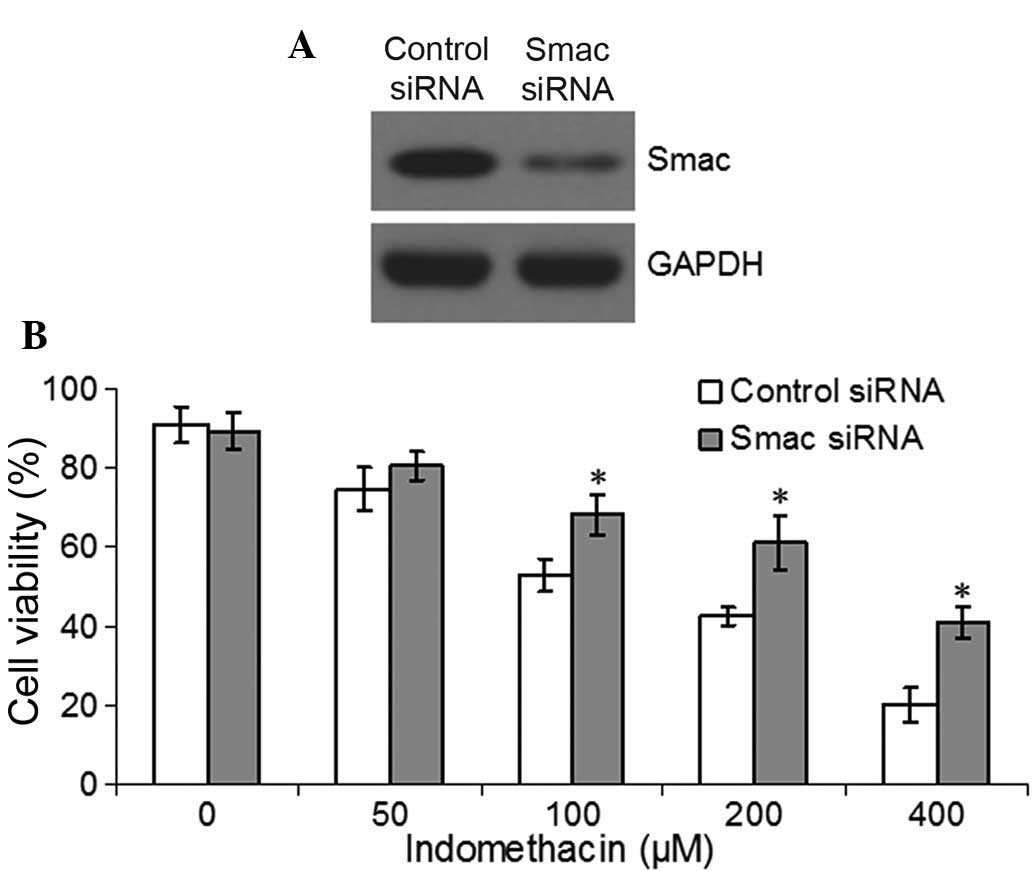
To further detect the function of Smac in the EC109
cells treated with indomethacin, siRNA of Smac or its control
segment was transfected into the EC109 cells for 48 h. Western
blotting confirmed that the expression of Smac in the
Smac-knockdown cells was reduced compared with the control cells
(Fig. 3A). The Smac-knockdown
EC109 cells and control cells were then treated with different
concentrations of indomethacin for 48 h and the cell viability was
assessed by MTT assay. The results identified that the viability of
the Smac-knockdown cells was significantly higher compared with the
control cells following treatment with 100, 200 or 400 μM
indomethacin (P<0.05, Fig.
3B).
