Introduction
Staphylococcus aureus is a bacterium that
grows in the human nose and skin and is a major pathogen that
causes skin and soft-tissue infections, which have previously been
treated with the antibiotic methicillin. Since its detection in
1961, methicillin-resistant S. aureus (MRSA) has become the
most problematic Gram-positive bacterium in the public health arena
(1). This pathogen is associated
with a variety of infectious diseases (2) and has an average mortality rate of
36–50% (3). With increasing
resistance to various antibiotics, combination therapy is a
potential alternative. It may prove particularly useful in
developing countries where availability of antibiotics is limited,
as it allows for a reduction in the dose of the antibiotic required
(4–6). Furthermore, MRSA bacteria are not
only resistant to β-lactam antibiotics but also to fluoroquinolones
and other families of antibiotics (4).
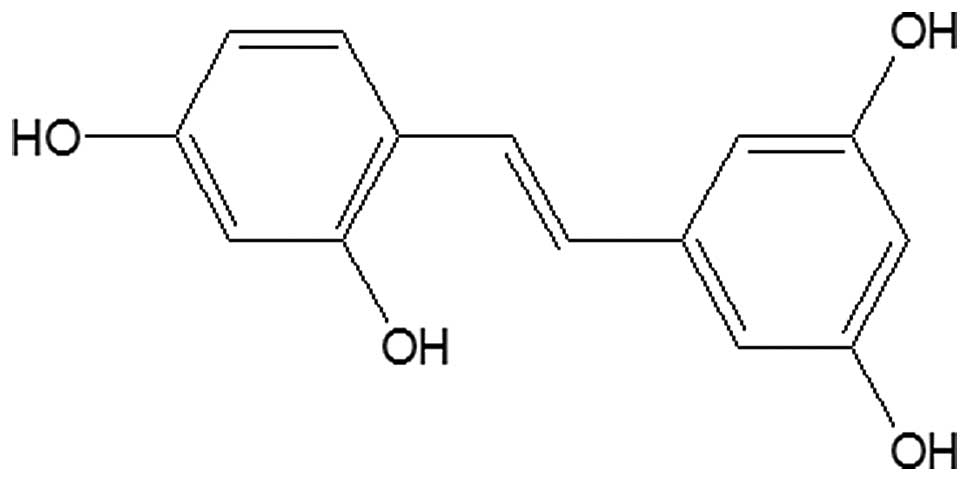
Oxyresveratrol (ORV) is an antioxidant (7), anthelmintic (8) tyrosinase inhibitor (9) and a cyclooxygenase inhibitor
(10,11). Various studies have indicated that
oxyresveratrol (Fig. 1) inhibits
apoptotic cell death in transient cerebral ischemia (12), is hepatoprotective (13) and is a potent free radical
scavenger (7). Oxyresveratrol has
been demonstrated to have an inhibitory effect on the herpes
simplex and varicella zoster virus (14,15).
In addition, the compound has been revealed to have skin-whitening
(16) and neuroprotective effects
(6,7,13,14).
However, the antimicrobial capacity of ORV against
Staphylococcus aureus remains unknown. Therefore, the
antibacterial activity of ORV alone and of ORV in conjunction with
commonly-used antibiotics was investigated in the present
study.
Materials and methods
Materials and chemicals
Ampicillin (AM), oxacillin (OX), gentamicin (GT),
vancomycin (VC), norfloxacin (NR) and ciprofloxacin (CP) (all
supplied by Sigma-Aldrich, St. Louis, MO, USA) were used.
Oxyresveratrol (>96.32%) was deposited at the Standardized
Material Bank for New Botanical Drugs (No NNMBP000018) at Wonkwang
University (Iksan, Republic of Korea). The twigs of Morus
alba were purchased from the herbal medicine co-operative
association of Jeonbuk Province, Korea, in October 2000. A voucher
specimen (no. WP 217) was deposited at the Herbarium of the College
of Pharmacy, Wonkwang University (Korea). Dried twigs of M.