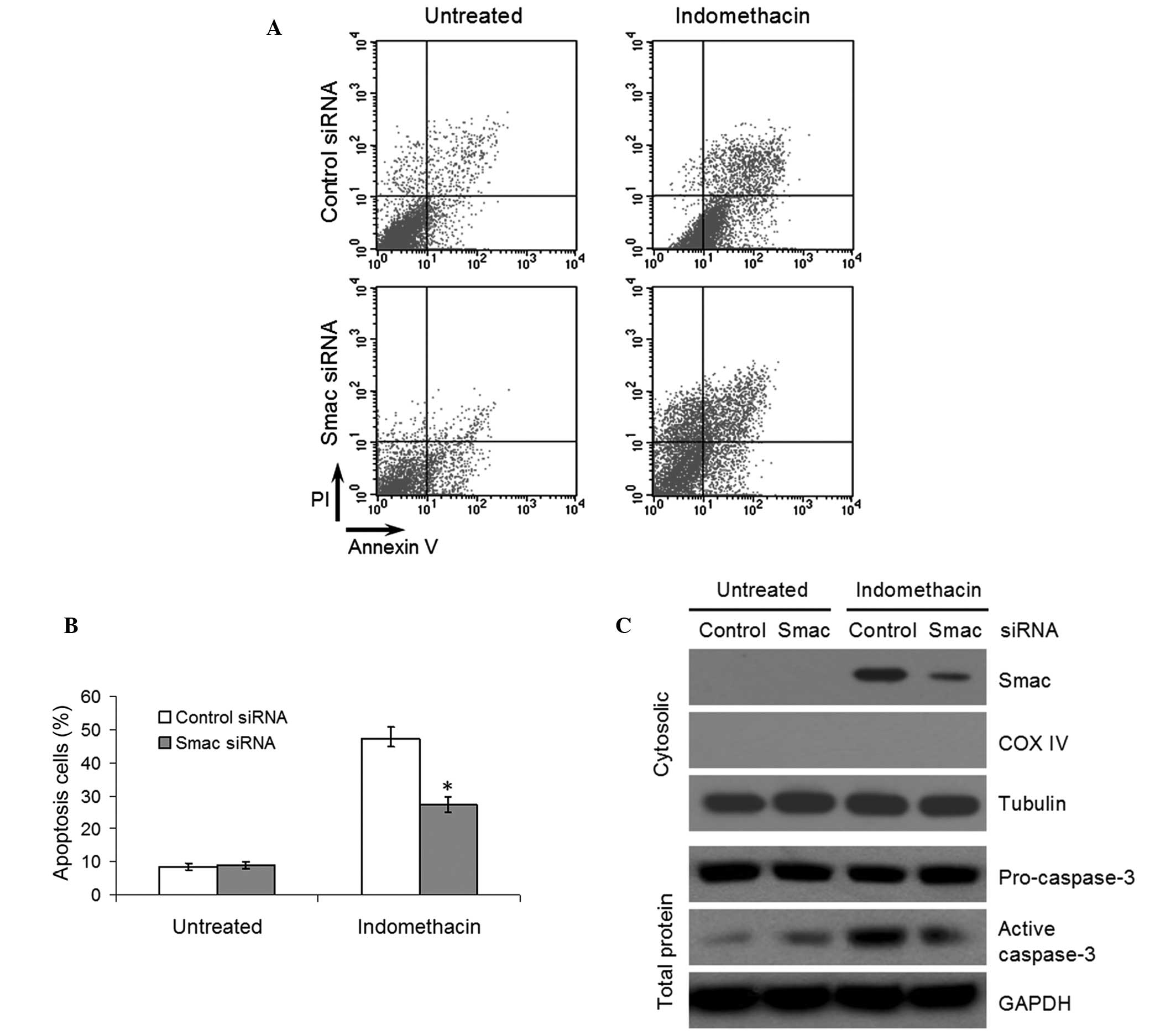
Following treatment with 400 μM indomethacin
for 48 h, the Smac-knockdown and control cells were stained with
propidium iodide and annexin V-FITC to assess the induction of
apoptosis by flow cytometry. In the indomethacin-treated cultures,
the number of apoptotic EC109 cells in the Smac-knockdown group was
significantly lower compared with the control group (P<0.05,
Fig. 4A and B). Furthermore,
western blotting demonstrated that the expression of Smac in the
cytosol and the activity of caspase-3 were reduced in the
Smac-knockdown cells treated with indomethacin, which indicated
that knockdown of Smac inhibited the activation of caspase-3,
resulting in a reduction in indomethacin-induced apoptosis
(Fig. 4C).
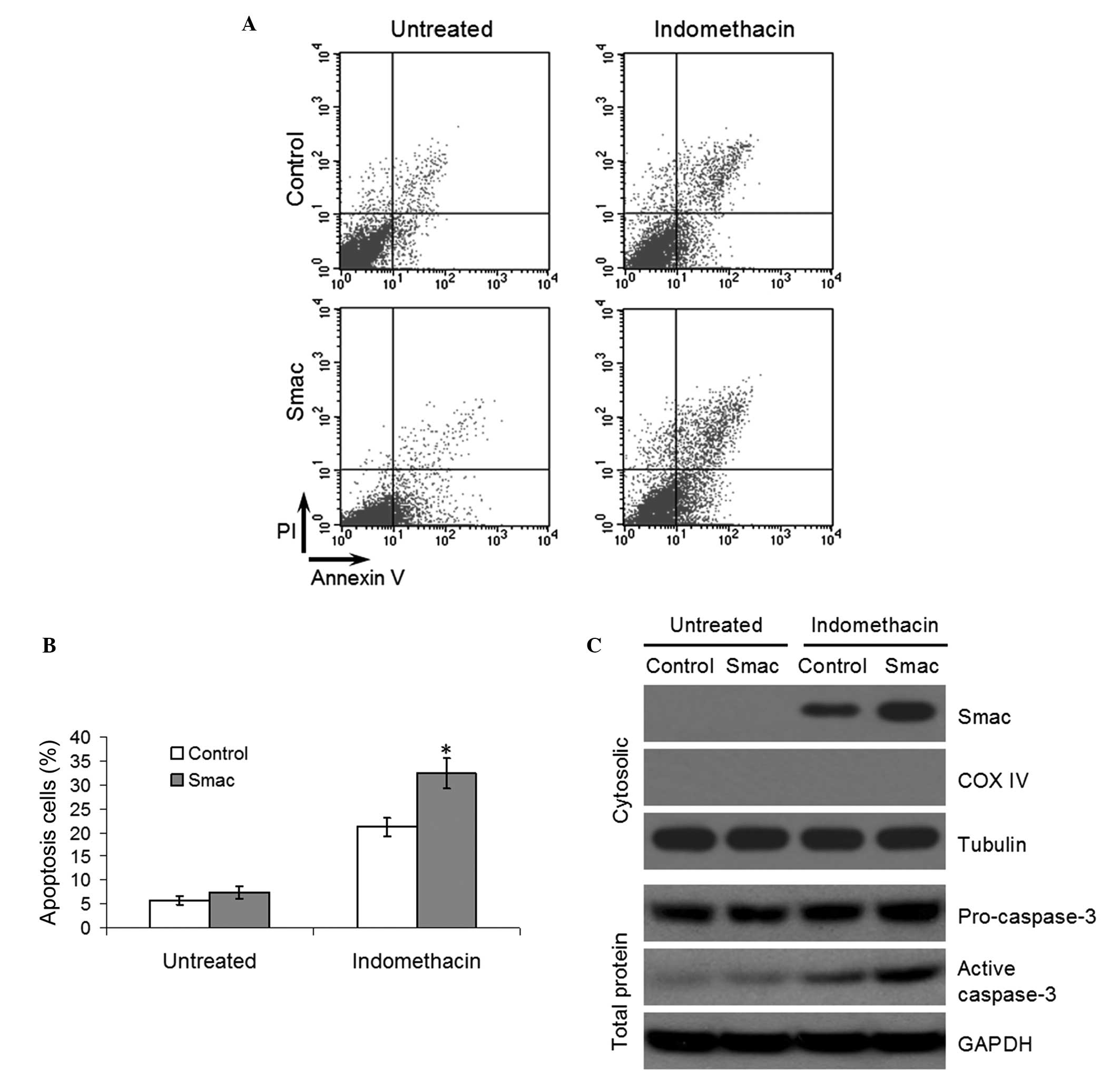
Overexpression of Smac enhances
indomethacin-induced apoptosis
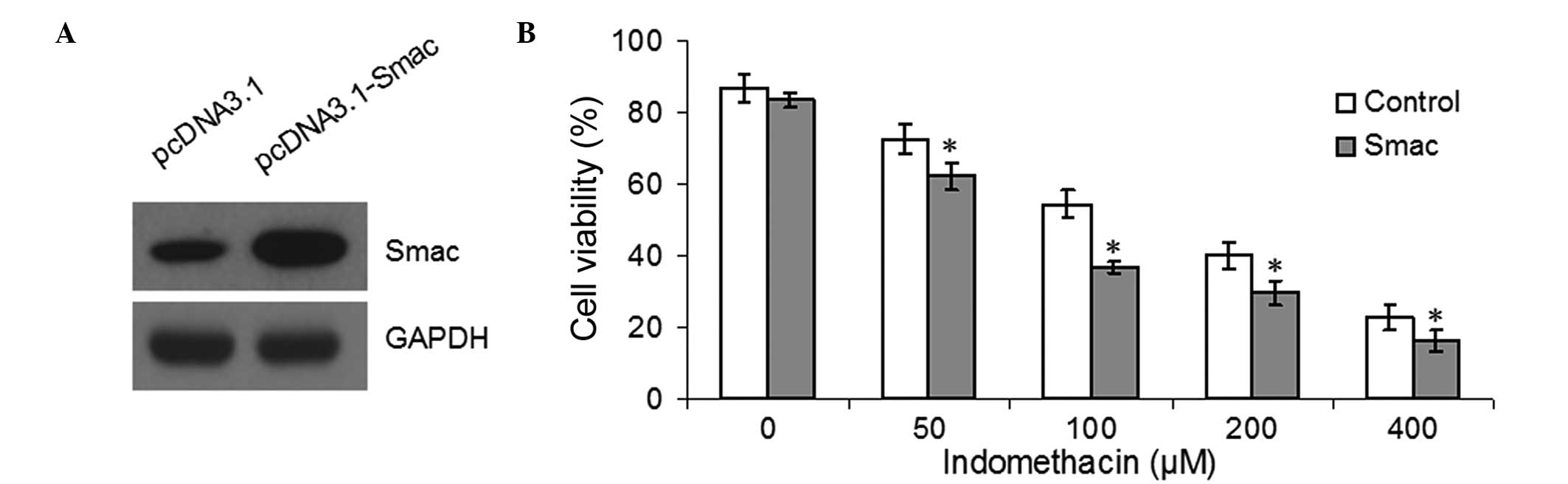
The EC109 cells were transfected with either
pcDNA3.1 or pcDNA3.1-Smac for 48 h and western blotting confirmed
that the overexpression of Smac was successful (Fig. 5A). Tthe cell viability was detected
by MTT following treatment with different concentrations of
indomethacin. Overexpression of Smac was found to reduce the
viability of EC109 following treatment with 50, 100, 200 or 400
μM indomethacin (P<0.05, Fig. 5B). Furthermore, flow cytometric
analysis indicated that the apoptotic rates of the
Smac-overexpressed EC109 cells were significantly higher compared
with the control group following treatment with 100 μM
indomethacin (P<0.05, Fig. 6A
and B). Finally, western blotting demonstrated that the increased
apoptotic rate in the Smac-overexpressing EC109 cells was due to
increased levels of cytosolic Smac and caspase-3 activation
(Fig. 6C).
Discussion
NSAIDs are a group of drugs with antipyretic,
analgesic, anti-inflammatory and anti-rheumatic properties
(15). In addition, previous
epidemiological surveys and preliminary clinical studies have
indicated that the long-term use of NSAIDs may reduce the risk of
colon, stomach, breast, prostate and lung cancer and other types of
malignant tumor (16–20). Several studies have demonstrated
that NSAIDs may inhibit tumor growth by inhibiting the expression
and activity of cyclooxygenase (COX), inducing apoptosis and
suppressing angiogenesis (21,22).