alba (2 kg) were extracted with EtOH (2 l) for 20 days at room
temperature. Dried residue of the EtOH extract (101 g) was
dissolved in 40% aqueous MeOH (1 L) and partitioned with n-hexane
(800 ml x2), CH2Cl2 (800 ml x2) and EtOAc
(800 ml x2), successively. The CH2Cl2 soluble
fraction (8.53 g) was chromatographed on Sephadex LH-20 column (5×
16 cm) using CH2Cl2-MeOH (4:1 - 1:1; each
volume, 300 ml) to obtain four fractions (Fr. A-D). The EtOAc
soluble fraction (4.83 g) was chromatographed on silica gel (250 g)
column using CH2Cl2-MeOH (8:1 - 4:1; each
volume, 600 ml) to obtain three fractions (Fr. D–F). Fr. E (2.77 g)
was chromatographed on silica gel (150 g) column (eluent:
n-hexane-acetone, 1:1) and further purified by Sephadex LH-20
column (2.5 × 20 cm) chromatography (eluent,
CH2Cl2-MeOH 4:1) to give oxyresveratrol (1.12
g, 0.056 w/w%). The structure of oxyresveratrol was identified by
analysis of nuclear magnetic resonance and mass spectra.
Bacterial strains and growth
conditions
Two clinical MRSA isolates were obtained from two
patients at Wonkwang University Hospital (Iksan, South Korea). The
other two strains were obtained from the American Type Culture
Collection (Manassas, VA, USA), and included S. aureus
(33591; methicillin-resistant strain) and S. aureus (25923;
methicillin-susceptible strain). Prior to each experiment, all
bacteria were stored in 30% glycerol and frozen at −70°C. The
bacteria were cultured in Difco™ Mueller-Hinton broth (MHB) and
Mueller-Hinton agar (MHA) (BD Biosciences, Franklin Lakes, NJ, USA)
by incubating at 37°C for 24 h.
Minimum inhibitory concentration
(MIC)
MICs were determined using the broth microdilution
method according to the guidelines of the Clinical and Laboratory
Standards Institute (17).
Briefly, a preparation of microorganism suspension was prepared by
growing the bacteria in broth for 24 h, which were adjusted to a
0.5 McFarland standard turbidity [~1.5×108 colony
forming units (CFU)/ml]. The final inoculums were adjusted to
1.5×106 CFU/ml. The serially diluted extracts were then
incubated along with the inoculum at 37°C for 18 h. The MIC was
defined as the lowest concentration of antibiotics and ORV that
prevented visible growth of the bacteria. At the end of the
incubation period, well plates were visually examined for
turbidity. Cloudiness indicated that bacterial growth had not been
inhibited.
Checkerboard dilution test
Synergistic combinations were investigated using the
preliminary checkerboard method according to the published
standards (5,18). The MIC was defined as the lowest
concentration of drug alone or in combination that inhibited
visible growth. In vitro interaction was quantified as the
fractional inhibitory concentration index (FICI), which was
calculated using the following formula: FICI = (MIC of drug A in
combination/MIC of drug A alone) + (MIC of drug B in
combination/MIC of drug B alone). FICIs were interpreted as
follows: <0.5, synergy; 0.5–0.75, partial synergy; 0.76–1.0,
additive effect; 1.0–4.0, indifference; and >4.0, antagonism.
The varying levels of synergy between two given agents were
determined (19). All experiments
were repeated three times.
MTT colorimetric assay
A colorimetric assay based on MTT for rapid
detection of the presence of bacteria was performed as previously
described (20–22). Briefly, a stock solution of 5 mg/ml
MTT (Sigma-Aldrich) was prepared in phosphate-buffered saline
(Sigma-Aldrich) and stored at −70°C. A final concentration of 1
mg/ml MTT was used in the assay. Following 24-h incubation at 37°C,
20 μl yellow MTT was added to a 96-well microtiter plate and
incubated for an additional 20 min. Blue color indicated the
presence of bacteria.
Time-kill curve assay
A time-kill curve assay was performed according to a
previous method (23) in order to
investigate the combined effect of time and antimicrobial agent
concentration on bacterial growth. For this assay, standard
inoculums of ~106 CFU/ml were used. ORV (0.5 MIC) was
used with various combinations of antibiotics (0.5 MIC). A test
plate containing MHB and inoculum was used as the control. Counts
of viable strains were conducted at different intervals up to 24 h
at 37°C. The rate and extent of bacterial death was determined by
plotting the viable colony counts (CFU/ml) against the time
cultured in MHA. All experiments were repeated three times.