Kohli et al (9) reported
that the NSAID-induced apoptosis in colon cancer cells was
dependent on the pro-apoptotic factor, Smac, as knockout or RNA
interference of the Smac gene in colon cancer cells significantly
reduced the apoptosis induced by NSAIDs. Increasing evidence
suggests that the use of NSAIDs also significantly reduces the risk
of esophageal cancer, although the mechanism by which NSAIDs
inhibit esophageal cancer growth remain to be elucidated (23,24).
Indomethacin is a commonly used NSAID. It has been
observed to induce the apoptosis of lung, head and neck cancer
cells (25,26). In the present study, different
concentrations of indomethacin were used to treat the EC109 cells.
The cell viability and cell growth were significantly inhibited by
indomethacin in a dose- and time-dependent manner. Subsequently,
western blot analysis revealed that Smac was released into the
cytosol and caspase-3 was activated in the EC109 cells treated with
indomethacin. Thus, it was hypothesized that indomethacin induced
apoptosis in the EC109 cells by activating the Smac-dependent
apoptotic pathway. A previous study demonstrated that the release
of Smac and cytochrome c occurred during sulindac-induced
apoptosis (10). Our previous
study described a similar function of Smac in cisplatin-induced
apoptosis, in which Smac led to the activation of caspase-3 and -9
in lung cancercells (27).
Smac is an important protein in the mitochondrial
apoptotic pathway. Previous studies have revealed a reduction or
loss in the expression of Smac in various tumor tissues compared
with their normal tissue counterparts, suggesting that Smac
functions as a tumor suppressor gene and its abnormally low level
of expression is associated with tumorigenesis (1,3,4). In
addition, Smac also inhibits tumor growth by promoting apoptosis
induced by the tumor necrosis factor-related apoptosis-inducing
ligand, enhancing antitumor immune response, inhibiting the cell
cycle and sensitizing tumor cells to radiotherapy or chemotherapy
(28,29). Reduced expression levels of Smac
have been observed in esophageal tumor cell lines and in tumor
specimens from patients with esophageal cancer (30). A lower levels of Smac expression
has been associated with a reduced sensitivity to chemotherapy in
the treatment of esophageal cancer (30). It has also been reported that the
overexpression of Smac sensitizes esophageal cancer cells to
cisplatin chemotherapy (31).
In the present study, the expression of Smac was
increased and decreased in order to further investigate the role of
Smac in the indomethacin-induced apoptosis of EC109 cells. The
results revealed that knockdown of Smac inhibited the
indomethacin-induced apoptosis in the EC109 cells, which were
transfected with Smac siRNA. Western blot analysis demonstrated
that indomethacin treatment of Smac-knockdown EC109 cells reduced
the cytosolic expression of Smac and the activation of caspase-3.
These results suggested that the translocation of Smac from the
mitochondria to the cytosol was important in promoting the
activation of caspase-3 and the indomethacin-induced apoptosis. To
further investigate the effects of indomethacin-induced apoptosis,
the apoptotic rates and activation of caspase-3 were measured
following overexpression of Smac in the EC109 cells. The results
were consistent with those of the Smac-knockdown experiments,
indicating that indomethacin-induced apoptosis is dependent on and
regulated by Smac and caspase-3. These observations regarding the
role of Smac in indomethacin-induced apoptosis in EC109 cells
provides a novel treatment strategy for esophageal cancer.
At present, Smac mimetics, which are composed of the
last four-eight N-terminal residues of Smac, have been successfully
applied in cancer treatment in the laboratory, and are being
assessed in early clinical studies (32,33).
Smac mimetics have also been combined with several chemotherapeutic
drugs to enhance the efficacy of chemotherapy and to overcome drug
resistance (34–36). However, at present, few studies
have been performed on the combined use of Smac peptides and
NSAIDs. NSAIDs are inexpensive and relatively non-toxic compared
with conventional chemotherapeutic drugs (37). Whether the combination of NSAIDs
and Smac peptides is more effective compared with either alone in
inducing apoptosis in esophageal cancer cells, with or without low
dose chemotherapeutic agents, remains to be elucidated and is of
interest.