Statistical analysis
All experiments were performed more than three
times. Data from the experiments are presented as the mean ±
standard error of the mean. Statistical analyses were performed
using one-way analysis of variance followed by Dunnett’s t-test
(SPSS software, version 19.0; IBM SPSS, Armonk, NY, USA).
P<0.001 was considered to indicate a statistically significant
difference.
Results
MIC determination and synergic
effect
Against all strains, the MIC was 125 μg/ml
for ORV. VC presented a range of MICs of 1.95–3.9 μg/ml in
the various strains. All strains were resistant to AM, OX, GT, CP
and NR, with MIC values ranging from 15.6 to 1,000 μg/ml.
ORV + antibiotic combinations all exhibited markedly lower MICs
than those when the drugs were used alone. The combined use of ORV
and VC or CP against the 33591 MRSA strain resulted in a FICI of
0.375 (synergy) (Table I),
indicating that an activity-enhancing effect was present. The
combined use of ORV and AM or OX against the 33591 MRSA strain
resulted in a FICI of 0.75–1, but against the 25923 MSSA strain
resulted in a FICI of 0.1875 (synergy). The combined use of ORV and
NR against all of the strains resulted in a FICI of 0.75–1 (partial
synergy or additive effect). None of the combinations exhibited an
antagonistic effect (FICI >4.0). These results demonstrated that
the method of combining ORV with antibiotics has potential to be
used to suppress MRSA growth.
 | Table IInterpreted FICI response against MRSA
and MSSA strains. |
Table I
Interpreted FICI response against MRSA
and MSSA strains.
A, Response for ORV
and AM
|
|---|
| Strain | ORVa
| AMa
| FICI |
|---|
| Alone | With AM | Alone | With ORV |
|---|
| ATCC 33591 | 125 | 62.5 | 1,000 | 250 | 0.75 |
| ATCC 25923 | 125 | 15.6 | 31.25 | 1.95 | 0.1875 |
| DPS-1 | 125 | 31.25 | 1,000 | 500 | 0.75 |
| DPS-2 | 125 | 62.5 | 1,000 | 500 | 1.00 |
B, Response for ORV
and OX
|
|---|
| Strain | ORVa
| OXa
| FICI |
|---|
| Alone | With OX | Alone | With ORV |
|---|
| ATCC 33591 | 125 | 62.5 | 500 | 250 | 1 |
| ATCC 25923 | 125 | 15.6 | 125 | 7.81 | 0.1875 |
| DPS-1 | 125 | 62.5 | 1,000 | 62.5 | 0.5625 |
| DPS-2 | 125 | 62.5 | 1,000 | 250 | 0.75 |
C, Response for ORV
and GT
|
|---|
| Strain | ORVa
| GTa
| FICI |
|---|
| Alone | With GT | Alone | With ORV |
|---|
| ATCC 33591 | 125 | 62.5 | 31.25 | 3.9 | 0.625 |
| ATCC 25923 | 125 | 31.25 | 62.5 | 7.8 | 0.375 |
| DPS-1 | 125 | 62.5 | 250 | 15.6 | 0.5625 |
| DPS-2 | 125 | 62.5 | 125 | 31.25 | 0.75 |
D, Response for ORV
and VC
|
|---|
| Strain | ORVa
| VCa
| FICI |
|---|
| Alone | With VC | Alone | With ORV |
|---|
| ATCC 33591 | 125 | 31.25 | 1.95 | 0.24 | 0.375 |
| ATCC 25923 | 125 | 62.5 | 3.9 | 1.95 | 1 |
| DPS-1 | 125 | 62.5 | 1.95 | 0.98 | 1 |
| DPS-2 | 125 | 31.25 | 3.9 | 0.98 | 0.5 |
E, Response for ORV
and CP
|
|---|
| Strain | ORVa
| CPa
| FICI |
|---|
| Alone | With CP | Alone | With ORV |
|---|
| ATCC 33591 | 125 | 31.25 | 500 | 62.5 | 0.375 |
| ATCC 25923 | 125 | 15.6 | 31.25 | 3.9 | 0.25 |
| DPS-1 | 125 | 15.6 | 125 | 31.25 | 0.375 |
| DPS-2 | 125 | 31.25 | 125 | 31.25 | 0.5 |
F, Response for ORV
and NR
|
|---|
| Strain | ORVa
| NRa
| FICI |
|---|
| Alone | With NR | Alone | With ORV |
|---|
| ATCC 33591 | 125 | 62.5 | 250 | 62.5 | 0.75 |
| ATCC 25923 | 125 | 62.5 | 15.6 | 3.9 | 0.75 |
| DPS-1 | 125 | 62.5 | 31.25 | 15.6 | 1 |
| DPS-2 | 125 | 62.5 | 31.25 | 7.8 | 0.75 |
The controls displayed no reduction in CFU counts,
and the use of ORV or antibiotics alone did not induce cell death
at 24 h. When used in combination, ORV and antibiotics led to a
marked reduction in bacterial counts. In particular, the
combination of ORV + GT and ORV + CP completely inhibited growth of
S. aureus after 24 h. These were the most effective
treatments and thus were selected for further analysis.
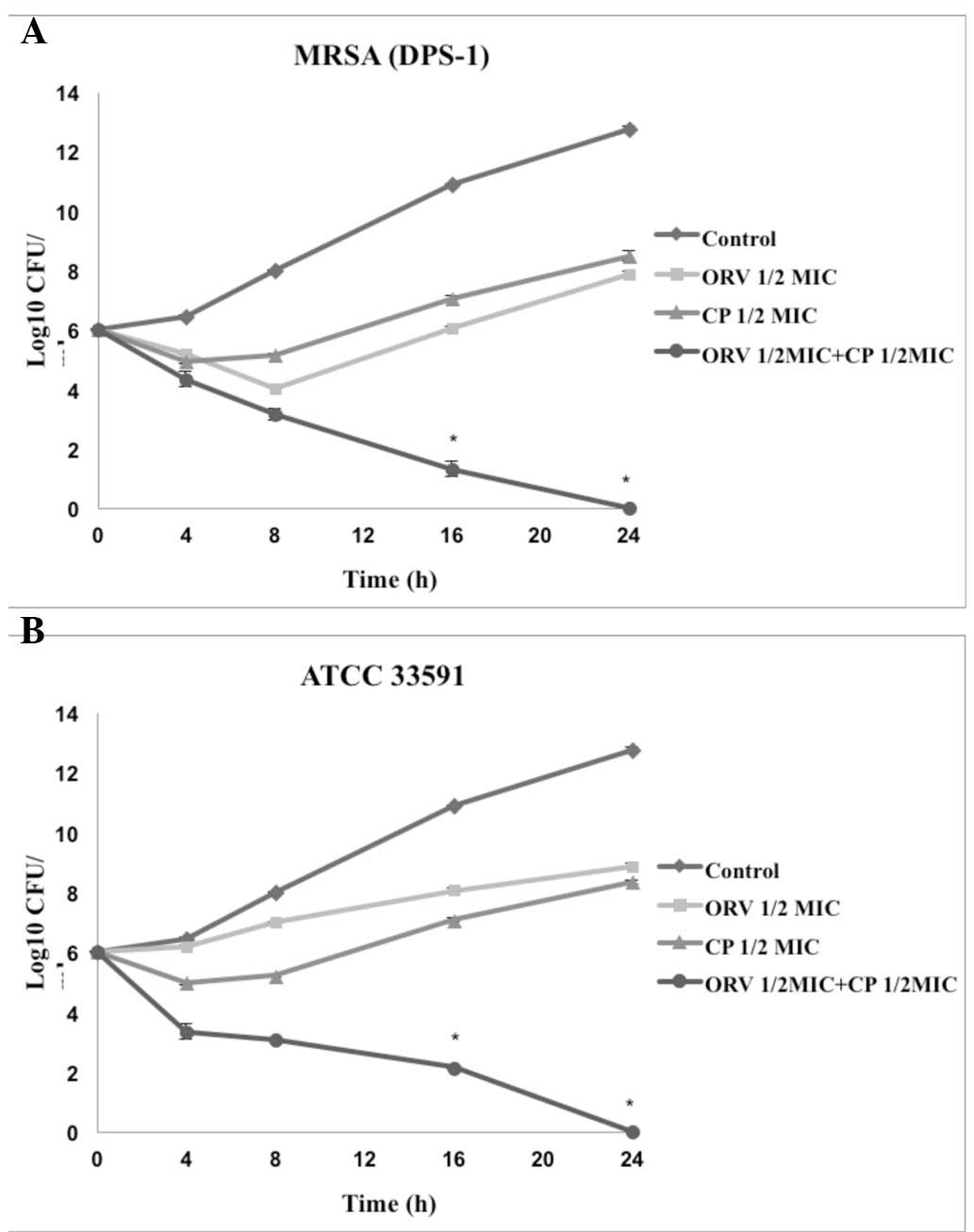
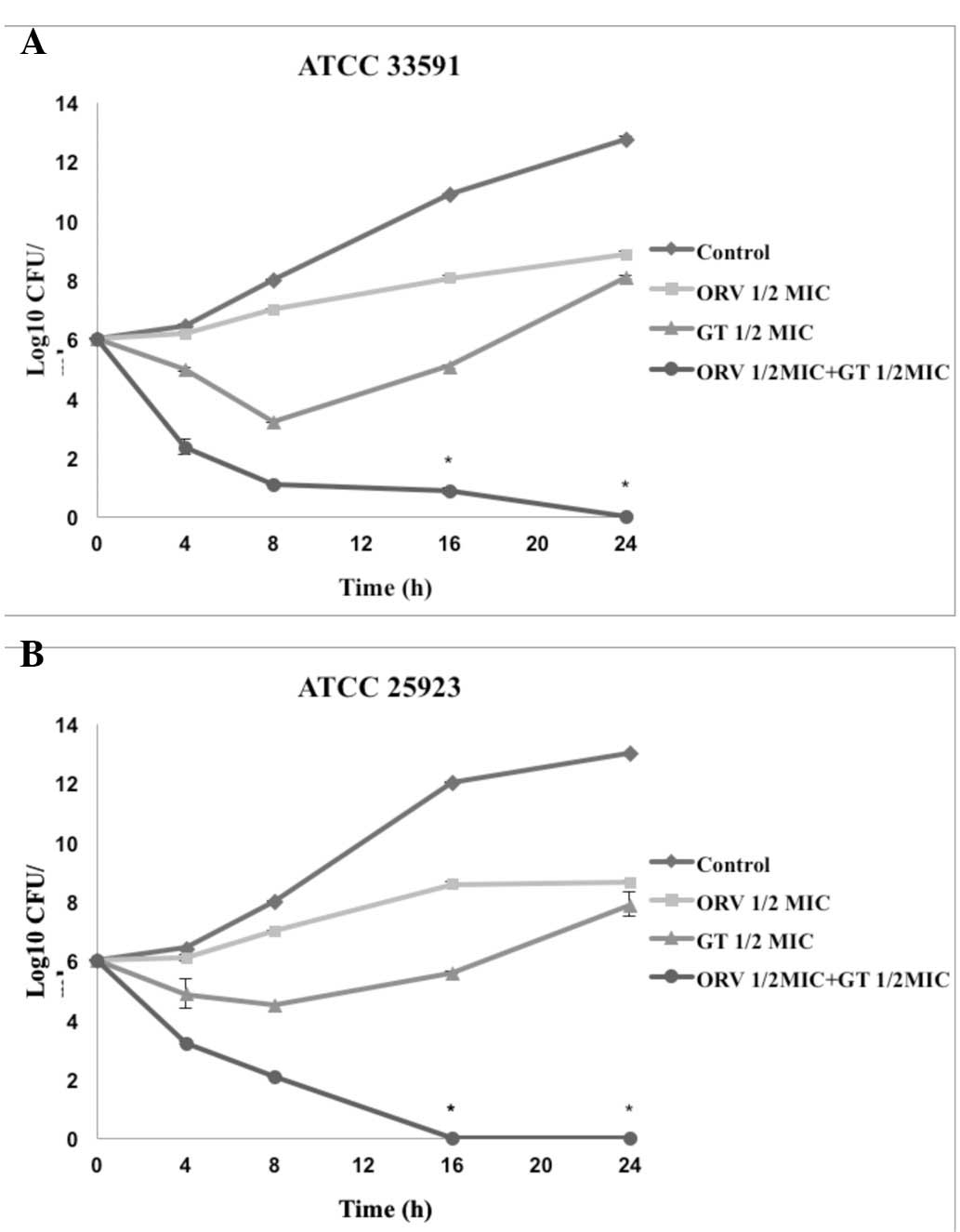
Time-kill curve assay
Time-kill tests were performed to investigate the
synergistic effects of ORV in combination with antibiotics and the
effect of length of treatment on cell viability. The control
displayed no reduction in CFU counts, and the use of ORV or
antibiotics alone did not induce cell death at 24 h. When used in
combination, ORV and antibiotics caused a marked reduction in
bacterial counts. In particular, the combination of ORV + GT and
ORV + CP completely inhibited growth of S. aureus after 24 h
(Figs. 2 and 3).