Acknowledgments
The present study was supported by the Shannxi
Province Science and Technology Fund (grant no. 2010K01-133) and
the Chinese National Natural Science Foundation (grant nos.
81272418 and 81402506).
References
|
1
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verhagen AM, Ekert PG, Pakusch M, et al:
Identification of DIABLO, a mammalian protein that promotes
apoptosis by binding to and antagonizing IAP proteins. Cell.
102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qin S, Yang C, Li S, Xu C, Zhao Y and Ren
H: Smac: Its role in apoptosis induction and use in lung cancer
diagnosis and treatment. Cancer Lett. 318:9–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinez-Ruiz G, Maldonado V,
Ceballos-Cancino G, Grajeda JP and Melendez-Zajgla J: Role of
Smac/DIABLO in cancer progression. J Exp Clin Cancer Res.
27:482008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Srinivasula SM, Hegde R, Saleh A, et al: A
conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO
regulates caspase activity and apoptosis. Nature. 410:112–116.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu WW, Liu Y, Liang S, Wu JH, Wang ZC and
Gong SL: Hypoxia- and radiation-induced overexpression of Smac by
an adenoviral vector and its effects on cell cycle and apoptosis in
MDA-MB-231 human breast cancer cells. Exp Ther Med. 6:1560–1564.
2013.PubMed/NCBI
|
|
7
|
Liu BH, Chen L, Li SR, Wang ZX and Cheng
WG: Smac/DIABLO regulates the apoptosis of hypertrophic scar
fibroblasts. Int J Mol Med. 32:615–622. 2013.PubMed/NCBI
|
|
8
|
Thun MJ, Jacobs EJ and Patrono C: The role
of aspirin in cancer prevention. Nat Rev Clin Oncol. 9:259–267.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohli M, Yu J, Seaman C, et al:
SMAC/Diablo-dependent apoptosis induced by nonsteroidal anti
inflammatory drugs (NSAIDs) in colon cancer cells. Proc Natl Acad
Sci USA. 101:16897–16902. 2004. View Article : Google Scholar
|
|
10
|
Bank A, Wang P, Du C, Yu J and Zhang L:
SMAC mimetics sensitize nonsteroidal anti-inflammatory drug-induced
apoptosis by promoting caspase-3-mediated cytochrome c release.
Cancer Res. 68:276–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bardou M, Barkun AN, Ghosn J, Hudson M and
Rahme E: Effect of chronic intake of NSAIDs and cyclooxygenase
2-selective inhibitors on esophageal cancer incidence. Clin
Gastroenterol Hepatol. 2:880–887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corley DA, Kerlikowske K, Verma R and
Buffler P: Protective association of aspirin/NSAIDs and esophageal
cancer: a systematic review and meta-analysis. Gastroenterology.
124:47–56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin SD, Ren H, Li XJ, et al: Construction
and expression of eukaryotic expression plasmid pcDNA3.1-smac. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 27:146–149. 2011.In Chinese.
PubMed/NCBI
|
|
15
|
Gurpinar E, Grizzle WE and Piazza GA:
NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res.
20:1104–1113. 2014. View Article : Google Scholar :
|
|
16
|
Antonakopoulos N and Karamanolis DG: The
role of NSAIDS in colon cancer prevention. Hepatogastroenterology.
54:1694–1700. 2007.PubMed/NCBI
|
|
17
|
Zhang YJ, Dai Q, Wu SM, et al:
Susceptibility for NSAIDs-induced apoptosis correlates to p53 gene
status in gastric cancer cells. Cancer Invest. 26:868–877. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brasky TM, Bonner MR, Moysich KB, et al:
Non-steroidal anti-inflammatory drugs (NSAIDs) and breast cancer
risk: differences by molecular subtype. Cancer Causes Control.
22:965–975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng I, Liu X, Plummer SJ, Krumroy LM,
Casey G and Witte JS: COX2 genetic variation, NSAIDs, and advanced
prostate cancer risk. Br J Cancer. 97:557–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skriver MV, Nørgaard M, Poulsen AH, et al:
Use of nonaspirin NSAIDs and risk of lung cancer. Int J Cancer.