Discussion
The most effective method to develop antibiotics
that produce minimal toxic effects or side effects is to use
natural products. Therefore, there is a requirement for the
development of alternative antimicrobial drugs against infectious
diseases. Combination therapy is the most commonly recommended
empirical treatment for bacterial infections in intensive care
units where monotherapy may not be effective against all potential
pathogens or for the prevention of antibacterial resistance
(24). When combined, certain
antibiotics are known to markedly increase bactericidal effects
(5,24,25).
To the best of our knowledge, the current study was
the first to investigate the potentiation of antibiotics by ORV
against MRSA. The MIC assay is considered to be the standard method
for determining the susceptibility of various microorganisms to
antibacterial agents. The in vitro results of the present
study determined the MIC values of ORV and antibiotics against
S. aureus.
Synergistic or partially synergistic effects of ORV
in combination with the antibiotic agents VC, GT and CP strongly
supported this explanation. The time-kill curves and FICI scores
confirmed the ability of ORV to synergistically reduce bacterial
counts below the lowest detectable limit within 24 h. Therefore,
ORV may be a potential antibacterial drug candidate for clinical
use against MRSA. The results of the present study are promising
and may increase the use of natural products as drugs.
Acknowledgments
The current study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education, Science and Technology (grant
no. 2012-0004337) and by the Basic Science Research Program through
the National Research Foundation of Korea(NRF) funded by the
Ministry of Education (grant no. 2013060380).
References
|
1
|
Witte W: Antibiotic resistance in
gram-positive bacteria: epidemiological aspects. J Antimicrob
Chemoth. 44(Suppl A): 1–9. 1999. View Article : Google Scholar
|
|
2
|
Baltch AL, Ritz WJ, Bopp LH, Michelsen PB
and Smith RP: Antimicrobial activities of daptomycin, vancomycin,
and oxacillin in human monocytes and of daptomycin in combination
with gentamicin and/or rifampin in human monocytes and in broth
against Staphylococcus aureus. Antimicrob Agents Chemother.
51:1559–1562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dancer SJ: The effect of antibiotics on
methicillin-resistant Staphylococcus aureus. J Antimicrob
Chemother. 61:246–253. 2008. View Article : Google Scholar
|
|
4
|
Aqil F, Ahmad I and Owais M: Evaluation of
anti-methicillin-resistant Staphylococcus aureus (MRSA) activity
and synergy of some bioactive plant extracts. Biotechnol J.
1:1093–1102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miranda-Novales G, Leaños-Miranda BE,
Vilchis-Pérez M and Solórzano-Santos F: In vitro activity effects
of combinations of cephalothin, dicloxacillin, imipenem, vancomycin
and amikacin against methicillin-resistant Staphylococcus spp.
strains. Ann Clin Microbiol Antimicrob. 5:252006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kastoris AC, Rafailidis PI, Vouloumanou
EK, Gkegkes ID and Falagas ME: Synergy of fosfomycin with other
antibiotics for Gram-positive and Gram-negative bacteria. Eur J
Clin Pharmacol. 66:359–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenz P, Roychowdhury S, Engelmann M,
Wolf G and Horn TF: Oxyresveratrol and resveratrol are potent
antioxidants and free radical scavengers: effect on nitrosative and
oxidative stress derived from microglial cells. Nitric Oxide.
9:64–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saowakon N, Tansatit T, Wanichanon C, et
al: Fasciola gigantica: anthelmintic effect of the aqueous extract
of Artocarpus lakoocha. Exp Parasitol. 122:289–298. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YM, Yun J, Lee CK, et al:
Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on
tyrosinase and mechanism of action. J Biol Chem. 227:16340–16344.
2002. View Article : Google Scholar
|
|
10
|
Shin NH, Ryu SY, Choi EJ, et al:
Oxyresveratrol as the potent inhibitor on dopa oxidase activity of
mushroom tyrosinase. Biochem Biophys Res Commun. 243:801–803. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin NH, Ryu SY, Lee HS, Min KR and Kim
YS: Inhibitory effects of hydroxystilbenes on cyclooxygenase from
sheep seminal vesicles. Planta Med. 64:283–284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ban JY, Jeon SY, Nguyen TT, et al:
Neuroprotective effect of oxyresveratrol from smilacis chinae
rhizome on amyloid Beta protein (25–35)-induced neurotoxicity in
cultured rat cortical neurons. Biol Pharm Bull. 29:2419–2424. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oh H, Ko EK, Jun JY, et al:
Hepatoprotective and free radical scavenging activities of
prenylflavonoids, coumarin, and stilbene from Morus alba. Planta
Med. 68:932–934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuanasa T, Phromjai J, Lipipun V, et al:
Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol
derived from Thai medicinal plant: mechanism of action and
therapeutic efficacy on cutaneous HSV-1 infection in mice.
Antiviral Res. 80:62–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasivimolphan P, Lipipun V,
Likhitwitayawuid K, et al: Inhibitory activity of oxyresveratrol on
wild-type and drug-resistant varicella-zoster virus replication in
vitro. Antiviral Res. 84:95–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Likhitwitayawuid K: Stilbene with
tyrosinase inhibitory activity. Curr Sci. 94:44–52. 2008.
|
|
17
|
Clinical and Laboratory Standards
Institute (CLSI): CLSI documents M7-A5: Methods for dilution
antimicrobial susceptibility tests for bacteria that grow
aerobically; approved standard. 5th edition. CLSI; Wayne, PA, USA:
2000
|
|
18
|
Odds FC: Synergy, antagonism, and what the
chequerboard puts between them. J Antimicrob Chemother. 52:12003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazumdar K, Dutta NK, Kumar KA and
Dastidar SG: In vitro and in vivo synergism between tetracycline
and the cardiovascular agent oxyfedrine HCl against common
bacterial strains. Biol Pharm Bull. 28:713–717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scheuber PH, Mossmann H, Beck G and Hammer
DK: Direct skin test in highly sensitized guinea pigs for rapid and
sensitive determination of staphylococcal enterotoxin B. Appl
Environ Microbiol. 46:1351–1356. 1983.PubMed/NCBI
|
|
21
|
Abate G, Mshana RN and Miörner H:
Evaluation of a colorimetric assay based on
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
for rapid detection of rifampicin resistance in Mycobacterium
tuberculosis. Int J Tuberc Lung Dis. 2:1011–1016. 1998.PubMed/NCBI
|
|
22
|
Shi YJ, Chen J and Xu M: A new method for
antimicrobial susceptibility testing of in vitro-cultured bacteria
by means of resonance light scattering technique. J Microbiol
Biotechnol. 18:118–123. 2008.PubMed/NCBI
|
|
23
|
Nascimento AM, Brandão MG, Oliveira GB,
Fortes IC and Chartone-Souza E: Synergistic bactericidal activity
of Eremanthus erythropappus oil or beta-bisabolene with ampicillin
against Staphylococcus aureus. Antonie Van Leeuwenhoek. 92:95–100.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Drago L, De Vecchi E, Nicola L and
Gismondo MR: In vitro evaluation of antibiotics’ combinations for
empirical therapy of suspected methicillin resistant Staphylococcus
aureus severe respiratory infections. BMC Infect Dis. 7:1112007.
View Article : Google Scholar
|
|
25
|
Liu IX, Durham DG and Richards RM:
Baicalin synergy with beta-lactam antibiotics against
methicillin-resistant Staphylococcus aureus and other
beta-lactam-resistant strains of S. aureus. J Pharm Pharmacol.
52:361–366. 2000. View Article : Google Scholar : PubMed/NCBI
|