117:873–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jana NR: NSAIDs and apoptosis. Cell Mol
Life Sci. 65:1295–1301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tarnawski AS and Jones MK: Inhibition of
angiogenesis by NSAIDs: molecular mechanisms and clinical
implications. J Mol Med (Berl). 81:627–636. 2003. View Article : Google Scholar
|
|
23
|
Sun L and Yu S: Meta-analysis:
non-steroidal anti-inflammatory drug use and the risk of esophageal
squamous cell carcinoma. Dis Esophagus. 24:544–549. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao LM, Vaughan TL, Corley DA, et al:
Nonsteroidal anti-inflammatory drug use reduces risk of
adenocarcinomas of the esophagus and esophagogastric junction in a
pooled analysis. Gastroenterology. 142:442–485. 2012. View Article : Google Scholar
|
|
25
|
Cai JB, Zhang CX, Luo JW and Wang D: A
study of 131iodine labeling of indomethacin, its in vivo
biological distribution in Lewis-bearing lung cancer, and its
induction of apoptosis in lung cancer. Saudi Med J. 32:15–22.
2011.PubMed/NCBI
|
|
26
|
Pelzmann M, Thurnher D, Gedlicka C,
Martinek H and Knerer B: Nimesulide and indomethacin induce
apoptosis in head and neck cancer cells. J Oral Pathol Med.
33:607–613. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin S, Yang C, Wang X, et al:
Overexpression of Smac promotes Cisplatin-induced apoptosis by
activating caspase-3 and caspase-9 in lung cancer A549 cells.
Cancer Biother Radiopharm. 28:177–182. 2013. View Article : Google Scholar
|
|
28
|
Zhang XD, Zhang XY, Gray CP, Nguyen T and
Hersey P: Tumor necrosis factor-related apoptosis-inducing
ligand-induced apoptosis of human melanoma is regulated by
Smac/DIABLO release from mitochondria. Cancer Res. 61:7339–7348.
2001.PubMed/NCBI
|
|
29
|
Greer RM, Peyton M, Larsen JE, et al: SMAC
mimetic (JP1201) sensitizes non-small cell lung cancers to multiple
chemotherapy agents in an IAP-dependent but TNF-α-independent
manner. Cancer Res. 71:7640–7648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Y, Zhou L, Huang J, et al: Role of Smac
in determining the chemotherapeutic response of esophageal squamous
cell carcinoma. Clin Cancer Res. 17:5412–5422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du N, Yang B, Hu L, et al: Overexpression
of Smac gene erhanced chemotherapeutic sensitivity of esophageal
cancer cell line Eca109 to cisplatin. Xi Bao Yu Fen Mian Yi Xue Za
Zhi. 28:344–346. 2012.In Chinese.
|
|
32
|
Fulda S: Molecular pathways: targeting
death receptors and smac mimetics. Clin Cancer Res. 20:3915–3920.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai L, Smith DC and Wang S: Small-molecule
SMAC mimetics as new cancer therapeutics. Pharmacol Ther.
144:82–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li L, Thomas RM, Suzuki H, De Brabander
JK, Wang X and Harran PG: A small molecule Smac mimic potentiates
TRAIL- and TNFalpha-mediated cell death. Science. 305:1471–1474.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Petersen SL, Wang L, Yalcin-Chin A, et al:
Autocrine TNFalpha signaling renders human cancer cells susceptible
to smac-mimetic-induced apoptosis. Cancer Cell. 12:445–456. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mao HL, Pang Y, Zhang X, et al: Smac
peptide potentiates TRAIL- or paclitaxel-mediated ovarian cancer
cell death in vitro and in vivo. Oncol Rep. 29:515–522. 2013.
|
|
37
|
Bahadur S, Keshri L and Pathak K: Adverse
drug reactions and safety considerations of NSAIDs: clinical
analysis. Curr Drug Saf. 6:310–7. 2011. View Article : Google Scholar
|